Idaho Medicaid Drug Utilization Review Program 23 August














































































- Slides: 123

Idaho Medicaid Drug Utilization Review Program 23 August 2012

Follow-up to Previous Reviews Citalopram High Dose DUR 2

Citalopram High Dose DUR FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide) On August 24, 2011, the Food and Drug Administration (FDA) released a Safety Announcement addressing the high dose of citalopram and potential adverse effects it can have on the heart. The maximum daily dose is now recommended to be 40 mg per day when it was previously 60 mg per day. 3

Citalopram High Dose DUR On March 28, 2012 the FDA sent out a revised Drug Safety Communication with updated recommendations: Recognition that although citalopram use should be avoided, if possible, in patients with certain conditions because of the risk of QT prolongation, ECG monitoring and/or electrolyte monitoring is recommended if citalopram must be used in such patients. Patients with congenital long QT syndrome are at particular risk of Torsade de Pointes, ventricular tachycardia, and sudden death when given drugs that prolong the QT interval. Nevertheless, the labeling recommendation for patients with congenital long QT syndrome has been changed from “contraindicated” to “not recommended, ” because it is recognized that there may be some patients with this condition who could benefit from a low dose of citalopram and who lack viable alternatives. The maximum recommended dose of citalopram is 20 mg per day for patients over the age of 60. Citalopram is recommended to be discontinued in patients who are found to have persistent QTc measurements greater than 500 ms. 4

Citalopram High Dose DUR It was decided at the last DUR Board Meeting that we would look at the numbers again once a little time had past after the FDA Safety Announcement. 5

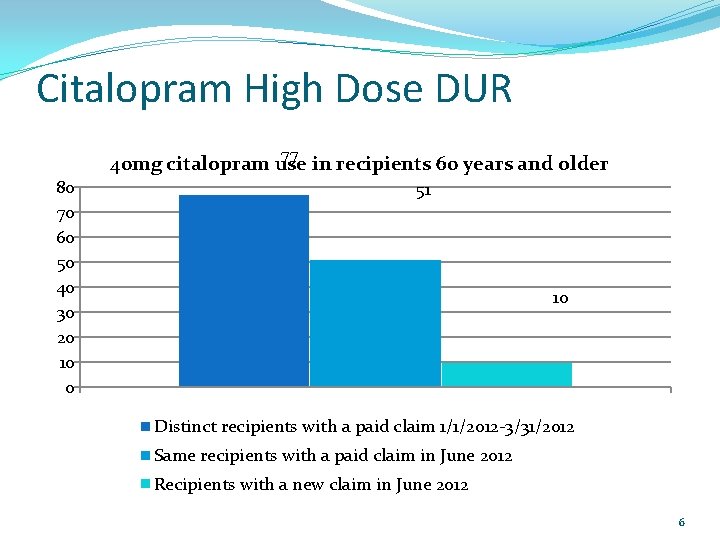
Citalopram High Dose DUR 77 40 mg citalopram use in recipients 60 years and older 80 70 60 50 40 30 20 10 0 51 10 Distinct recipients with a paid claim 1/1/2012 -3/31/2012 Same recipients with a paid claim in June 2012 Recipients with a new claim in June 2012 6

Citalopram High Dose DUR Next steps ? ? ? 7

Current Interventions/Outcomes Studies P&T Committee Narcotic Analgesic Studies Aliskiren DUR Lupron DUR Ciprofloxacin DUR Synagis DUR – Medical Claims Data Growth Hormone DUR Psychotropic Medications in Foster Children Update Five (5) or more psychotropic medications prescribed concomitantly 8

Narcotic Patterns of Use in Chronic Non-Malignant Pain Complete Report 9

Profile Review Generated profiles for the top 150 recipients by total narcotic claim count from the recipients who had at least one narcotic claim in each of the 24 months of the period ending December 2011 Time Period: May 1, 2011 through December 31, 2011 Evaluated 144 Cancer Diagnosis found in 6 All profiles were hand reviewed by Idaho Medicaid Pharmacists 10

Review Focus Years of opioid use Number of different opioids used Daily morphine equivalents Number of different prescribers Other concurrent potentially addictive drugs Diagnosis or indication for chronic opioid use Average days between refills History of abuse diagnosis Currently in lock-in program? Additional opioid use outside of Medicaid

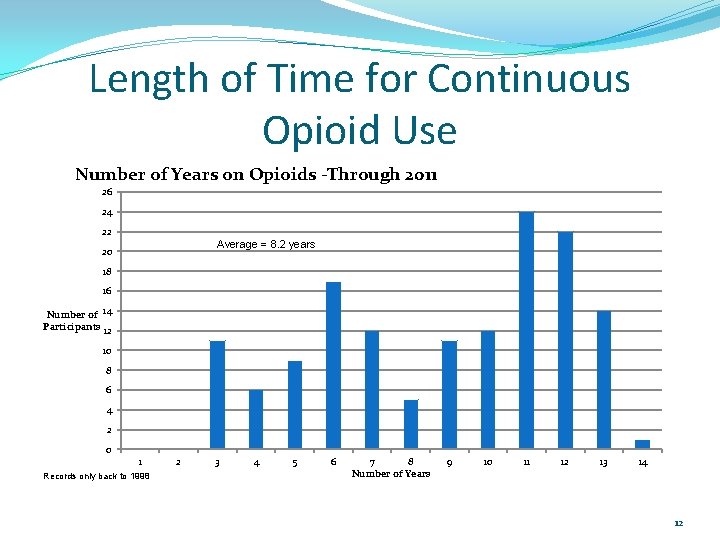
Length of Time for Continuous Opioid Use Number of Years on Opioids -Through 2011 26 24 22 Average = 8. 2 years 20 18 16 Number of 14 Participants 12 10 8 6 4 2 0 1 Records only back to 1998 2 3 4 5 6 7 8 Number of Years 9 10 11 12 13 14 12

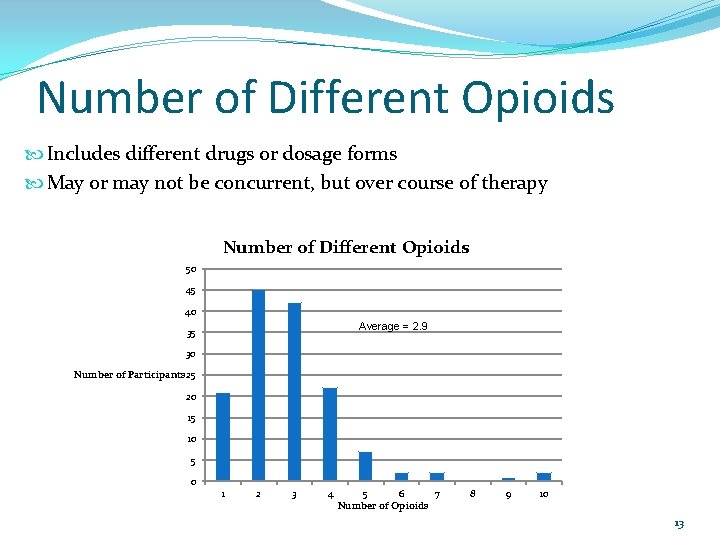
Number of Different Opioids Includes different drugs or dosage forms May or may not be concurrent, but over course of therapy Number of Different Opioids 50 45 40 Average = 2. 9 35 30 Number of Participants 25 20 15 10 5 0 1 2 3 4 5 6 7 Number of Opioids 8 9 10 13

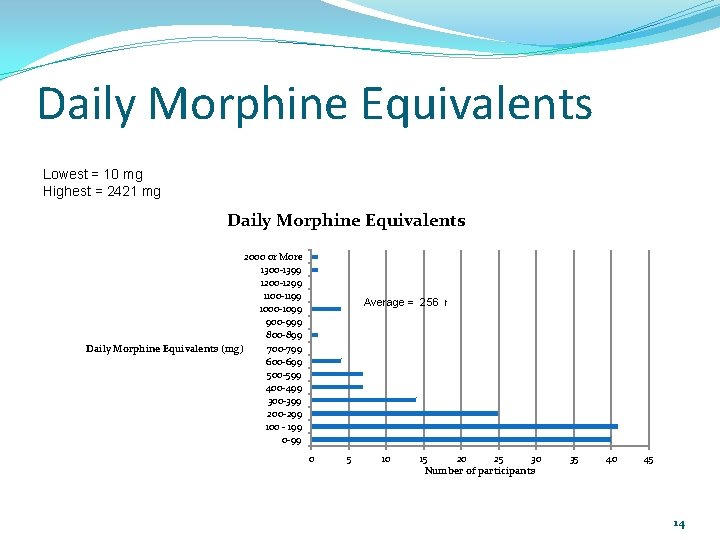
Daily Morphine Equivalents Lowest = 10 mg Highest = 2421 mg Daily Morphine Equivalents 2000 or More 1300 -1399 1200 -1299 1100 -1199 1000 -1099 900 -999 800 -899 Daily Morphine Equivalents (mg) 700 -799 600 -699 500 -599 400 -499 300 -399 200 -299 100 - 199 0 -99 Average = 256 mg equivalents 0 5 10 15 20 25 30 Number of participants 35 40 45 14

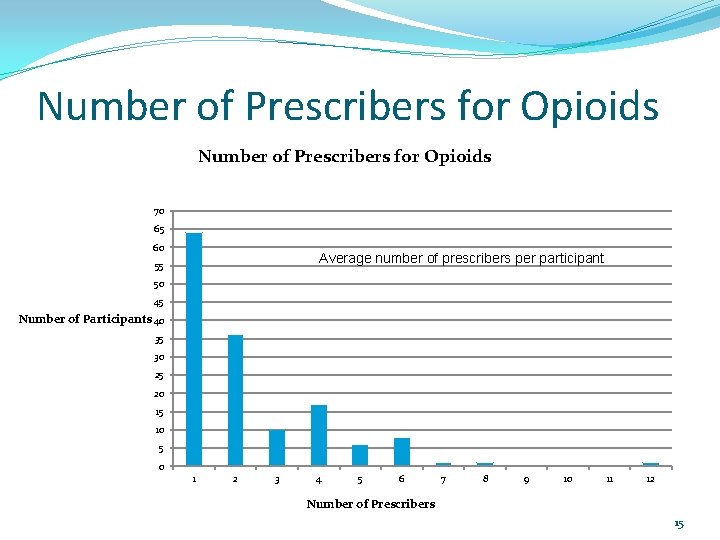
Number of Prescribers for Opioids 70 65 60 Average number of prescribers per participant is 2 55 50 45 Number of Participants 40 35 30 25 20 15 10 5 0 1 2 3 4 5 6 7 8 9 10 11 12 Number of Prescribers 15

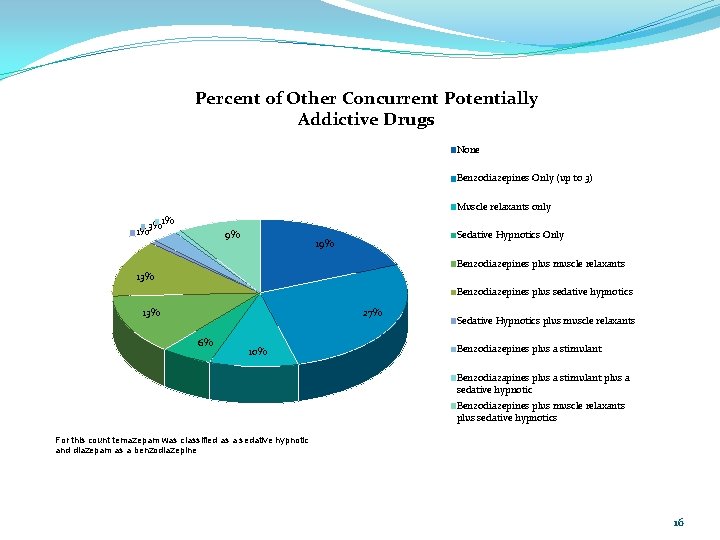
Percent of Other Concurrent Potentially Addictive Drugs None Benzodiazepines Only (up to 3) Muscle relaxants only 3% 1% 1% 9% Sedative Hypnotics Only 19% Benzodiazepines plus muscle relaxants 13% Benzodiazepines plus sedative hypnotics 13% 27% 6% 10% Sedative Hypnotics plus muscle relaxants Benzodiazepines plus a stimulant Benzodiazapines plus a stimulant plus a sedative hypnotic Benzodiazepines plus muscle relaxants plus sedative hypnotics For this count temazepam was classified as a sedative hypnotic and diazepam as a benzodiazepine 16

