Idaho Medicaid Drug Utilization Review Program 17 January










































- Slides: 69

Idaho Medicaid Drug Utilization Review Program 17 January 2013 1

Follow-up to Previous Reviews Immune Globulin (IV and SC) Atopic Dermatitis 2

Immune Globulin (IV and SC) Additional Responses to DUR letters sent in August 2012 One doctor’s office had charged Idaho Medicaid for immune globulin (brand name Privigen) 500 mg as a single dose for four separate patients. Patients had actually received promethazine 50 mg injectable. Doctor’s office has been asked to correct billing error 3

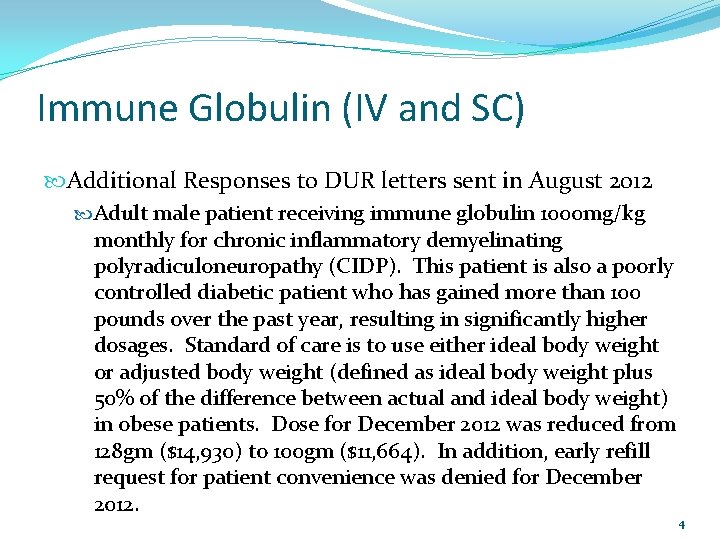
Immune Globulin (IV and SC) Additional Responses to DUR letters sent in August 2012 Adult male patient receiving immune globulin 1000 mg/kg monthly for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). This patient is also a poorly controlled diabetic patient who has gained more than 100 pounds over the past year, resulting in significantly higher dosages. Standard of care is to use either ideal body weight or adjusted body weight (defined as ideal body weight plus 50% of the difference between actual and ideal body weight) in obese patients. Dose for December 2012 was reduced from 128 gm ($14, 930) to 100 gm ($11, 664). In addition, early refill request for patient convenience was denied for December 2012. 4

Immune Globulin (IV and SC) Additional Responses to DUR letters sent in August 2012 Pregnant woman whose first child died secondary to congenital hemochromatosis (2008). Second child survived (2010) – mother had been treated with IVIG weekly from Weeks 14 -37. Third pregnancy (due date February 2013) – mother is being treated with IVIG weekly starting at week 14 (September 2012). Have approved IVIG to continue throughout this third pregnancy. 5

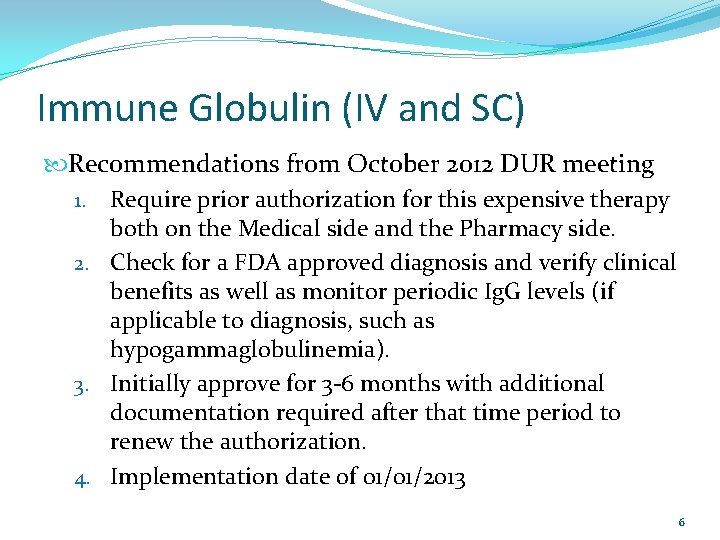
Immune Globulin (IV and SC) Recommendations from October 2012 DUR meeting 1. Require prior authorization for this expensive therapy both on the Medical side and the Pharmacy side. 2. Check for a FDA approved diagnosis and verify clinical benefits as well as monitor periodic Ig. G levels (if applicable to diagnosis, such as hypogammaglobulinemia). 3. Initially approve for 3 -6 months with additional documentation required after that time period to renew the authorization. 4. Implementation date of 01/01/2013 6

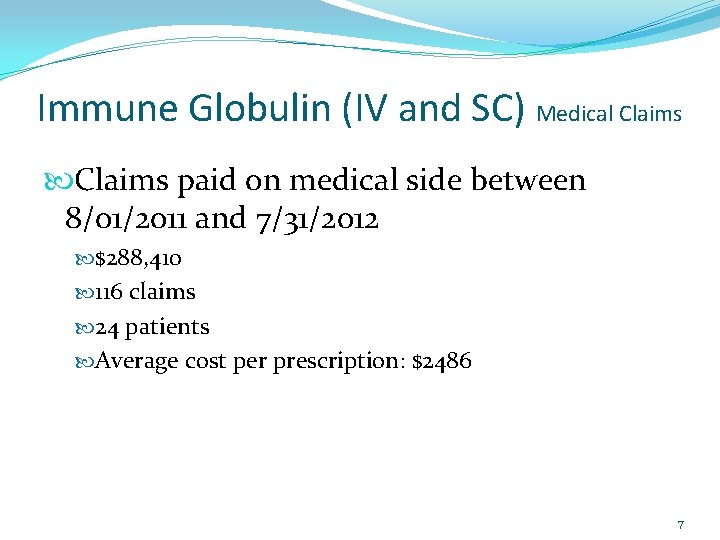
Immune Globulin (IV and SC) Medical Claims paid on medical side between 8/01/2011 and 7/31/2012 $288, 410 116 claims 24 patients Average cost per prescription: $2486 7

Immune Globulin (IV and SC) Medical Claims Requiring prior authorization for applicable J-codes effective 01/01/2013. Notification of new prior authorization requirements published in Medic Aide Pharmacy Unit will be processing these prior authorization requests. 8

Immune Globulin (IV and SC)Pharmacy Claims Reviewing outpatient prescription claims between 8/01/2011 and 7/31/2012 $279, 527 79 claims 14 patients Average cost per prescription: $3538 9

Immune Globulin (IV and SC)Pharmacy Claims Requiring prior authorization effective 01/01/2013. Notification letter sent out to current prescribers and pharmacies of patients receiving immune globulin between September – November 2012(letters sent second week of December) and to new patients receiving immune globulin in December 2012 (letters sent early January 2013). 10

Immune Globulin (IV and SC) Reference: Intravenous Immune Globulin in Autoimmune and Inflammatory Disease. NEJM 2012; 367: 2015 -25. Review article in NEJM published in November 2012. Medicare or a commercial insurer has approved reimbursement for such therapy [autoimmune conditions], often conditionally, requiring documentation of contraindications to or a lack of response to conventional therapies. 11

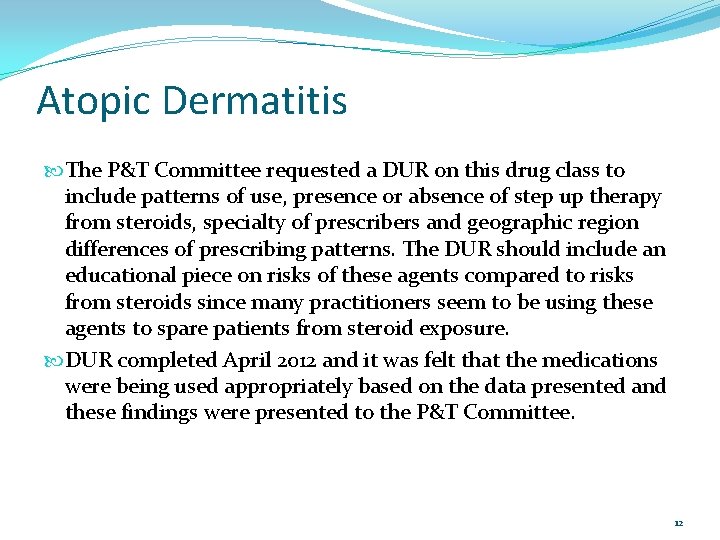
Atopic Dermatitis The P&T Committee requested a DUR on this drug class to include patterns of use, presence or absence of step up therapy from steroids, specialty of prescribers and geographic region differences of prescribing patterns. The DUR should include an educational piece on risks of these agents compared to risks from steroids since many practitioners seem to be using these agents to spare patients from steroid exposure. DUR completed April 2012 and it was felt that the medications were being used appropriately based on the data presented and these findings were presented to the P&T Committee. 12

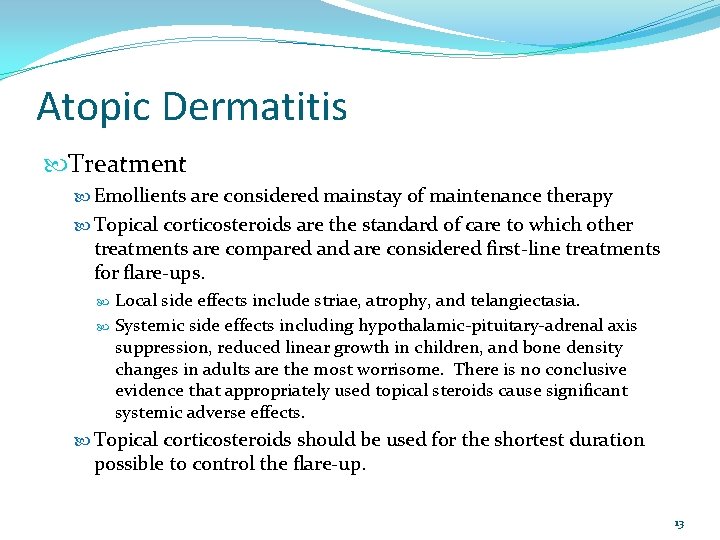
Atopic Dermatitis Treatment Emollients are considered mainstay of maintenance therapy Topical corticosteroids are the standard of care to which other treatments are compared and are considered first-line treatments for flare-ups. Local side effects include striae, atrophy, and telangiectasia. Systemic side effects including hypothalamic-pituitary-adrenal axis suppression, reduced linear growth in children, and bone density changes in adults are the most worrisome. There is no conclusive evidence that appropriately used topical steroids cause significant systemic adverse effects. Topical corticosteroids should be used for the shortest duration possible to control the flare-up. 13

Atopic Dermatitis Treatment Sedating antihistamines are useful when patients have sleep disturbances and concomitant allergic conditions. Antibiotics should be reserved for patients with acutely infected lesions. Topical calcineurin inhibitors should be second-line treatment for flare-ups and maintenance. Local side effects include skin burning and irritation. Patients should also be counseled on proper sun protection. Black Box Warning – discussed on next slide 14

Atopic Dermatitis 15

Atopic Dermatitis In March 2010, the FDA issued a public health advisory about the potential cancer risk associated with the use of Elidel (pimecrolimus) and Protopic (tacrolimus) products applied to the skin. This was based off of information from animal studies, case reports in a small amount of patients, and how the drugs work. The FDA recommends that healthcare providers, patients, and caregivers consider the following: Use these products only as second-line agents as short term and intermittent treatment. Avoid the use in children under the age of 2. Use for a short period of time, not continuously. Children and adults with a weakened or compromised immune system should not use these products. Use the minimum amount of the products needed to control the patient’s symptoms. 16

Atopic Dermatitis References Hanifin, J. M. , Cooper, K. D. , Ho, V. C. , Kang, S. , et al. Guidelines of care for atopic dermatitis. Journal of the American Academy of Dermatology. 2004; 50: 391 -404. Peterson, J. D. , Chan, L. S. , A Comprehensive Management Guide for Atopic Dermatitis. Dermatology Nursing. 2006; 18(6): 531 -542. Buys, L. M. , Treatment Options for Atopic Dermatitis. Am Fam Physician. 2007; Feb 15; 75(4): 523 -528. http: //www. fda. gov/Drugs/Drug. Safety/Postmarket. Drug. Safety. Informationfor. Patient sand. Providers/ucm 153525. htm. Retrieved March 16, 2012. http: //www. fda. gov/Drugs/Drug. Safety/Postmarket. Drug. Safety. Informationfor. Patient sand. Providers/ucm 153941. htm. Retrieved March 16, 2012. Elidel [package insert]. East Hanover, NJ; Novartis Pharmaceuticals Corp. ; July 2010. Protopic [package insert]. Deerfield, IL; Astellas Pharma US, Inc. ; November 2011. 17