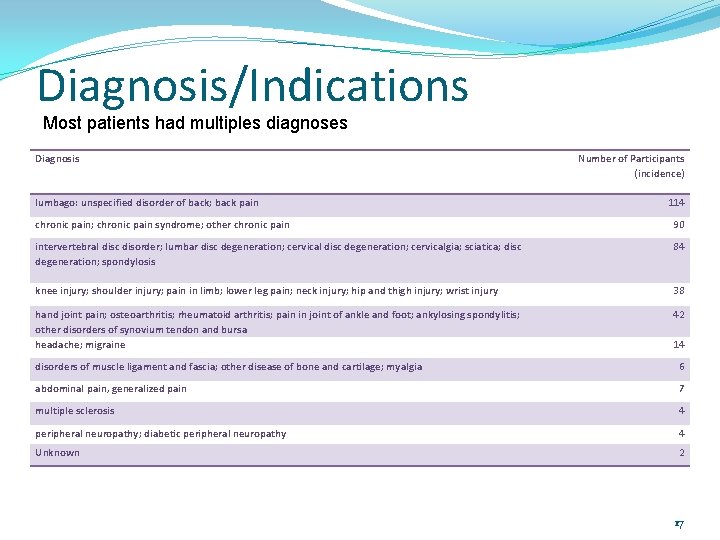
Diagnosis/Indications Most patients had multiples diagnoses Diagnosis lumbago: unspecified disorder of back; back pain Number of Participants (incidence) 114 chronic pain; chronic pain syndrome; other chronic pain 90 intervertebral disc disorder; lumbar disc degeneration; cervicalgia; sciatica; disc degeneration; spondylosis 84 knee injury; shoulder injury; pain in limb; lower leg pain; neck injury; hip and thigh injury; wrist injury 38 hand joint pain; osteoarthritis; rheumatoid arthritis; pain in joint of ankle and foot; ankylosing spondylitis; other disorders of synovium tendon and bursa headache; migraine 42 14 disorders of muscle ligament and fascia; other disease of bone and cartilage; myalgia 6 abdominal pain, generalized pain 7 multiple sclerosis 4 peripheral neuropathy; diabetic peripheral neuropathy 4 Unknown 2 17

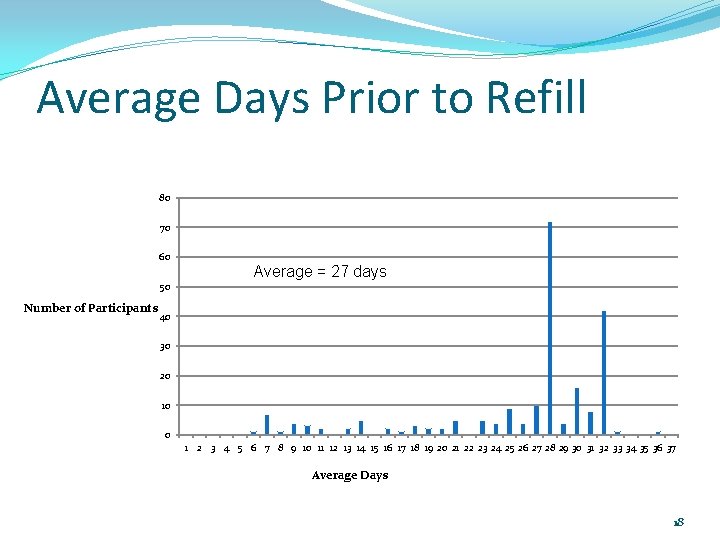
Average Days Prior to Refill 80 70 60 Average = 27 days 50 Number of Participants 40 30 20 10 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 Average Days 18

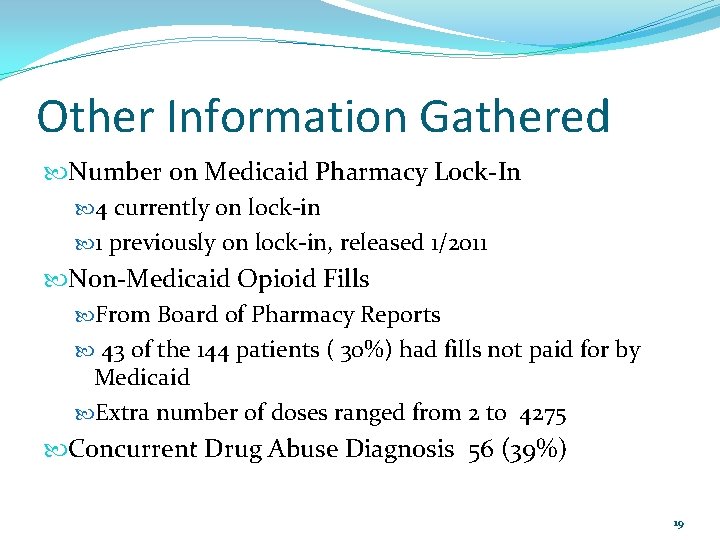
Other Information Gathered Number on Medicaid Pharmacy Lock-In 4 currently on lock-in 1 previously on lock-in, released 1/2011 Non-Medicaid Opioid Fills From Board of Pharmacy Reports 43 of the 144 patients ( 30%) had fills not paid for by Medicaid Extra number of doses ranged from 2 to 4275 Concurrent Drug Abuse Diagnosis 56 (39%) 19

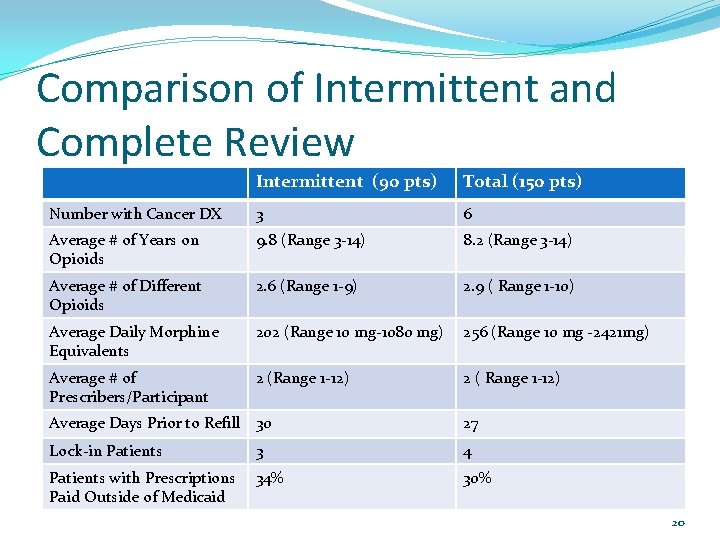
Comparison of Intermittent and Complete Review Intermittent (90 pts) Total (150 pts) Number with Cancer DX 3 6 Average # of Years on Opioids 9. 8 (Range 3 -14) 8. 2 (Range 3 -14) Average # of Different Opioids 2. 6 (Range 1 -9) 2. 9 ( Range 1 -10) Average Daily Morphine Equivalents 202 (Range 10 mg-1080 mg) 256 (Range 10 mg -2421 mg) Average # of Prescribers/Participant 2 (Range 1 -12) 2 ( Range 1 -12) Average Days Prior to Refill 30 27 Lock-in Patients 3 4 Patients with Prescriptions Paid Outside of Medicaid 34% 30% 20

Narcotic Analgesics DUR Board Recommendations April 2012 Complete Review for the rest of top 150 patients Research other state restrictions and initiatives P&T Recommendations May 2012 Long- Acting Narcotic Analgesics Tighten up definition of “failure of a preferred agent” beyond just a prior fill of preferred agent Removal of history of preferred oral agent for fentanyl transdermal 21

Review of External Programs and Recommendations 22

PROP – Physicians for Responsible Opioid Prescribing www. responsibleopioidprescribing. org Mission Statement Our mission is to reduce morbidity and mortality resulting from prescribing of opioids and to promote cautious, safe and responsible opioid prescribing practices. Disclosure Statement PROP does not accept financial support from pharmaceutical companies, medical device companies, urine toxicology laboratories or any other entities that could be perceived as a potential conflict of interest. Cautious Evidence Based Opioid Prescribing Four page handout included in your packet. Video: Effectiveness for Chronic Pain: http: //www. supportprop. org/educational/index. html 23

FDA Recommendations July 9, 2012 FDA introduced new safety measures for extended release and long acting opioid medications as part of a federal initiative to address the prescription drug abuse, misuse, and overdose epidemic. Key components to REMS (risk evaluation and mitigation strategies): Training for prescribers Updated Medication Guide and patient counseling document Assessment/Auditing 24

Training for Prescribers Training for prescribers is OPTIONAL at this time. The Obama Administration is pursuing legislative changes to link mandatory training on responsible opioid prescribing to DEA registration. Companies will be required to make education programs available to prescribers based on an FDA blueprint at no or a nominal cost. Companies will be required to perform periodic assessments of the implementation of the REMS and the success of the program in meeting its goals. FDA will review these assessments and may require additional elements to achieve the goals of the program. 25

Updated Medication Guide Consumer friendly information on the safe use, storage, and disposal of long acting opioids Signs of potential overdose Specific advice on safe storage to prevent accidental exposure to family members and household visitors. 26

Assessment/Auditing Companies will be expected to achieve certain FDAestablished goals for the percentage of prescribers of ER/LA opioids who complete the training (25% at the end of the first year, 50% after two years, 60% after four years). Assess prescriber’s understanding of important risk information. Assess whether the REMS is adversely affecting patient access to necessary pain medications. 27

PROP Response to FDA Letter sent by PROP to the Food and Drug Administration on July 25, 2012 Recommendations Strike the term “moderate” from the indication for non -cancer pain. Add a maximum daily dose, equivalent to 100 mg of morphine, for non-cancer pain. Add a maximum duration of 90 days for continuous (daily) use for non-cancer pain. 28

PROP Future Activities National Summit on Opioid Safety October 31 – November 1, 2012 Seattle, WA Keynote Speakers Roger Chou MD, MPH Oregon Health & Science University Jane Ballantyne, MD, MPH University of Washington 29

Current Activities Revision of long-acting opioid criteria Manual Review (automatic POS denial) for all new patients with prescriptions for the following Oxycodone ER Fentanyl transdermal Butrans Review of Other State Programs Alaska therapeutic duplication edit Washington second opinion by pain specialist Montana case management Oregon back pain management program 30

Possible Intervention Education on patients receiving > 500 morphine equivalents per day From review n = 17 Content ? 31

Aliskiren DUR The U. S. Food and Drug Administration (FDA) came out with a safety announcement on April 20, 2012 warning of the possible risks when using aliskiren containing products with ACEIs or ARBs in patients with diabetes or kidney impairment. (see packet for safety announcement) A report was run to identify patients who had received an aliskiren containing product within the past 90 days. 32

Aliskiren DUR Letters were sent to 17 prescribers about 13 patients on 4/30/2012. As of 8/10/2012, 4 responses have been received (24% response rate. ) See packet for a copy of the letters. 33

Aliskiren DUR response detail as of 8/10/2012 Note that providers may choose more than one selection per response. Reviewed and have or will modify the treatment I will use this information in the care of future pts My patient, but I did not prescribe this Not useful to my practice Info regarding this pt appears to be correct 1 1 2 1 1 34

Aliskiren DUR response detail as of 8/10/2012 “I am recipient’s PCP. Dr Rahim from Idaho Kidney Institute manages her BP medications because of her nephrotic syndrome. There are frequent changes made and he was the one who started her on aliskiren. He did decrease her lisinopril to 20 mg. She is being monitored closely with frequent lab and f/u with the specialist. Thank you for the information. ” “She is no longer on aliskiren. ” “no longer on this combo. Also original rx was by the patient’s nephrologist Dr Davidson. ” “before sending information please do appropriate research. Pt has stage 5 CKD and is no longer approved for ACE or ARB therapy. ” Please refer to packet for recipient’s profile 35

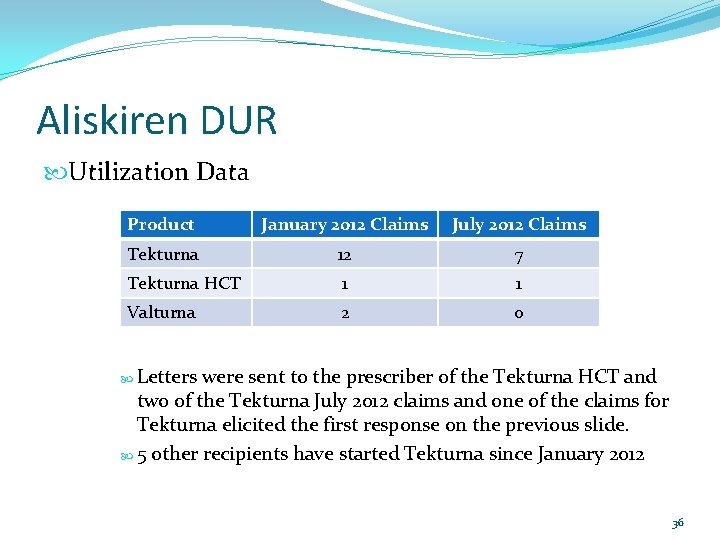
Aliskiren DUR Utilization Data Product January 2012 Claims July 2012 Claims Tekturna 12 7 Tekturna HCT 1 1 Valturna 2 0 Letters were sent to the prescriber of the Tekturna HCT and two of the Tekturna July 2012 claims and one of the claims for Tekturna elicited the first response on the previous slide. 5 other recipients have started Tekturna since January 2012 36

Aliskiren DUR Comments/Suggestions ? ? ? 37

Lupron DUR Reason for DUR Project – Received prior authorization request for Lupron for a 14 year old genetically male patient who is in the process of becoming a girl. Currently lives in another state – “he” and his mother are planning on moving to Idaho so this prior authorization request was submitted as “he” is now eligible for Idaho Medicaid. Nothing specific in Idaho Medicaid’s rules about paying or not paying for transgender procedures. Would fall under “medical necessity” review. Chart note was submitted with prior authorization request – nothing written in endocrinologist’s chart note about chromosomal mosaicism or genetic abnormality. Request was denied as not medically necessary. 38

Lupron DUR Previously Lupron claims would pay at pharmacy with prior authorization not needed. Have since instituted prior authorization requirements. FDA approved indications Precocious Puberty Girls less than 8 years old; boys less than 9 years old Monthly dose 7. 5 mg – 15 mg; Can switch to every 3 month depot formulation Uterine leiomyomata associated with anemia, in conjunction with iron supplementation 3. 75 mg monthly x 3 months or 11. 25 mg depot single injection 39

Lupron DUR FDA approved indications continued Endometriosis 3. 75 mg monthly x 3 months or 11. 25 mg depot single injection Premenstrual syndrome 3. 75 mg monthly x 3 months Prostate cancer, advanced (palliative treatment) 7. 5 mg monthly (indefinitely) or 22. 5 mg depot every 3 months or 30 mg depot every 4 months or 45 mg depot every 6 months In vitro fertilization Not a diagnosis covered by Idaho Medicaid (do not cover fertility treatment) 40

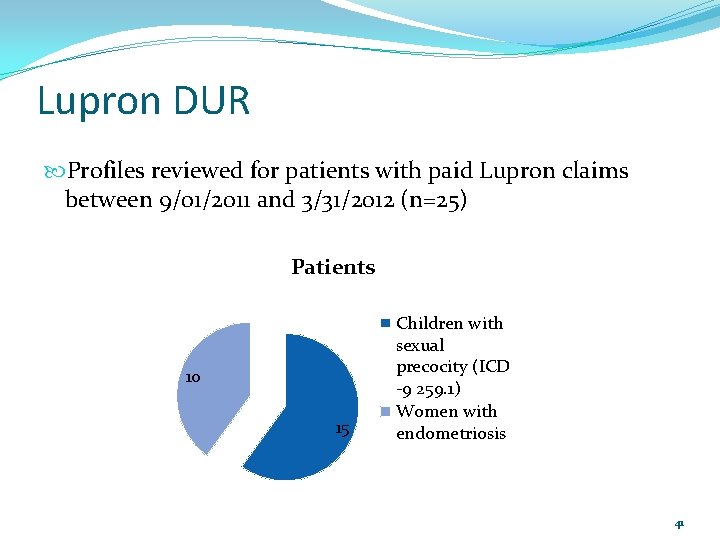
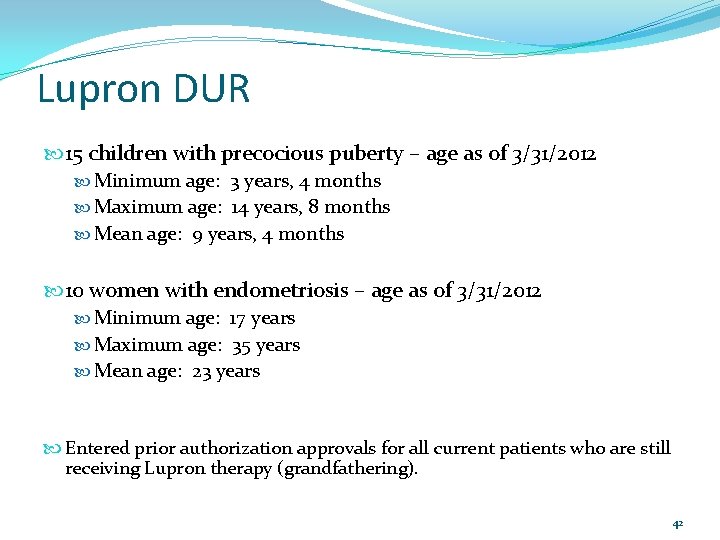
Lupron DUR Profiles reviewed for patients with paid Lupron claims between 9/01/2011 and 3/31/2012 (n=25) Patients 10 15 Children with sexual precocity (ICD -9 259. 1) Women with endometriosis 41

Lupron DUR 15 children with precocious puberty – age as of 3/31/2012 Minimum age: 3 years, 4 months Maximum age: 14 years, 8 months Mean age: 9 years, 4 months 10 women with endometriosis – age as of 3/31/2012 Minimum age: 17 years Maximum age: 35 years Mean age: 23 years Entered prior authorization approvals for all current patients who are still receiving Lupron therapy (grandfathering). 42

Lupron DUR Cost of Lupron therapy (all strengths) from 4/1/2011 – 3/31/2012 through outpatient prescription drug program: $289, 971. 77 (49 patients total) Cost of Lupron therapy (all strengths) from 4/1/2011 – 3/31/2012 through J-code billing on medical side: $12, 179. 79 (24 patients total) Cost of Lupron therapy for requested patient (7. 5 mg monthly): ~$880 per month - $10, 560 per year 43

Lupron DUR Will be instituting auto-PA rule so that claims for children with precocious puberty and women with endometriosis would still pay at Point of Sale with no prior authorization paperwork required IF the applicable diagnosis is in the patient’s electronic profile at the time that the pharmacy claim is run. 44

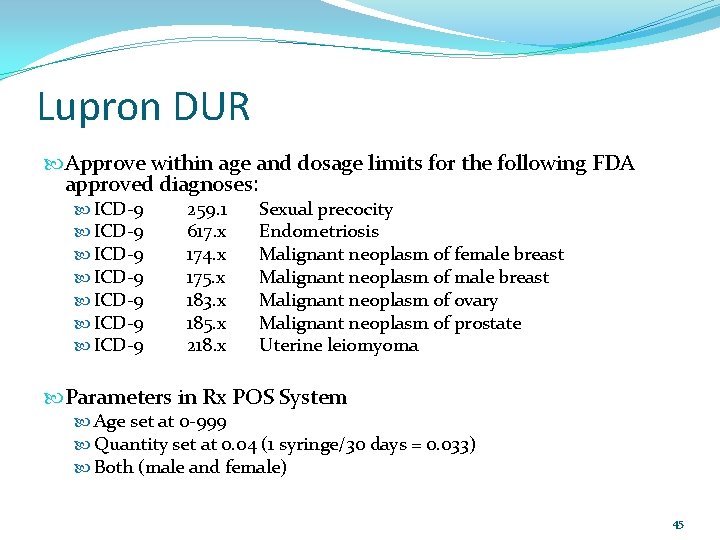
Lupron DUR Approve within age and dosage limits for the following FDA approved diagnoses: ICD-9 ICD-9 259. 1 617. x 174. x 175. x 183. x 185. x 218. x Sexual precocity Endometriosis Malignant neoplasm of female breast Malignant neoplasm of ovary Malignant neoplasm of prostate Uterine leiomyoma Parameters in Rx POS System Age set at 0 -999 Quantity set at 0. 04 (1 syringe/30 days = 0. 033) Both (male and female) 45

Lupron DUR References Abbott Laboratories. Lupron Depot-PED prescribing information. Cigna Medical Coverage Policy – Effective date 1/15/2011. Texas Medicaid Policy. 46

Ciprofloxacin DUR Rationale for study: Idaho Medicaid was requested to look at why ciprofloxacin was prescribed for pediatric patients less than 16 years old. Currently ciprofloxacin will pay at the pharmacy with no age limitations while levofloxacin currently requires an age override prior authorization for pediatric patients less than 16 years old. 47

Ciprofloxacin DUR Ciprofloxacin Package Insert: FDA approved for pediatric patients 1 -17 years of age with complicated urinary tract infections and pyelonephritis due to E coli and for pediatric patients (age not specified) with inhalational anthrax. Levaquin Package Insert: FDA approved for pediatric patients (6 months of age and older) only for the prevention of inhalational anthrax (post-exposure) and for plague. 48

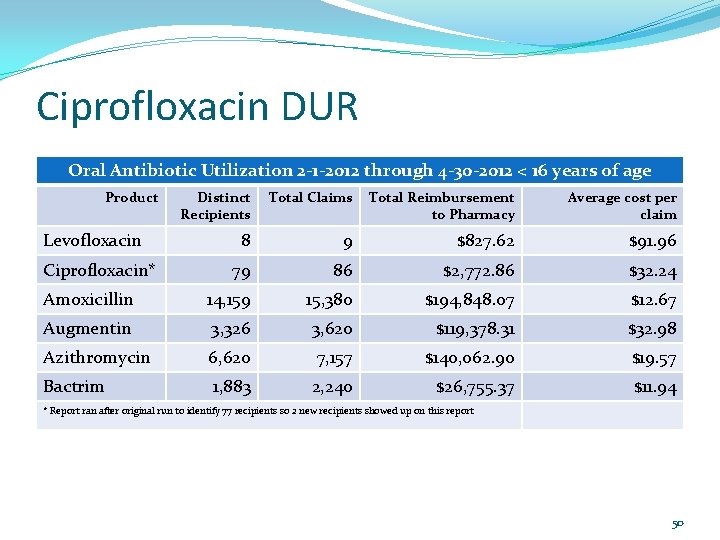
Ciprofloxacin DUR Retrospective DUR was done on patients less than 16 years of age with at least one paid claim between 2 -112 and 4 -30 -12. N=77, mean age 10. 3, std deviation 4. 0 years. 49

Ciprofloxacin DUR Oral Antibiotic Utilization 2 -1 -2012 through 4 -30 -2012 < 16 years of age Product Distinct Recipients Total Claims Total Reimbursement to Pharmacy Average cost per claim 8 9 $827. 62 $91. 96 79 86 $2, 772. 86 $32. 24 Amoxicillin 14, 159 15, 380 $194, 848. 07 $12. 67 Augmentin 3, 326 3, 620 $119, 378. 31 $32. 98 Azithromycin 6, 620 7, 157 $140, 062. 90 $19. 57 Bactrim 1, 883 2, 240 $26, 755. 37 $11. 94 Levofloxacin Ciprofloxacin* * Report ran after original run to identify 77 recipients so 2 new recipients showed up on this report 50