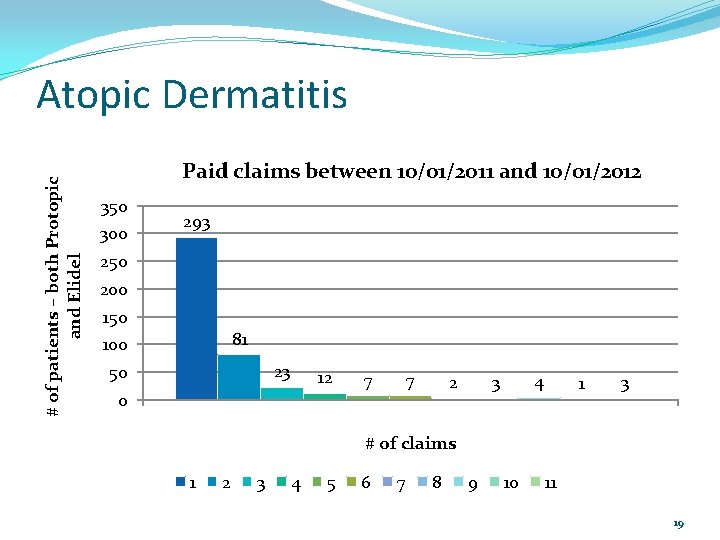
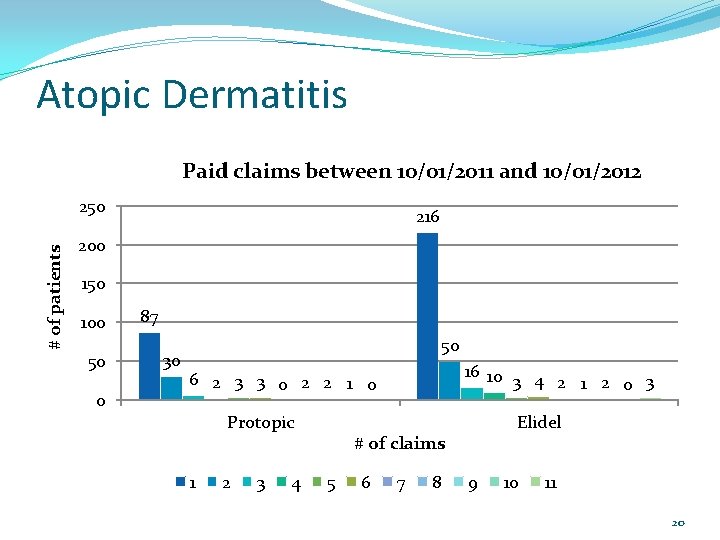
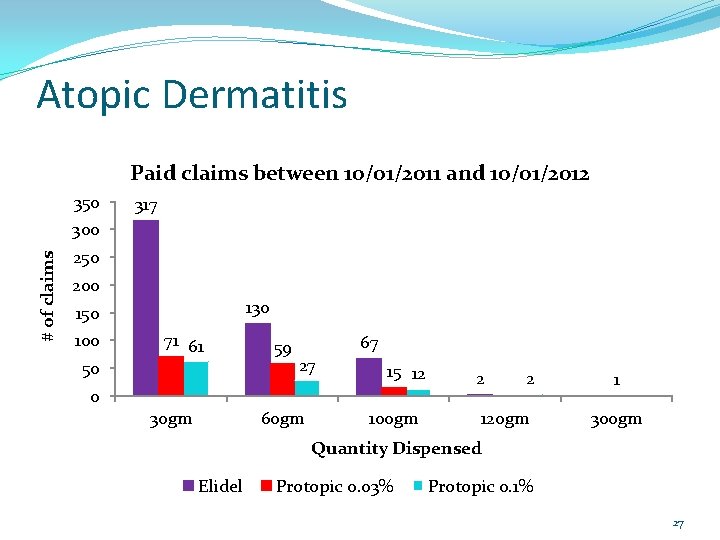
Atopic Dermatitis The P&T Committee asked at their October 2012 meeting for the DUR Board to look at how frequently these medications were being filled. A review of paid claims between 10/01/2011 and 10/01/2012 was done. 18

# of patients – both Protopic and Elidel Atopic Dermatitis Paid claims between 10/01/2011 and 10/01/2012 350 300 293 250 200 150 81 100 23 50 12 0 7 7 4 3 2 1 3 # of claims 1 2 3 4 5 6 7 8 9 10 11 19

Atopic Dermatitis Paid claims between 10/01/2011 and 10/01/2012 # of patients 250 216 200 150 100 50 0 87 30 50 16 10 3 4 2 1 2 0 3 6 2 3 3 0 2 2 1 0 Protopic 1 2 3 4 Elidel # of claims 5 6 7 8 9 10 11 20

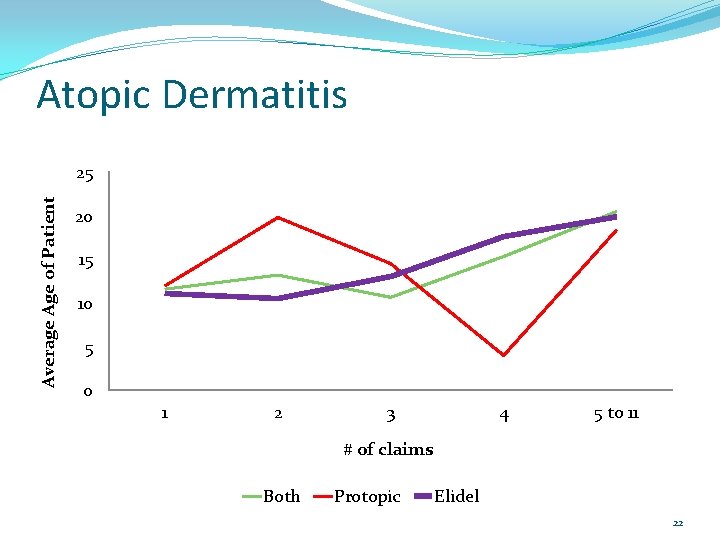
Atopic Dermatitis # of claims Age-both Age-Protopic Age-Elidel 1 11. 58 ± 10. 62 11. 97 ± 12. 23 11. 09 ± 9. 79 2 13. 20 ± 15. 56 19. 79 ± 20. 96 10. 51 ± 11. 77 3 10. 65 ± 7. 91 14. 50 ± 13. 70 13. 06 ± 11. 91 4 15. 33 ± 15. 09 4. 00 ± 2. 83 17. 60 ± 15. 59 5 to 11 20. 48 ± 20. 31 18. 36 ± 19. 07 19. 87 ± 20. 66 21

Atopic Dermatitis Average Age of Patient 25 20 15 10 5 0 1 2 3 4 5 to 11 # of claims Both Protopic Elidel 22