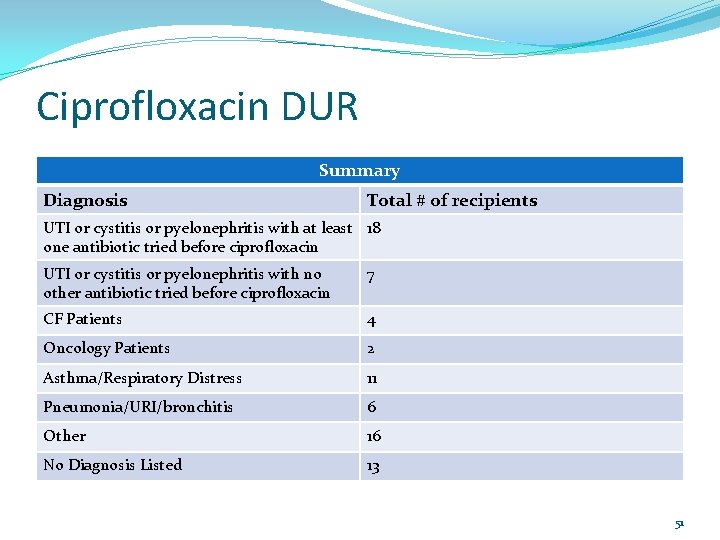
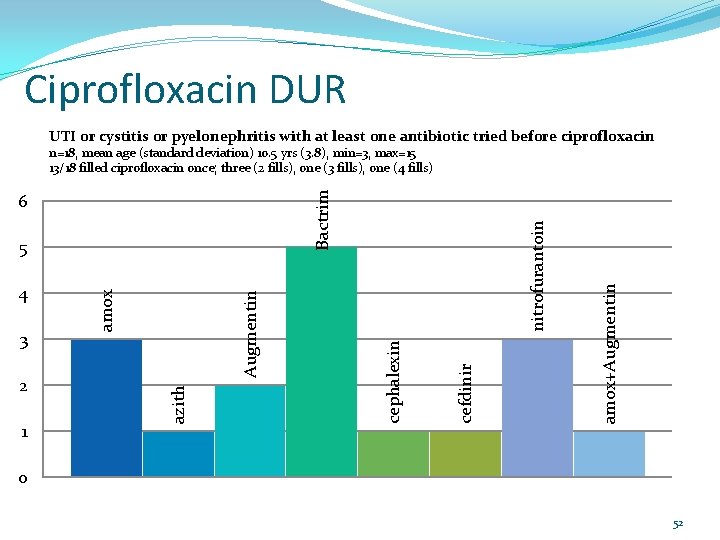
Ciprofloxacin DUR Summary Diagnosis Total # of recipients UTI or cystitis or pyelonephritis with at least 18 one antibiotic tried before ciprofloxacin UTI or cystitis or pyelonephritis with no other antibiotic tried before ciprofloxacin 7 CF Patients 4 Oncology Patients 2 Asthma/Respiratory Distress 11 Pneumonia/URI/bronchitis 6 Other 16 No Diagnosis Listed 13 51

Ciprofloxacin DUR UTI or cystitis or pyelonephritis with at least one antibiotic tried before ciprofloxacin amox+Augmentin 1 cefdinir 2 Augmentin 3 azith 4 amox 5 cephalexin 6 nitrofurantoin Bactrim n=18, mean age (standard deviation) 10. 5 yrs (3. 8), min=3, max=15 13/18 filled ciprofloxacin once; three (2 fills), one (3 fills), one (4 fills) 0 52

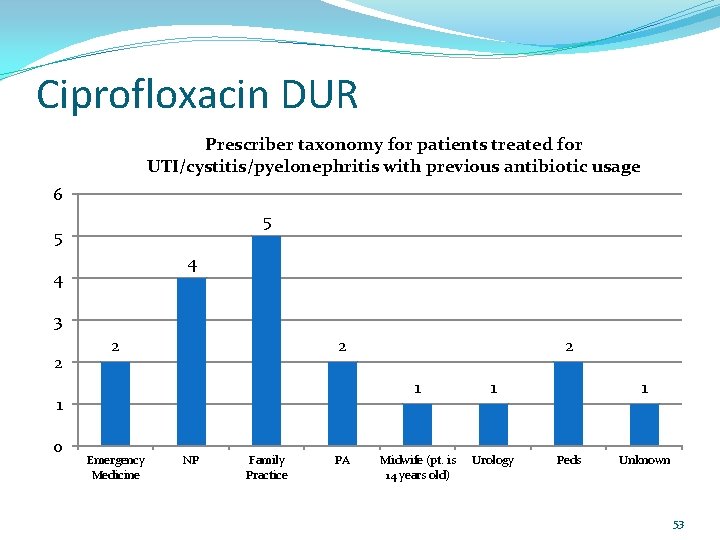
Ciprofloxacin DUR Prescriber taxonomy for patients treated for UTI/cystitis/pyelonephritis with previous antibiotic usage 6 5 5 4 4 3 2 2 2 1 0 Emergency Medicine NP Family Practice PA 2 1 1 Midwife (pt. is 14 years old) Urology 1 Peds Unknown 53

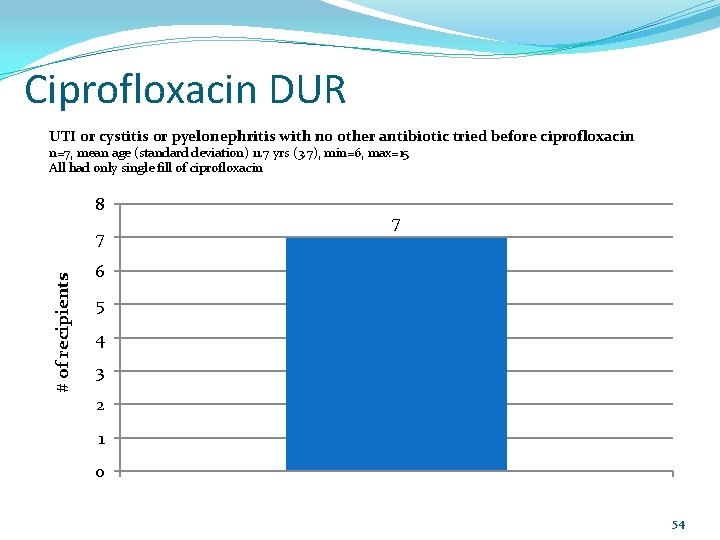
Ciprofloxacin DUR UTI or cystitis or pyelonephritis with no other antibiotic tried before ciprofloxacin n=7, mean age (standard deviation) 11. 7 yrs (3. 7), min=6, max=15 All had only single fill of ciprofloxacin 8 # of recipients 7 7 6 5 4 3 2 1 0 54

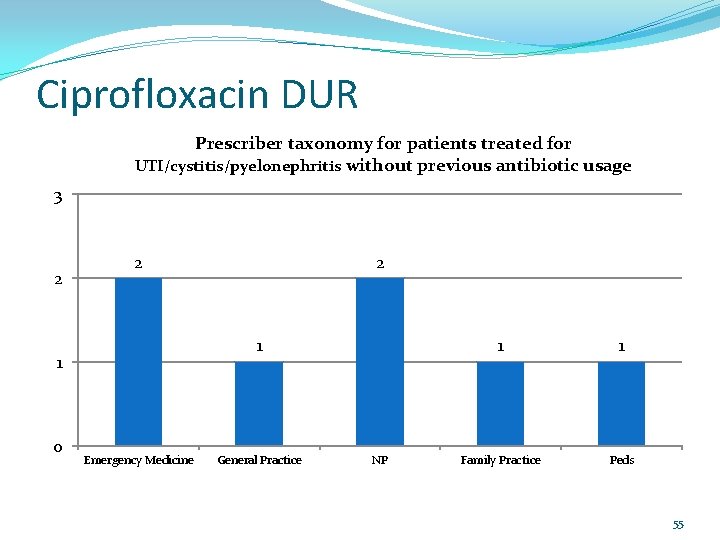
Ciprofloxacin DUR Prescriber taxonomy for patients treated for UTI/cystitis/pyelonephritis without previous antibiotic usage 3 2 2 1 1 0 2 Emergency Medicine General Practice NP 1 1 Family Practice Peds 55

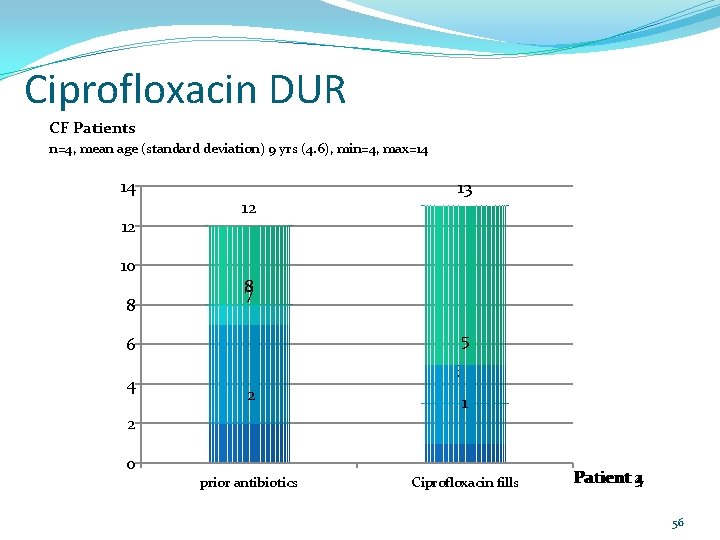
Ciprofloxacin DUR CF Patients n=4, mean age (standard deviation) 9 yrs (4. 6), min=4, max=14 14 12 10 8 12 87 5 6 4 13 3 2 2 1 0 prior antibiotics Ciprofloxacin fills Patient 4 231 56

Ciprofloxacin DUR Oncology Patients Both were 6 years old Kidney cancer with cellulitis of leg Bone cancer with infection of amputation stump Both had single fills of ciprofloxacin 57

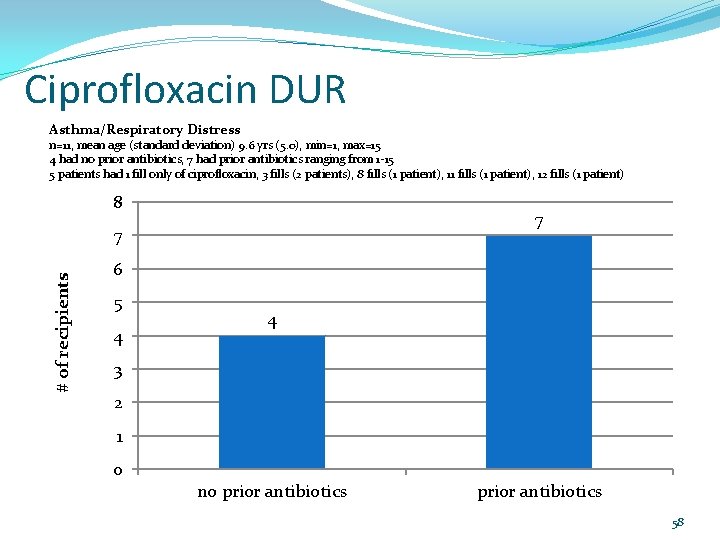
Ciprofloxacin DUR Asthma/Respiratory Distress n=11, mean age (standard deviation) 9. 6 yrs (5. 0), min=1, max=15 4 had no prior antibiotics, 7 had prior antibiotics ranging from 1 -15 5 patients had 1 fill only of ciprofloxacin, 3 fills (2 patients), 8 fills (1 patient), 11 fills (1 patient), 12 fills (1 patient) 8 7 # of recipients 7 6 5 4 4 3 2 1 0 no prior antibiotics 58

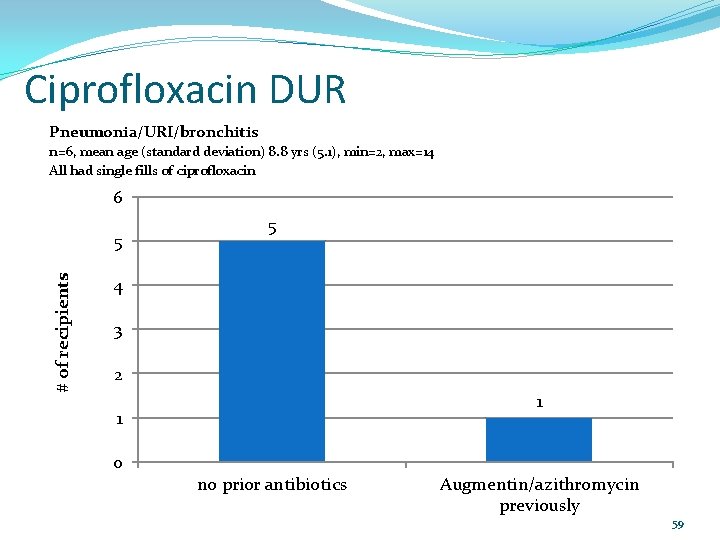
Ciprofloxacin DUR Pneumonia/URI/bronchitis n=6, mean age (standard deviation) 8. 8 yrs (5. 1), min=2, max=14 All had single fills of ciprofloxacin 6 # of recipients 5 5 4 3 2 1 1 0 no prior antibiotics Augmentin/azithromycin previously 59

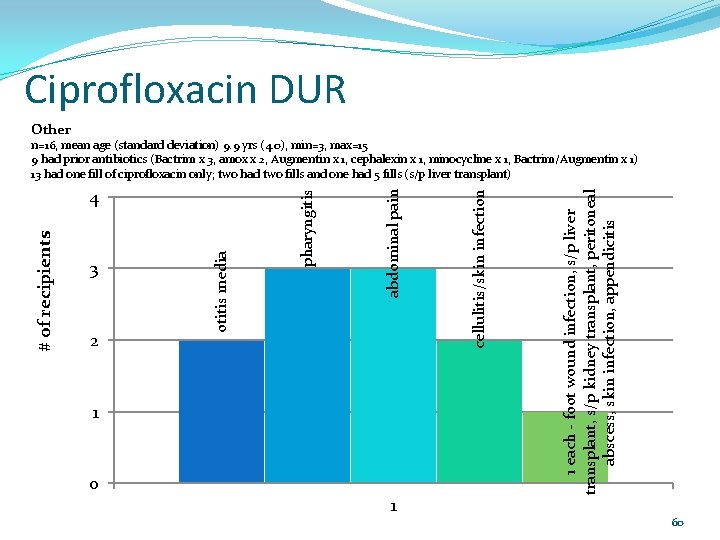
Ciprofloxacin DUR Other 0 1 1 each - foot wound infection, s/p liver transplant, s/p kidney transplant, peritoneal abscess, skin infection, appendicitis 1 cellulitis/skin infection 2 abdominal pain 3 otitis media # of recipients 4 pharyngitis n=16, mean age (standard deviation) 9. 9 yrs (4. 0), min=3, max=15 9 had prior antibiotics (Bactrim x 3, amox x 2, Augmentin x 1, cephalexin x 1, minocycline x 1, Bactrim/Augmentin x 1) 13 had one fill of ciprofloxacin only; two had two fills and one had 5 fills (s/p liver transplant) 60

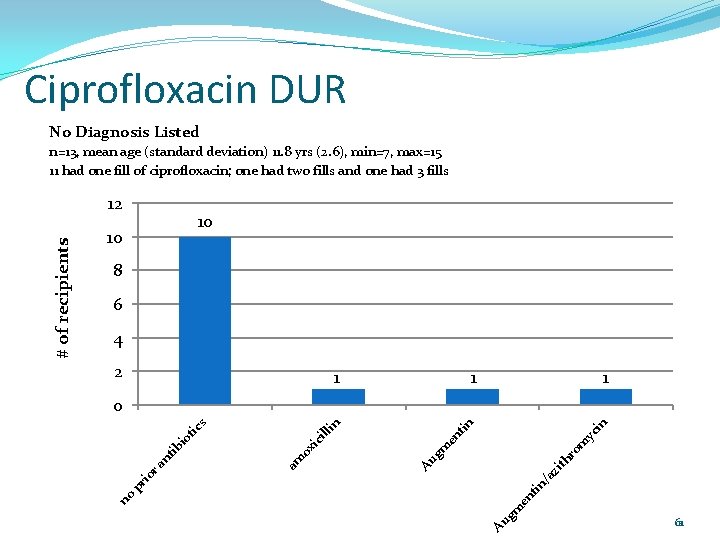
Ciprofloxacin DUR No Diagnosis Listed n=13, mean age (standard deviation) 11. 8 yrs (2. 6), min=7, max=15 11 had one fill of ciprofloxacin; one had two fills and one had 3 fills 10 10 8 6 4 2 1 1 1 in yc m th ro gm zi Au ox am en in ic ill tic s io ib nt tin pr /a io ra tin 0 Au gm en no # of recipients 12 61

Ciprofloxacin DUR American Academy of Pediatrics: The Use of Systemic and Topical Fluoroquinolones. John S. Bradley, Mary Anne Jackson and the Committee on Infectious Diseases. Pediatrics 2011; 128; e 1034 -e 1045. Quotes from the article: Use of fluoroquinolones in children should continue to be limited to treatment of infections for which no safe and effective alternative exists. Animal toxicology data available with the first quinolones compounds documented their propensity to create inflammation and subsequent destruction of weight-bearing joints in juvenile animals. No published reports exist of physician-diagnosed cartilage damage in children in the United States, either from controlled clinical trials of fluoroquinolones or from unsolicited reporting to the FDA or drug manufacturers. No reports of tendon rupture in pediatric patients exposed to any quinolone. 62

Ciprofloxacin DUR Study 1 – Prospective safety study performed at the request of the FDA by Bayer for ciprofloxacin. Studied rate of arthropathy 6 weeks after treatment with ciprofloxacin (n=335) or comparator antibiotic (n=349) in eight countries including the US. A difference was only detected in the US with a rate of arthropathy 21. 0% (n=62) with ciprofloxacin vs. 11. 3% (n=71) with comparator antibiotic. Mexico had a zero incidence of arthropathy in both ciprofloxacin and comparator antibiotic. The study used a non inferiority design to assess musculoskeletal complaints across all countries, and as analyzed, the groups were sufficiently different to suggest potential musculoskeletal toxicity with ciprofloxacin (9. 3%) vs. comparator (6. 0%). 63

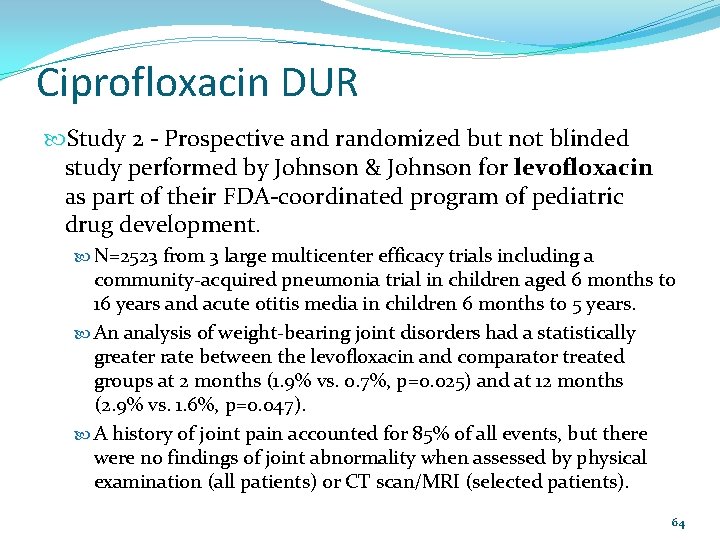
Ciprofloxacin DUR Study 2 - Prospective and randomized but not blinded study performed by Johnson & Johnson for levofloxacin as part of their FDA-coordinated program of pediatric drug development. N=2523 from 3 large multicenter efficacy trials including a community-acquired pneumonia trial in children aged 6 months to 16 years and acute otitis media in children 6 months to 5 years. An analysis of weight-bearing joint disorders had a statistically greater rate between the levofloxacin and comparator treated groups at 2 months (1. 9% vs. 0. 7%, p=0. 025) and at 12 months (2. 9% vs. 1. 6%, p=0. 047). A history of joint pain accounted for 85% of all events, but there were no findings of joint abnormality when assessed by physical examination (all patients) or CT scan/MRI (selected patients). 64

Ciprofloxacin DUR World Health Organization: What is the evidence of safety of quinolones use in children? International Child Health Review Collaboration. September 2, 2008. Summary Statement: Fluoroquinolones are efficacious antimicrobial agents with an important role in the treatment of a variety of pediatric infections. Ciprofloxacin is a particularly useful fluoroquinolone for dysentery and typhoid. There is grade A evidence to support both the overall safety of ciprofloxacin use in children and lack of joint toxicity. 65

Ciprofloxacin DUR Recommendations for prior authorization for age override for pediatric patients receiving ciprofloxacin and levofloxacin? Add age criteria to cipro and/or remove age criteria from levofloxacin? 66

Synagis DUR Medical claims for 2011 -2012 season: $274, 881. 27 Pharmacy claims for 2011 -2012 season: $1, 362, 626. 70 67

Synagis DUR – Medical Claims Data from 10 -1 -2011 through 6 -20 -2012 (RSV 2011 -2012 Season) 40 patients identified – ALL had prior authorization approvals. PA Request Marked as: Billing using CPT Code: 11 Pharmacy billing for drug: 7 Nothing specific marked on form: 22 Future Question: Should we inform Idaho medical unit of all prior authorizations for Synagis as the doctors’ offices are not doing a good job of informing Medicaid if claim will be paid as a medical claim or as a pharmacy claim? 68

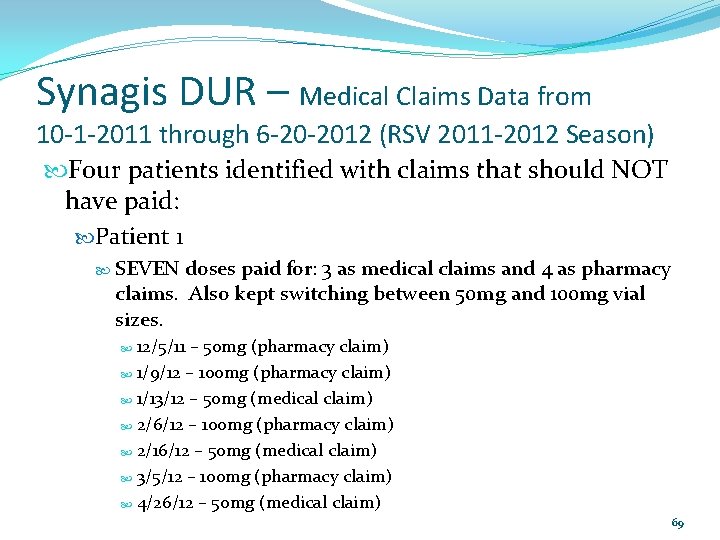
Synagis DUR – Medical Claims Data from 10 -1 -2011 through 6 -20 -2012 (RSV 2011 -2012 Season) Four patients identified with claims that should NOT have paid: Patient 1 SEVEN doses paid for: 3 as medical claims and 4 as pharmacy claims. Also kept switching between 50 mg and 100 mg vial sizes. 12/5/11 – 50 mg (pharmacy claim) 1/9/12 – 100 mg (pharmacy claim) 1/13/12 – 50 mg (medical claim) 2/6/12 – 100 mg (pharmacy claim) 2/16/12 – 50 mg (medical claim) 3/5/12 – 100 mg (pharmacy claim) 4/26/12 – 50 mg (medical claim) 69

Synagis DUR – Medical Claims Data from 10 -1 -2011 through 6 -20 -2012 (RSV 2011 -2012 Season) Patient 2 A total of 5 doses paid but two paid in same month (one as pharmacy claim and one as medical claim) plus four claims paid as 100 mg but third paid as 50 mg. 12/12/11 – 100 mg (pharmacy claim) 1/9/12 – 100 mg (pharmacy claim) 1/20/12 – 50 mg (medical claim) 2/6/12 – 100 mg (pharmacy claim) 3/5/12 – 100 mg (pharmacy claim) 70

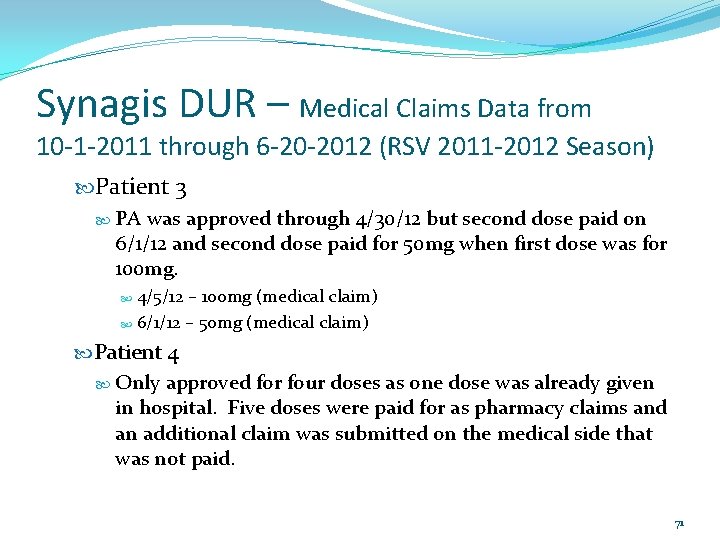
Synagis DUR – Medical Claims Data from 10 -1 -2011 through 6 -20 -2012 (RSV 2011 -2012 Season) Patient 3 PA was approved through 4/30/12 but second dose paid on 6/1/12 and second dose paid for 50 mg when first dose was for 100 mg. 4/5/12 – 100 mg (medical claim) 6/1/12 – 50 mg (medical claim) Patient 4 Only approved for four doses as one dose was already given in hospital. Five doses were paid for as pharmacy claims and an additional claim was submitted on the medical side that was not paid. 71

Synagis DUR – Medical Claims Data from 10 -1 -2011 through 6 -20 -2012 (RSV 2011 -2012 Season) Other Dosing Issues per each individual patient Claims paid on pharmacy side not medical side First dose paid for 150 mg but second through fifth doses paid for 100 mg Claims paid on medical side Doses 1, 2, 3, 5 – 150 mg but Dose 4 - 200 mg Claims paid on medical side Doses 1, 3, 4, 5 – 150 mg but Dose 2 - 100 mg Claims paid on pharmacy side Dose 1 – 150 mg but Dose 2 100 mg Claims paid on medical side Doses 1, 2, 4, 5 – 150 mg but Dose 3 100 mg 72

Synagis DUR – Medical Claims Data from 10 -1 -2011 through 6 -20 -2012 (RSV 2011 -2012 Season) Comments/suggestions ? ? ? 73

Synagis - July 2012 AAP Red Book Updates for Synagis Prophylaxis Gestational Age 32 weeks, 0 days through 34 weeks, 6 days AND chronological age less than 90 days Synagis prophylaxis (maximum of 3 monthly doses) may be considered for infants who have at least 1 of 2 risk factors: 1. 2. Infant attends child care, defined as home or facility where care is provided for any number of infants or young toddlers. One or more older siblings younger than 5 years of age or other children younger than 5 years of age lives permanently in the same household. Multiple births younger than 1 year of age do not qualify as fulfilling this 74 risk factor.