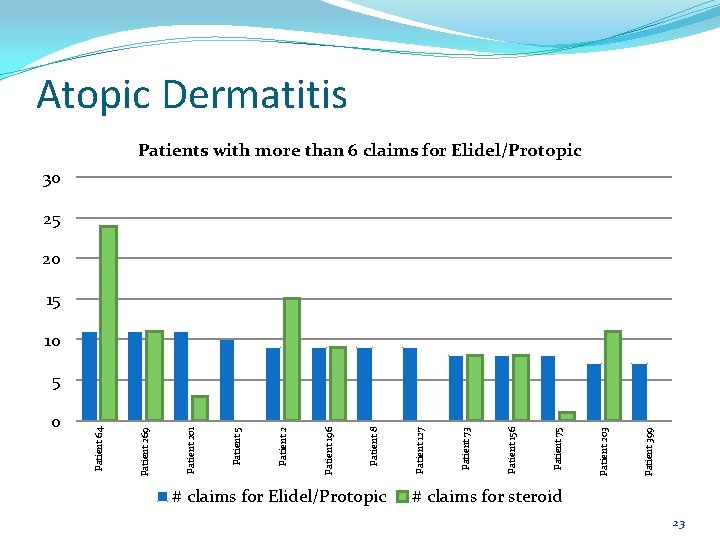
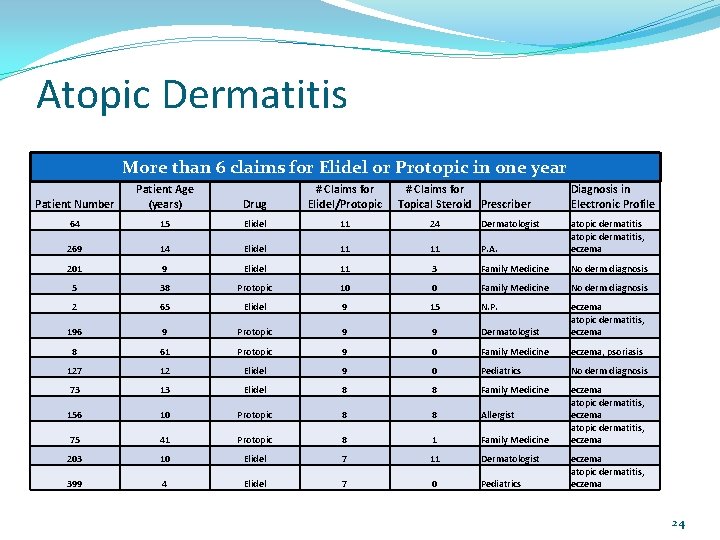
0 # claims for Elidel/Protopic Patient 399 Patient 203 Patient 75 Patient 156 Patient 73 Patient 127 Patient 8 Patient 196 Patient 2 Patient 5 Patient 201 Patient 269 Patient 64 Atopic Dermatitis Patients with more than 6 claims for Elidel/Protopic 30 25 20 15 10 5 # claims for steroid 23

Atopic Dermatitis More than 6 claims for Elidel or Protopic in one year Patient Number Patient Age (years) Drug # Claims for Elidel/Protopic # Claims for Topical Steroid Prescriber Diagnosis in Electronic Profile 64 15 Elidel 11 24 Dermatologist 269 14 Elidel 11 11 P. A. atopic dermatitis, eczema 201 9 Elidel 11 3 Family Medicine No derm diagnosis 5 38 Protopic 10 0 Family Medicine No derm diagnosis 2 65 Elidel 9 15 N. P. 196 9 Protopic 9 9 Dermatologist eczema atopic dermatitis, eczema 8 61 Protopic 9 0 Family Medicine eczema, psoriasis 127 12 Elidel 9 0 Pediatrics No derm diagnosis 73 13 Elidel 8 8 Family Medicine 156 10 Protopic 8 8 Allergist 75 41 Protopic 8 1 Family Medicine eczema atopic dermatitis, eczema 203 10 Elidel 7 11 Dermatologist 399 4 Elidel 7 0 Pediatrics eczema atopic dermatitis, eczema 24

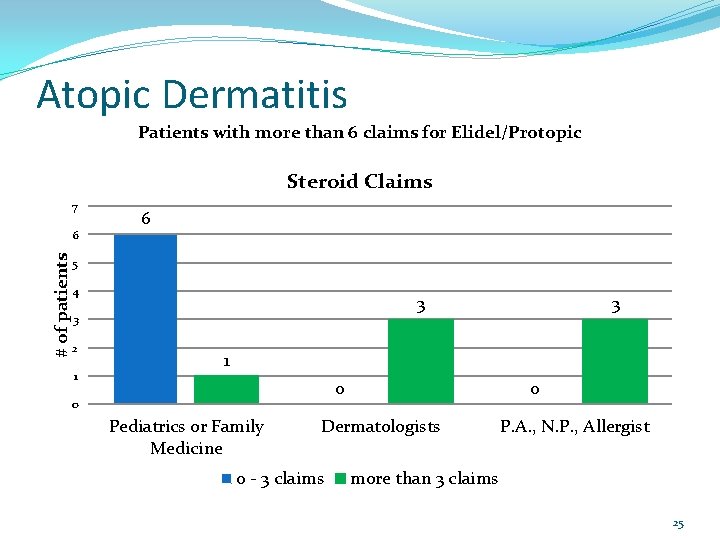
Atopic Dermatitis Patients with more than 6 claims for Elidel/Protopic Steroid Claims 7 # of patients 6 6 5 4 3 3 2 1 3 1 0 0 Pediatrics or Family Medicine 0 Dermatologists 0 - 3 claims P. A. , N. P. , Allergist more than 3 claims 25

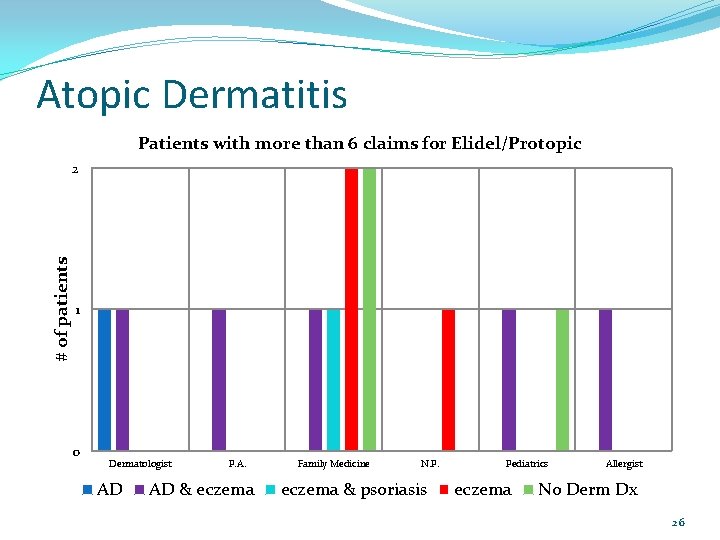
Atopic Dermatitis Patients with more than 6 claims for Elidel/Protopic # of patients 2 1 0 Dermatologist AD P. A. AD & eczema Family Medicine N. P. eczema & psoriasis Pediatrics eczema Allergist No Derm Dx 26