Synagis - July 2012 AAP Red Book Updates for Synagis Prophylaxis Infants with congenital abnormalities of the airway or neuromuscular disease Immunoprophylaxis may be considered for infants who have either congenital abnormalities of the airway or a neuromuscular condition that compromises handling of respiratory secretions. Infants and young children in this category should receive a maximum of 5 doses of palivizumab during the first year of life. 75

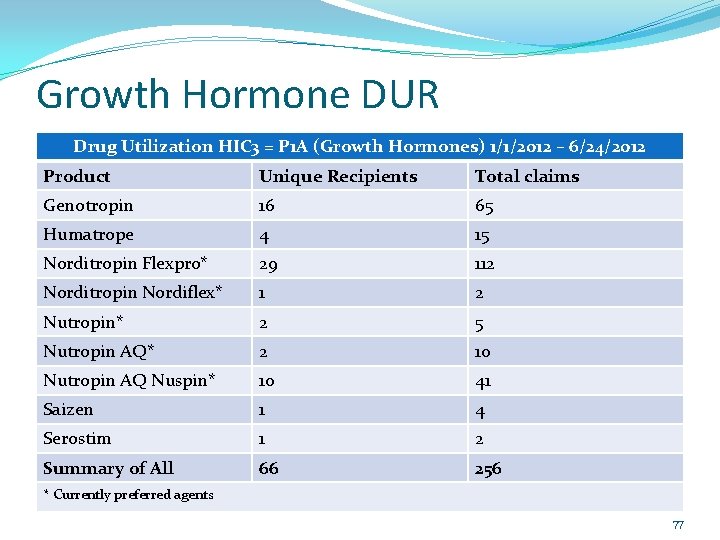
Growth Hormone DUR Idaho Medicaid’s Pharmacy & Therapeutics (P&T) Committee requested that the DUR Board look at the utilization numbers of the Growth Hormone class. The potential exists to save the State money should patients be switched from a non-preferred to a preferred agent. Previously patients have been grandfathered to allow them to remain on their current therapy. 76

Growth Hormone DUR Drug Utilization HIC 3 = P 1 A (Growth Hormones) 1/1/2012 – 6/24/2012 Product Unique Recipients Total claims Genotropin 16 65 Humatrope 4 15 Norditropin Flexpro* 29 112 Norditropin Nordiflex* 1 2 Nutropin* 2 5 Nutropin AQ* 2 10 Nutropin AQ Nuspin* 10 41 Saizen 1 4 Serostim 1 2 Summary of All 66 256 * Currently preferred agents 77

Growth Hormone DUR Comments/Suggestions ? ? ? 78

Foster Children Preliminary Idaho Medicaid Data 8/2012 79

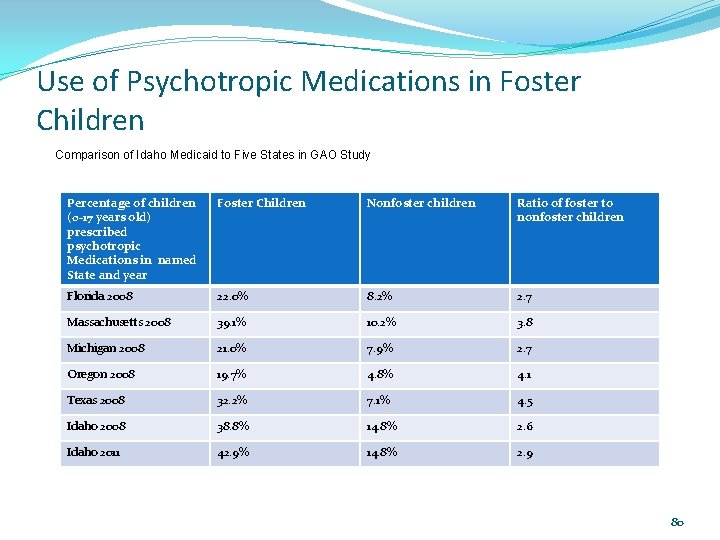
Use of Psychotropic Medications in Foster Children Comparison of Idaho Medicaid to Five States in GAO Study Percentage of children (0 -17 years old) prescribed psychotropic Medications in named State and year Foster Children Nonfoster children Ratio of foster to nonfoster children Florida 2008 22. 0% 8. 2% 2. 7 Massachusetts 2008 39. 1% 10. 2% 3. 8 Michigan 2008 21. 0% 7. 9% 2. 7 Oregon 2008 19. 7% 4. 8% 4. 1 Texas 2008 32. 2% 7. 1% 4. 5 Idaho 2008 38. 8% 14. 8% 2. 6 Idaho 2011 42. 9% 14. 8% 2. 9 80

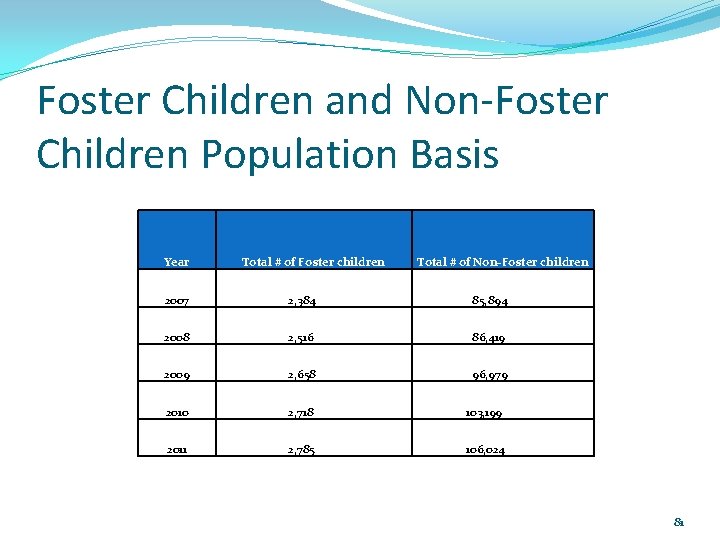
Foster Children and Non-Foster Children Population Basis Year Total # of Foster children Total # of Non-Foster children 2007 2, 384 85, 894 2008 2, 516 86, 419 2009 2, 658 96, 979 2010 2, 718 103, 199 2011 2, 785 106, 024 81

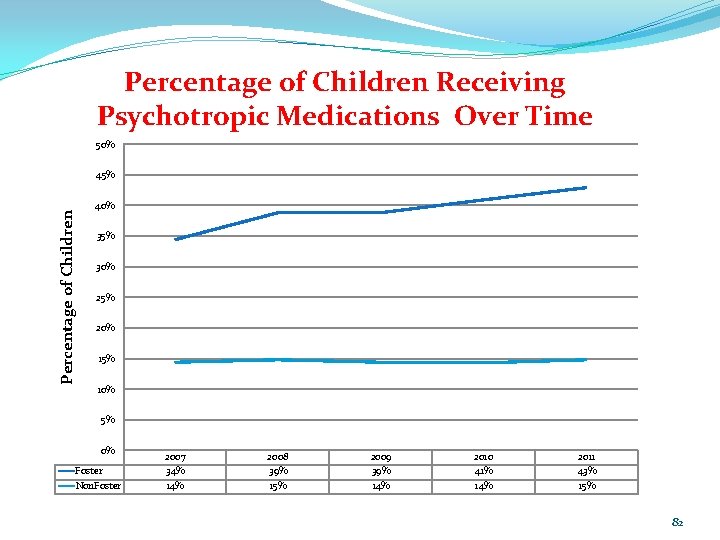
Percentage of Children Receiving Psychotropic Medications Over Time 50% Percentage of Children 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Foster Non. Foster 2007 34% 14% 2008 39% 15% 2009 39% 14% 2010 41% 14% 2011 43% 15% 82

Focus on Calendar Year 2011 83

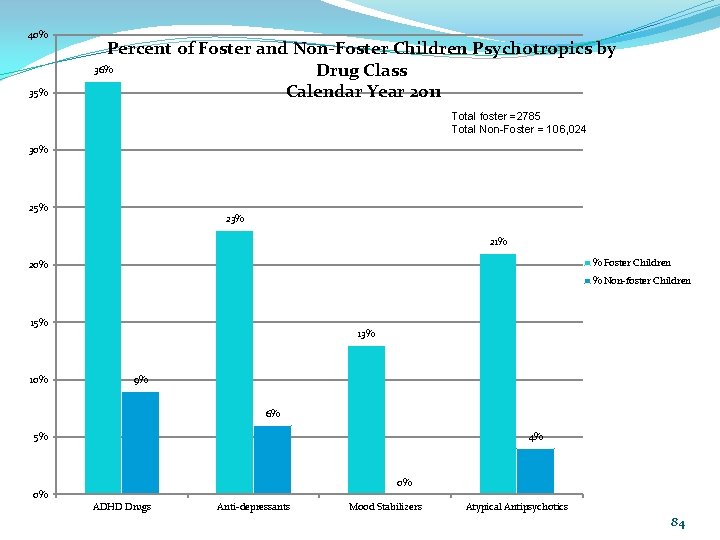
40% 35% Percent of Foster and Non-Foster Children Psychotropics by 36% Drug Class Calendar Year 2011 Total foster =2785 Total Non-Foster = 106, 024 30% 25% 23% 21% % Foster Children 20% % Non-foster Children 15% 10% 13% 9% 6% 4% 5% 0% 0% ADHD Drugs Anti-depressants Mood Stabilizers Atypical Antipsychotics 84

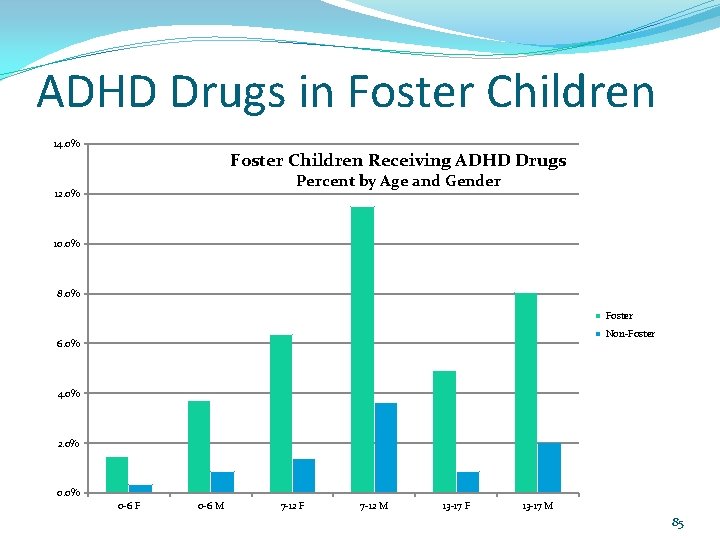
ADHD Drugs in Foster Children 14. 0% Foster Children Receiving ADHD Drugs Percent by Age and Gender 12. 0% 10. 0% 8. 0% Foster Non-Foster 6. 0% 4. 0% 2. 0% 0 -6 F 0 -6 M 7 -12 F 7 -12 M 13 -17 F 13 -17 M 85

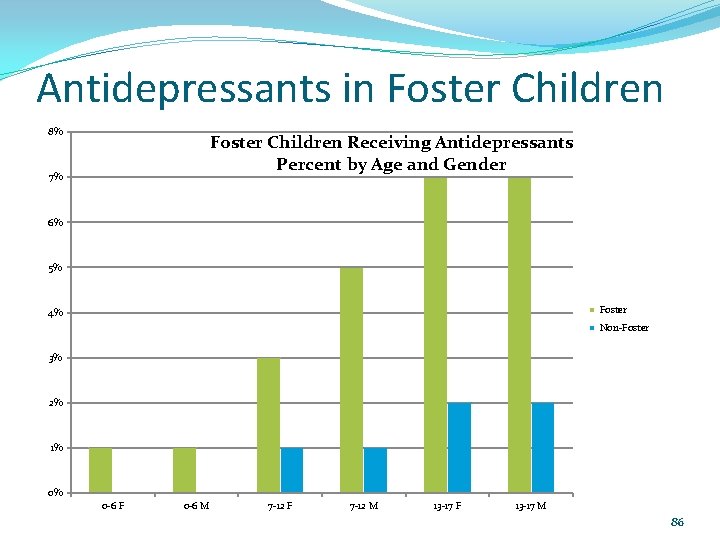
Antidepressants in Foster Children 8% Foster Children Receiving Antidepressants Percent by Age and Gender 7% 6% 5% Foster 4% Non-Foster 3% 2% 1% 0% 0 -6 F 0 -6 M 7 -12 F 7 -12 M 13 -17 F 13 -17 M 86

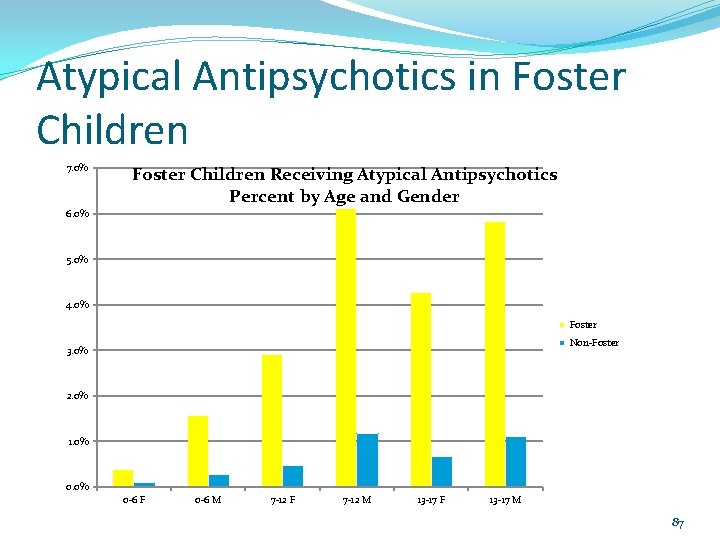
Atypical Antipsychotics in Foster Children 7. 0% Foster Children Receiving Atypical Antipsychotics Percent by Age and Gender 6. 0% 5. 0% 4. 0% Foster Non-Foster 3. 0% 2. 0% 1. 0% 0 -6 F 0 -6 M 7 -12 F 7 -12 M 13 -17 F 13 -17 M 87

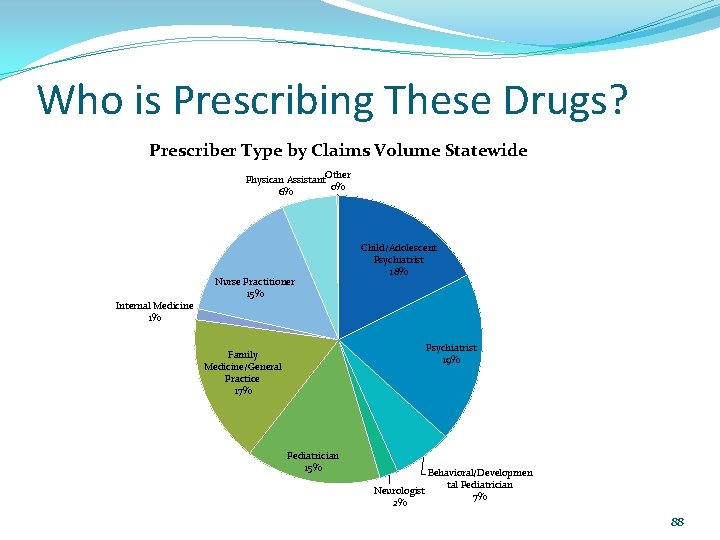
Who is Prescribing These Drugs? Prescriber Type by Claims Volume Statewide Physican Assistant. Other 0% 6% Internal Medicine 1% Nurse Practitioner 15% Child/Adolescent Psychiatrist 18% Psychiatrist 19% Family Medicine/General Practice 17% Pediatrician 15% Behavioral/Developmen tal Pediatrician Neurologist 7% 2% 88

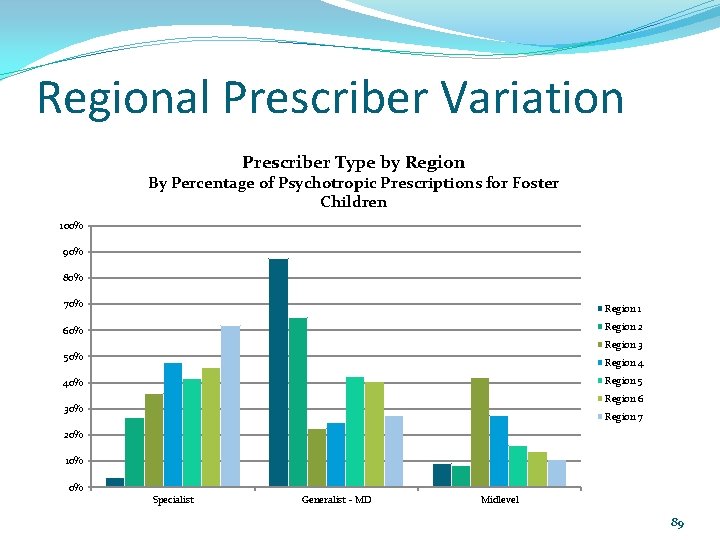
Regional Prescriber Variation Prescriber Type by Region By Percentage of Psychotropic Prescriptions for Foster Children 100% 90% 80% 70% Region 1 60% Region 2 Region 3 50% Region 4 Region 5 40% Region 6 30% Region 7 20% 10% 0% Specialist Generalist - MD Midlevel 89

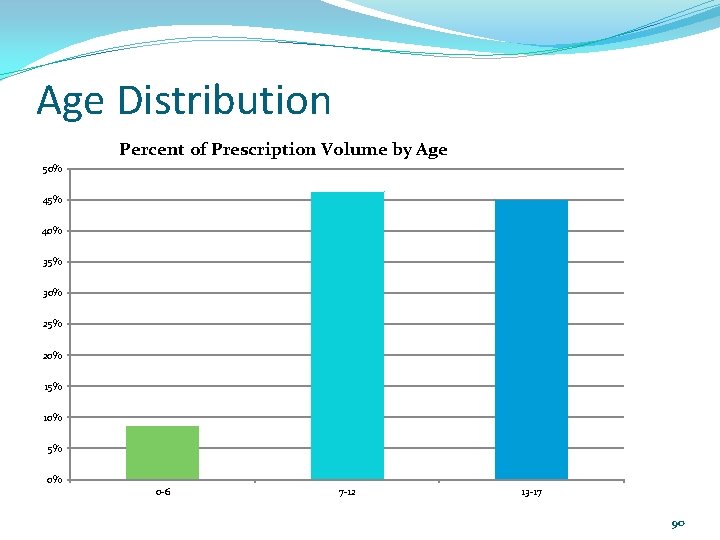
Age Distribution Percent of Prescription Volume by Age 50% 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% 0 -6 7 -12 13 -17 90

Foster Children Psychotropic Drugs Red Flags 8/21/2012 91

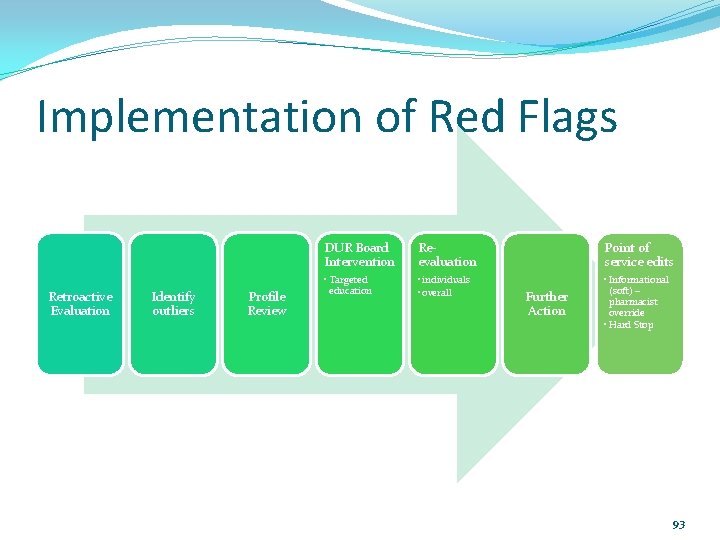
Red Flags Five (5) or more psychotropic medications prescribed concomitantly Two (2) or more concomitant antidepressants Two (2) or more concomitant antipsychotic medications Two(2) or more concomitant stimulant medications long-acting plus short-acting ok Three (3) or more concomitant mood stabilizer medications Psychotropic polypharmacy (2 or more agents) for a given mental disorder prescribed before utilizing psychotropic monotherapy 92

Implementation of Red Flags Retroactive Evaluation Identify outliers Profile Review DUR Board Intervention Reevaluation Point of service edits • Targeted education • individuals • overall • Informational (soft) – pharmacist override • Hard Stop Further Action 93

Foster Children with Five or More Psychotropic Medications August 2012 94

Study Parameters Foster Children with 5 or more distinct behavioral health drugs Service Dates between 11/1/2011 and 4/30/2012 (6 months) N = 5 95

Mental Health Drug List: Stimulants amphetamine mixed salts generic, Adderall XR dextroamphetamine generic, Dexedrine Spansule lisdexamfetamine Vyvanse methylphenidate generic, Ritalin, Metadate ER, Metadate CD, Methylin ER, Concerta, Daytrana TD dexmethlyphenidate generic, Focalin XR 96

Mental Health Drug List: Other ADHD Treatments atomoxetine Strattera clonidine generic, Catapres guanfacine generic, Tenex, Intuniv bupropion generic, Wellbutrin SR, Wellbutrin XR imipramine generic, Tofranil-PM nortriptyline generic, Aventyl, Pamelor 97

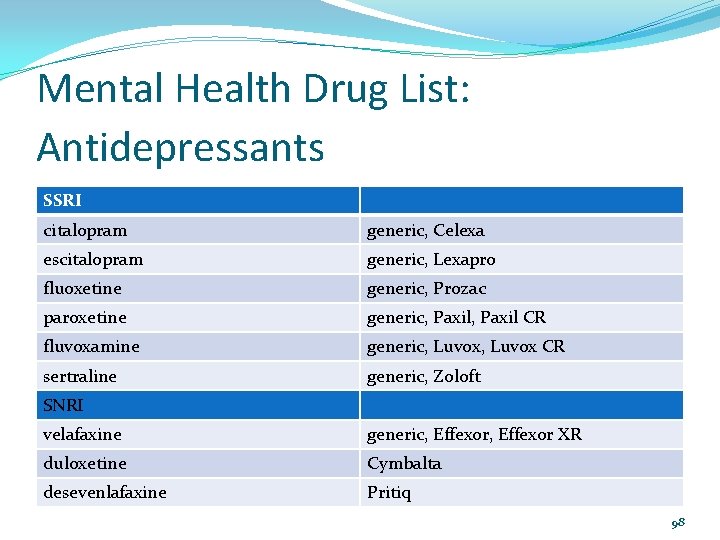
Mental Health Drug List: Antidepressants SSRI citalopram generic, Celexa escitalopram generic, Lexapro fluoxetine generic, Prozac paroxetine generic, Paxil CR fluvoxamine generic, Luvox CR sertraline generic, Zoloft SNRI velafaxine generic, Effexor XR duloxetine Cymbalta desevenlafaxine Pritiq 98