Atopic Dermatitis Paid claims between 10/01/2011 and 10/01/2012 350 317 # of claims 300 250 200 130 150 100 71 61 50 0 30 gm 59 67 27 60 gm 15 12 100 gm 2 2 120 gm 1 300 gm Quantity Dispensed Elidel Protopic 0. 03% Protopic 0. 1% 27

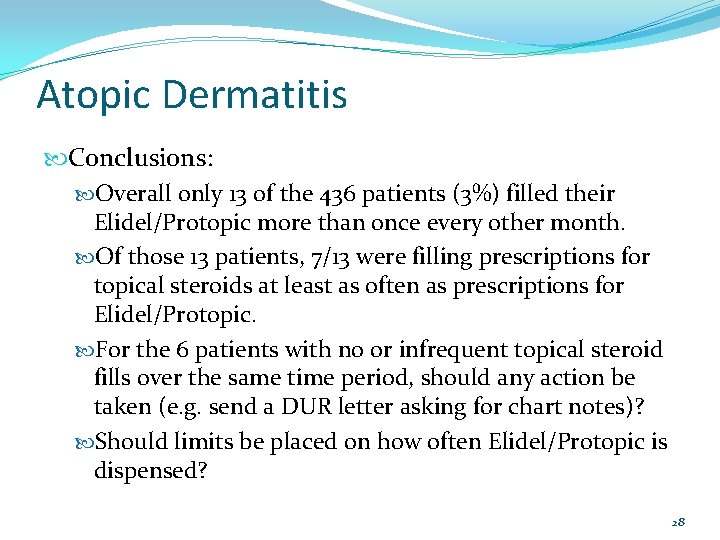
Atopic Dermatitis Conclusions: Overall only 13 of the 436 patients (3%) filled their Elidel/Protopic more than once every other month. Of those 13 patients, 7/13 were filling prescriptions for topical steroids at least as often as prescriptions for Elidel/Protopic. For the 6 patients with no or infrequent topical steroid fills over the same time period, should any action be taken (e. g. send a DUR letter asking for chart notes)? Should limits be placed on how often Elidel/Protopic is dispensed? 28

Atopic Dermatitis Recommendations of DUR Board 29

Current Interventions/Outcomes Studies P&T Committee Narcotic Analgesic Studies National Summit on Opioid Safety Psychotropic Medications in Foster Children Two (2) or more concomitant antipsychotics Synagis Update Revatio 30

National Summit on Opioid Safety October 31 - November 1, 2012 Seattle, Washington 31

Principles for more selective and cautious opioid prescribing* Principles for All Chronic Non-Cancer Pain Patients 1. 2. 3. 4. Self-care is the foundation for effective chronic noncancer pain care Your relationship with the patient supports effective self-care Guide care by progress toward resuming activities Prioritize long-term effectiveness over short-term pain relief * These principles are not intended for palliative care of chronic pain at end of life. 32

Principles for more selective and cautious opioid prescribing* Principles When Considering Long-term Use of Opioids Put patient safety first 2. Think twice before prescribing long-term opioids for axial low back pain, headache and fibromyalgia 3. Systematically evaluate risks 4. Consider intermittent opioid use 5. Do not sustain opioid use long-term without decisive benefits 6. Keep opioid doses as low as possible 1. * These principles are not intended for palliative care of chronic pain at end of life. 33

Principles for more selective and cautious opioid prescribing* Principles for Patients Using Opioids Long-term 1. 2. 3. 4. 5. Clearly communicate standardized expectations to reduce risks Adhere to recommended precautions Avoid prescribing opioids and sedatives concurrently Revisit discontinuing opioids or lowering dose Identify and treat prescription opioid misuse disorders * These principles are not intended for palliative care of chronic pain at end of life. 34

Principles for more selective and cautious opioid prescribing* Prepared by the faculty of the National Summit for Opioid Safety The National Summit had support from the Group Health Foundation. It was co-sponsored by Group Health Research Institute; Project ROAM (Dept. of Family Medicine, University of Washington); and Physicians for Responsible Opioid Prescribing (PROP). * These principles are not intended for palliative care of chronic pain at end of life. 35

Foster Children Psychotropic Drugs Red Flags 1/17/2013 36

Red Flags Five (5) or more psychotropic medications prescribed concomitantly (reviewed August 2012) Two (2) or more concomitant antidepressants (reviewed October 2013) Two (2) or more concomitant antipsychotic medications (current) Two(2) or more concomitant stimulant medications long-acting plus short-acting ok Three (3) or more concomitant mood stabilizer medications Psychotropic polypharmacy (2 or more agents) for a given mental disorder prescribed before utilizing psychotropic monotherapy 37

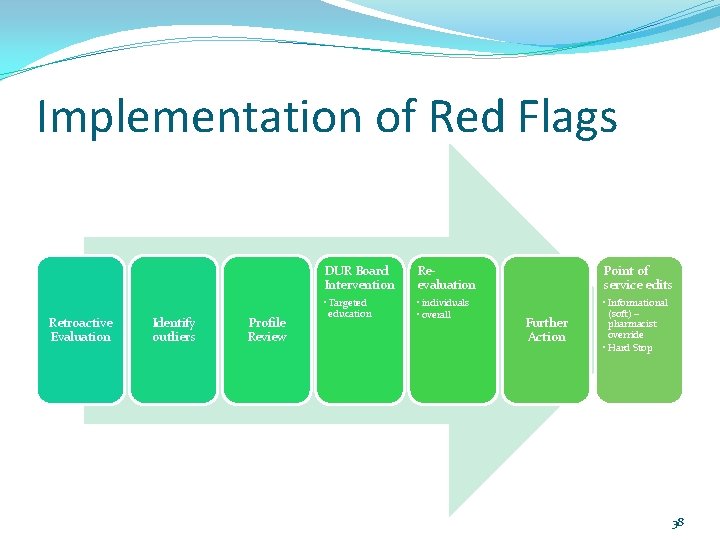
Implementation of Red Flags Retroactive Evaluation Identify outliers Profile Review DUR Board Intervention Reevaluation Point of service edits • Targeted education • individuals • overall • Informational (soft) – pharmacist override • Hard Stop Further Action 38

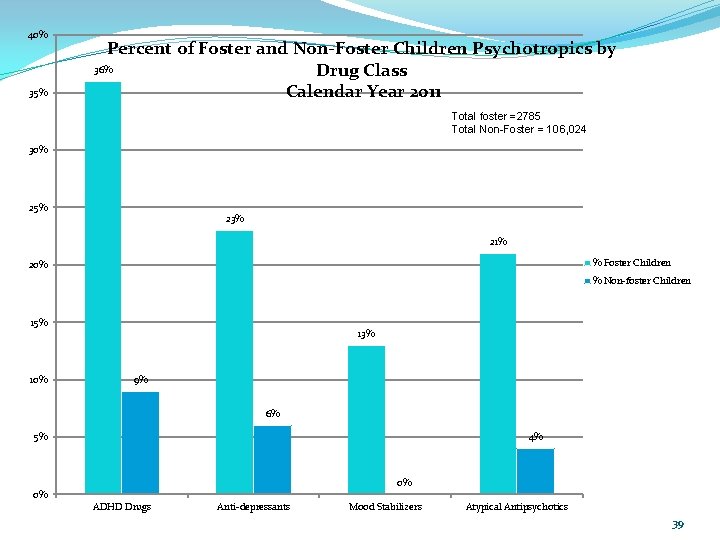
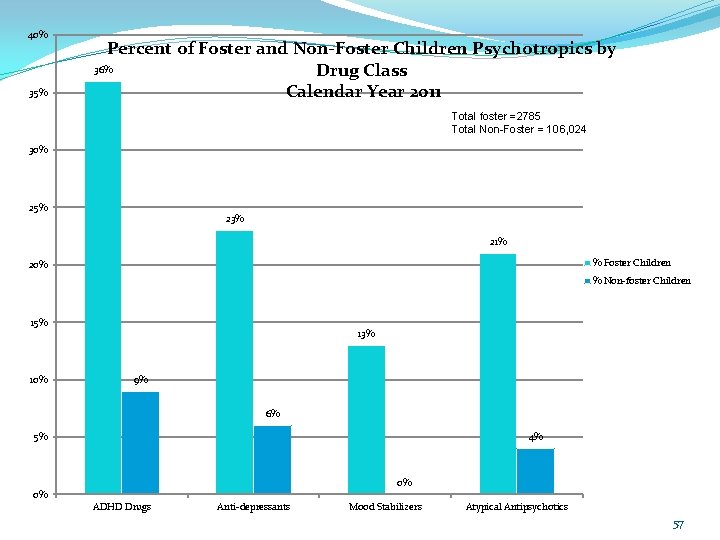
40% 35% Percent of Foster and Non-Foster Children Psychotropics by 36% Drug Class Calendar Year 2011 Total foster =2785 Total Non-Foster = 106, 024 30% 25% 23% 21% % Foster Children 20% % Non-foster Children 15% 10% 13% 9% 6% 4% 5% 0% 0% ADHD Drugs Anti-depressants Mood Stabilizers Atypical Antipsychotics 39

Foster Children Receiving Two or More Concurrent Antipsychotics 40

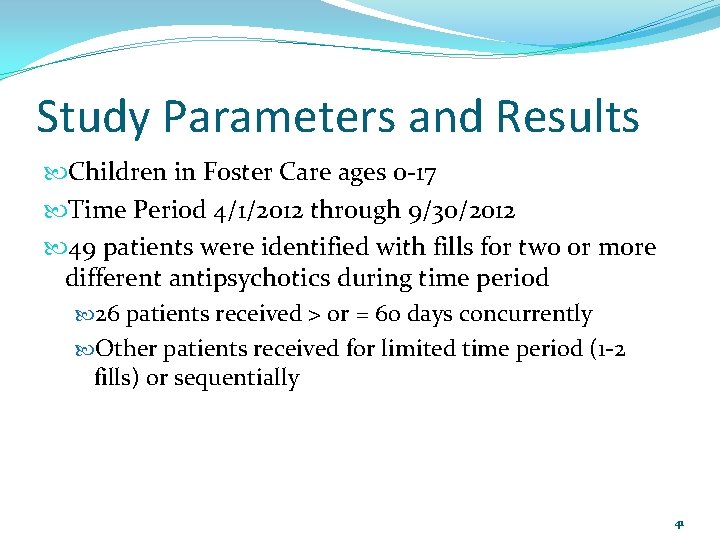
Study Parameters and Results Children in Foster Care ages 0 -17 Time Period 4/1/2012 through 9/30/2012 49 patients were identified with fills for two or more different antipsychotics during time period 26 patients received > or = 60 days concurrently Other patients received for limited time period (1 -2 fills) or sequentially 41

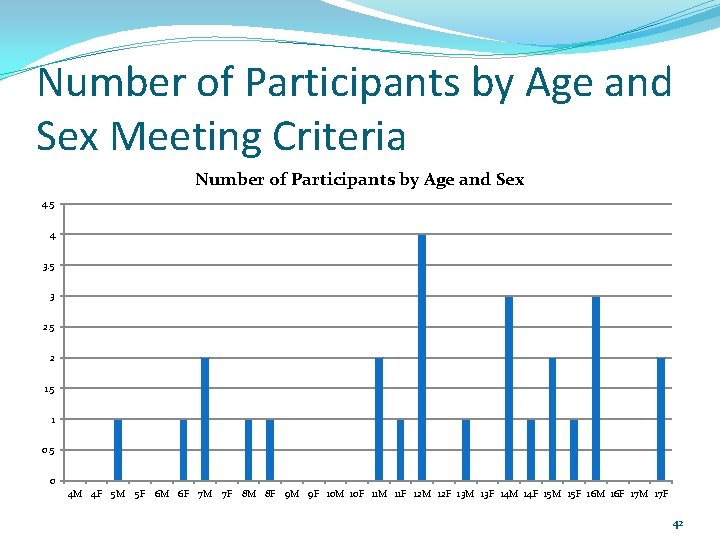
Number of Participants by Age and Sex Meeting Criteria Number of Participants by Age and Sex 4. 5 4 3. 5 3 2. 5 2 1. 5 1 0. 5 0 4 M 4 F 5 M 5 F 6 M 6 F 7 M 7 F 8 M 8 F 9 M 9 F 10 M 10 F 11 M 11 F 12 M 12 F 13 M 13 F 14 M 14 F 15 M 15 F 16 M 16 F 17 M 17 F 42

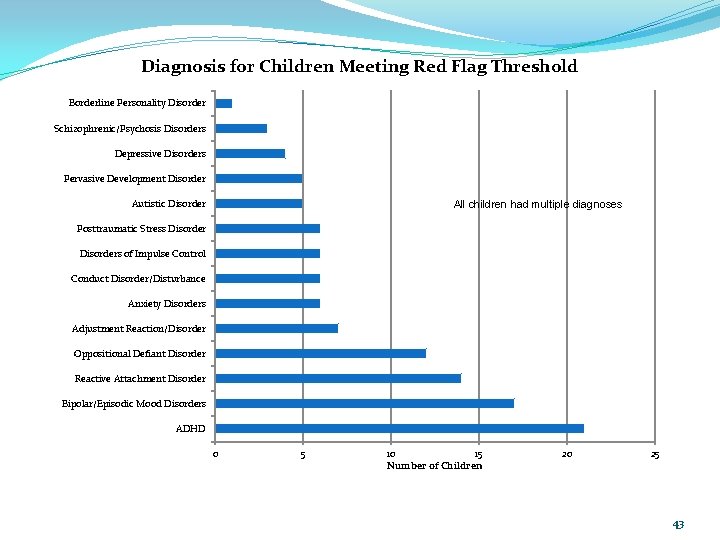
Diagnosis for Children Meeting Red Flag Threshold Borderline Personality Disorder Schizophrenic/Psychosis Disorders Depressive Disorders Pervasive Development Disorder Autistic Disorder All children had multiple diagnoses Posttraumatic Stress Disorders of Impulse Control Conduct Disorder/Disturbance Anxiety Disorders Adjustment Reaction/Disorder Oppositional Defiant Disorder Reactive Attachment Disorder Bipolar/Episodic Mood Disorders ADHD 0 5 10 15 Number of Children 20 25 43