Mental Health Drug List: Mood Stabilizers carbamazepine generic, Carbatol, Tegretol XR divalproex generic, Depakote lithium generic, Eskalith CR, Lithobid lamotrigine generic, Lamictal 99

Mental Health Drug List: Second Generation (atypical) Antipsychotics Second Generation Antipsychotics aripiprizole Abilify asenapine Saphris clozapine generic, Clozaril iloperidone Fanapt lurasidone Latuda olanzapine generic, Zyprexa paliperidone Invega quetiapine generic, Seroquel XR risperidone generic, Risperdal ziprasidone generic, Geodon 100

Mental Health Drug List: First Generation (Typical) Antipsychotics First Generation Antipsychotics chlorpromazine generic, Thorazine haloperidol generic, Haldol perphenazine generic, Trilafon pimozide Orap 101

Patient 101 15 year old female Mental Health Diagnoses Pervasive Developmental Disorder Encephalopathy, other Oppositional Defiant Disorder Attention Deficit with Hyperactivity Posttraumatic Stress Disorder Drug Duration Vyvanse 70 mg #30 2 years 5 months Amphetamine Salt Combo 10 mg #60 3 years 3 months Clonidine 0. 1 mg #30 10 months Fluoxetine 10 mg #30 6 months Geodon 80 mg #60 1 year 3 months Geodon 60 mg #30 1 year 7 months 102

Patient 105 16 year old female Mental Health Diagnoses Reactive Attachment Disorder Posttraumatic Stress Disorder Bipolar Disorder, Unspecified Drug Duration Amphetamine Salt Combo 20 mg #62 2 years Sertraline 100 mg #30 1 year 5 months Oxcarbazepine 600 mg #60 4 years Lithium 300 mg #120 4 years Risperidone 0. 5 mg #60 1 year 11 months Quetiapine 400 mg #30 6 years 103

Patient 113 8 year old female Mental Health Diagnoses Bipolar I Disorder, Unspecified Bipolar I Disorder, Mixed Severe Attention Deficit Disorder with Hyperactivity Reactive Attachment Disorder Unspecified Delay in Development 104

Patient 113 Drug Duration Adderall XR 20 mg #30 3 years 8 months Amphetamine Salt Combo 10 mg #30 1 year 7 months Vyvanse 70 mg #30 1 year 8 months Clonidine 0. 1 mg #120 1 year 11 months Geodon 40 mg 1 month Abilify 30 mg #30 1 year 7 months Quetiapine 100 mg #60 Quetiapine 200 mg #60 Quetiapine 300 mg #30 Quetiapine 400 mg #30 Quetiapine was started 5/20/2011. Dose during the evaluated 6 month period 700 mg daily. 105

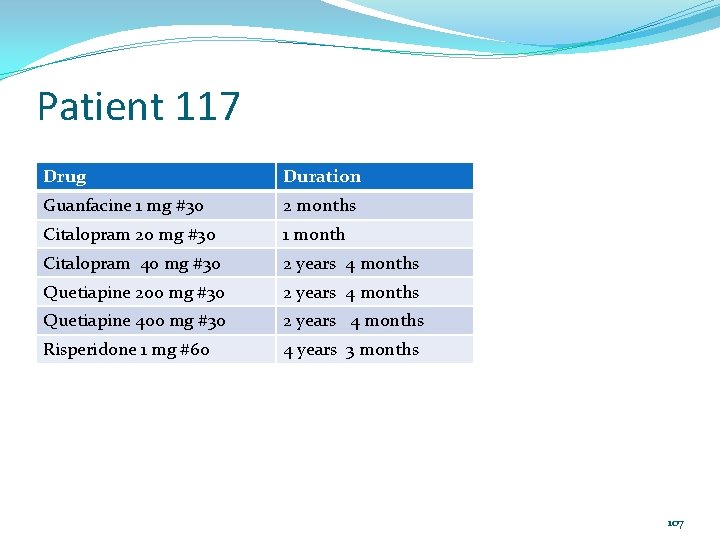
Patient 117 17 year old female Mental Health Diagnoses Moderate Intellectual Disability Unspecified Developmental Delay Bipolar Disorder, Unspecified 106

Patient 117 Drug Duration Guanfacine 1 mg #30 2 months Citalopram 20 mg #30 1 month Citalopram 40 mg #30 2 years 4 months Quetiapine 200 mg #30 2 years 4 months Quetiapine 400 mg #30 2 years 4 months Risperidone 1 mg #60 4 years 3 months 107

Patient 120 11 year old male Mental Health Diagnoses Attention Deficit Disorder with Hyperactivity Unspecified Episodic Mood Disorder Unspecified Emotional Disturbance of Childhood or Adolescence 108

Patient 120 Drug Duration Concerta 54 mg #30 3 years 8 months Strattera 60 mg # 30 10 months Clonidine 0. 1 mg #90 2 years 7 months Abilify 5 mg #30 2 months Quetiapine * 50 mg #90 1 month Quetiapine * 200 mg #60 4 months Quetiapine * 300 mg #60 2 years 8 months Patient has been on varying doses of quetiapine since 9/16/2008 109

Proposed Studies for Next Quarter: P&T Committee Narcotic Analgesic Studies – Next Steps Leukotrienes vs. inhaled corticosteroids in children with asthma Use of Psychotropic Medications in Foster Children Two (2) or more concomitant antidepressants Migraine Prevention Prophylaxis Utilization in Chronic Triptan Utilizers Topiramate PA, Medical Claim and Triptan Use Mismatch IVIG 110

Leukotrienes vs. inhaled corticosteroids in children with asthma Number of recipients < 18 years of age with paid claim for leukotriene: Date # of recipients 7/1/2011 – 9/30/2011 3, 369 1/1/2012 – 3/31/2012 3, 059 Number of recipients < 18 years of age with paid claim for inhaled corticosteroid: Date # of recipients 7/1/2011 – 9/30/2011 1, 595 1/1/2012 – 3/31/2012 2, 156 111

Use of Psychotropic Medications in Foster Children The U. S. Government Accountability Office released the results from a study that they performed examining the rates of psychotropic medications for foster and nonfoster children in 2008. It was determined that HHS Guidance Could Help States Improve Oversight of Psychotropic Prescriptions. 112

Use of Psychotropic Medications in Foster Children Medication Classes included in the report ADHD drugs Anti-anxiety Anticonvulsant Antidepressants Anti-enuretic (just desmopressin acetate) Antiparkinson Antipsychotics Combination anti-anxiety and antidepressant Hypnotic Mood stabilizer (just lithium) Sleep aid (just melatonin) 113

Use of Psychotropic Medications in Foster Children: Next Steps Two (2) or more concomitant antidepressants 114

Migraine Prevention Prophylaxis Utilization in Chronic Triptan Utilizers See packet for summary handout 115

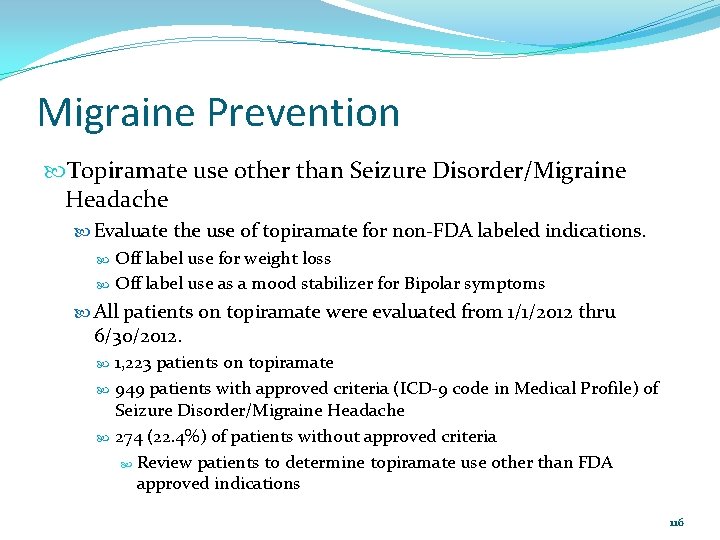
Migraine Prevention Topiramate use other than Seizure Disorder/Migraine Headache Evaluate the use of topiramate for non-FDA labeled indications. Off label use for weight loss Off label use as a mood stabilizer for Bipolar symptoms All patients on topiramate were evaluated from 1/1/2012 thru 6/30/2012. 1, 223 patients on topiramate 949 patients with approved criteria (ICD-9 code in Medical Profile) of Seizure Disorder/Migraine Headache 274 (22. 4%) of patients without approved criteria Review patients to determine topiramate use other than FDA approved indications 116

IVIG – Intravenous Immune Globulin Currently prior authorization is not needed for either an outpatient prescription (as long as cost per claim is less than $7500) or for a claim on the medical side. 117

IVIG – Intravenous Immune Globulin Reviewing outpatient prescription claims between 8/01/2011 and 7/31/2012 $279, 527 79 claims 14 patients Average cost per prescription: $3538 Claims paid on medical side between 1/01/2011 and 12/31/2011 $106, 414 136 claims 37 patients Average cost per prescription: $783 118

IVIG – Intravenous Immune Globulin In the process of sending out letters to gather medical information: Diagnosis Dosing regimen Response to therapy Will review information at October 2012 DUR meeting. Future question to answer: Should IVIG require prior authorization? 119

Prospective DUR Report History Errors: Non-History Errors: • DD – drug-to-drug • PA – drug-to-age • PG – drug to pregnancy • HD – high dose • TD – therapeutic duplication • LD – low dose • ER – early refill • SX – drug-to-gender • MC – drug-to-disease 120

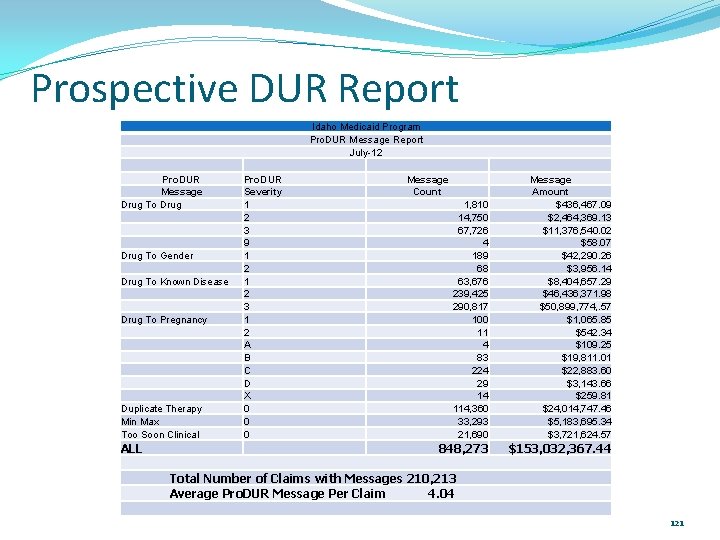
Prospective DUR Report Idaho Medicaid Program Pro. DUR Message Report July-12 Pro. DUR Message Drug To Gender Drug To Known Disease Drug To Pregnancy Duplicate Therapy Min Max Too Soon Clinical ALL Pro. DUR Severity 1 2 3 9 1 2 3 1 2 A B C D X 0 0 0 Message Count 1, 810 14, 750 67, 726 4 189 68 63, 676 239, 425 290, 817 100 11 4 83 224 29 14 114, 360 33, 293 21, 690 Message Amount $436, 467. 09 $2, 464, 369. 13 $11, 376, 540. 02 $58. 07 $42, 290. 26 $3, 956. 14 $8, 404, 657. 29 $46, 436, 371. 98 $50, 899, 774, . 57 $1, 065. 85 $542. 34 $109. 25 $19, 811. 01 $22, 883. 60 $3, 143. 66 $259. 81 $24, 014, 747. 46 $5, 183, 695. 34 $3, 721, 624. 57 848, 273 $153, 032, 367. 44 Total Number of Claims with Messages 210, 213 Average Pro. DUR Message Per Claim 4. 04 121

DUR Summer Newsletter Copy of Spring Newsletter in packet Brainstorm for new topics 122

Medicaid Update 123