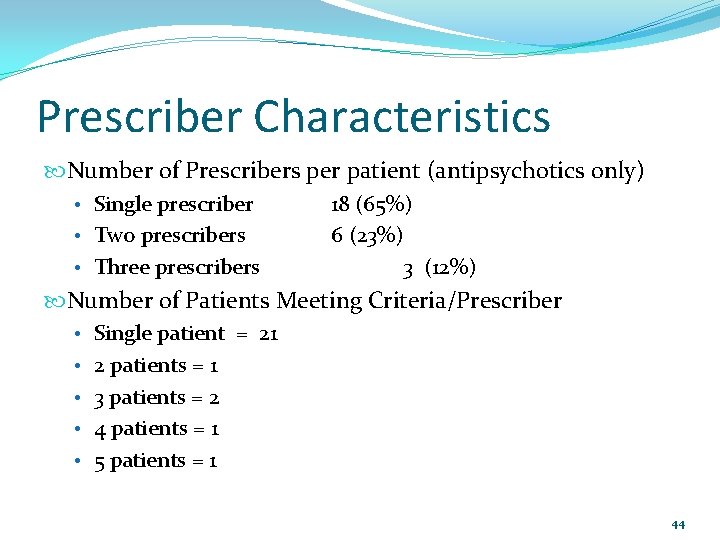
Prescriber Characteristics Number of Prescribers per patient (antipsychotics only) • Single prescriber • Two prescribers • Three prescribers 18 (65%) 6 (23%) 3 (12%) Number of Patients Meeting Criteria/Prescriber • Single patient = 21 • 2 patients = 1 • 3 patients = 2 • 4 patients = 1 • 5 patients = 1 44

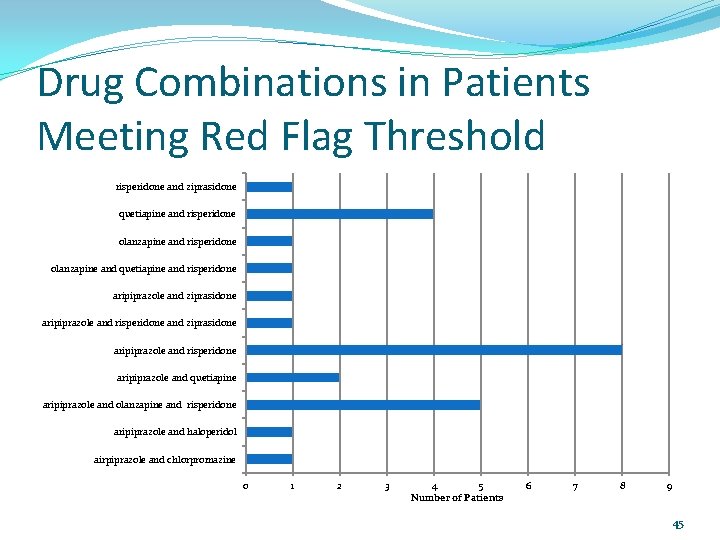
Drug Combinations in Patients Meeting Red Flag Threshold risperidone and ziprasidone quetiapine and risperidone olanzapine and quetiapine and risperidone aripiprazole and ziprasidone aripiprazole and risperidone aripiprazole and quetiapine aripiprazole and olanzapine and risperidone aripiprazole and haloperidol airpiprazole and chlorpromazine 0 1 2 3 4 5 Number of Patients 6 7 8 9 45

Next Steps ? 46

Synagis Update Idaho Medicaid’s outpatient prescription drug program authorized payment for eligible patients for the 2012 -2013 RSV season as of December 1, 2012. Many hospitals started dosing Synagis in November 2012. Doses given in the hospital are subtracted from the total doses approved by Idaho Medicaid outpatient prescription drug program. AAP recommends a maximum of five monthly doses – recommend utilizing Idaho specific epidemiology to maximize drug benefit. After the fifth dose of Synagis, most patients will have adequate RSV antibody titers for six to seven weeks. The antibody levels do not plummet to zero thirty days after the fifth Synagis dose. 47

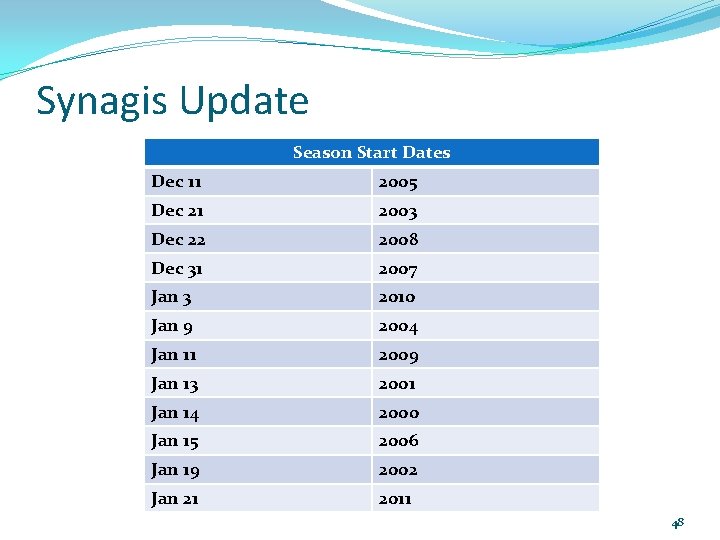
Synagis Update Season Start Dates Dec 11 2005 Dec 21 2003 Dec 22 2008 Dec 31 2007 Jan 3 2010 Jan 9 2004 Jan 11 2009 Jan 13 2001 Jan 14 2000 Jan 15 2006 Jan 19 2002 Jan 21 2011 48

Synagis Update Season End Dates Mar 28 2004 Apr 10 2006 Apr 13 2003 Apr 24 2005 May 5 2008 May 6 2001 May 7 2011 May 9 2010 May 12 2002 May 19 2012 May 24 2009 May 28 2007 49

Synagis Update In Idaho, Respiratory Syncytial Virus (RSV) season officially began the week ending December 8, 2012. The definition for season onset is adapted from the National Respiratory and Enteric Virus Surveillance System (NREVSS). RSV is considered widespread in Idaho in the first of two consecutive weeks during which the reported total percent of specimens testing positive for antigen is ≥ 10%. 50

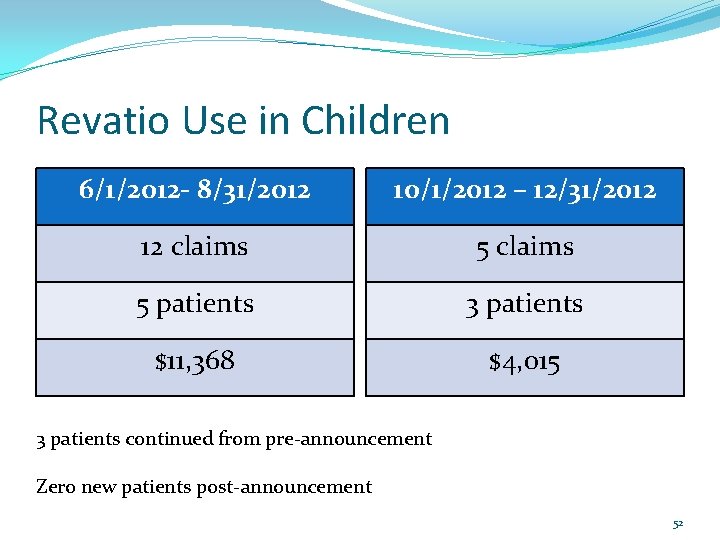
Revatio Use in Children On August 30, 2012, the U. S. Food and Drug Administration (FDA) sent out a safety announcement recommending against the use of Revatio in children with pulmonary hypertension. (handout in packet) Revatio claims in Idaho Medicaid patients were reviewed prior to and after the announcement for comparison. 51

Revatio Use in Children 6/1/2012 - 8/31/2012 10/1/2012 – 12/31/2012 12 claims 5 patients 3 patients $11, 368 $4, 015 3 patients continued from pre-announcement Zero new patients post-announcement 52

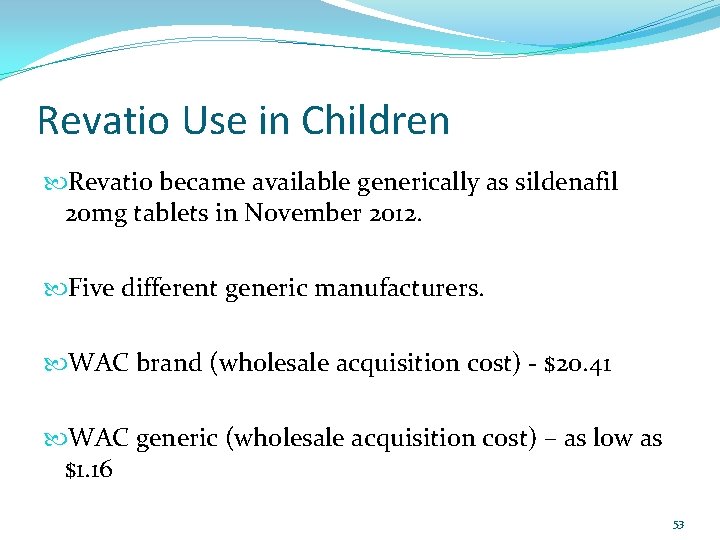
Revatio Use in Children Revatio became available generically as sildenafil 20 mg tablets in November 2012. Five different generic manufacturers. WAC brand (wholesale acquisition cost) - $20. 41 WAC generic (wholesale acquisition cost) – as low as $1. 16 53

Proposed Studies for Next Quarter: P&T Committee Narcotic Analgesic Studies – Next Steps Use of Psychotropic Medications in Foster Children – Next Steps Two(2) or more concomitant stimulant medications long-acting plus short-acting ok Migraine Prevention Prophylaxis Utilization in Chronic Triptan Utilizers Botulinumtoxin Products Testosterone enanthate Testosterone cypionate Antipsychotic Indication Evaluation- Hold for Future AAP and DVTs- Hold for future 54

P&T Committee Narcotic Analgesic Studies – Next Steps 55

Use of Psychotropic Medications in Foster Children The U. S. Government Accountability Office released the results from a study that they performed examining the rates of psychotropic medications for foster and nonfoster children in 2008. It was determined that HHS Guidance Could Help States Improve Oversight of Psychotropic Prescriptions. 56

40% 35% Percent of Foster and Non-Foster Children Psychotropics by 36% Drug Class Calendar Year 2011 Total foster =2785 Total Non-Foster = 106, 024 30% 25% 23% 21% % Foster Children 20% % Non-foster Children 15% 10% 13% 9% 6% 4% 5% 0% 0% ADHD Drugs Anti-depressants Mood Stabilizers Atypical Antipsychotics 57

Use of Psychotropic Medications in Foster Children: Next Steps Two(2) or more concomitant stimulant medications long-acting plus short-acting ok 58

Migraine Prevention Prophylaxis Utilization in Chronic Triptan Utilizers See packet for summary handout 59

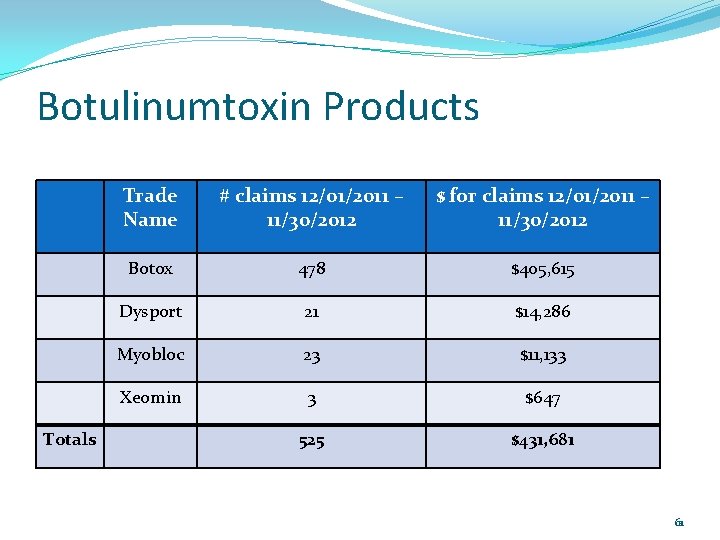
Botulinumtoxin Products Botulinumtoxin products are excluded from coverage by the outpatient pharmacy prescription drug program – these medications are only administered by health care professionals and are not safe for patients to pick up and “brown bag” to the doctor’s office. Botulinumtoxin products are currently payable without prior authorization on the medical side using J codes. 60

Botulinumtoxin Products Totals Trade Name # claims 12/01/2011 – 11/30/2012 $ for claims 12/01/2011 – 11/30/2012 Botox 478 $405, 615 Dysport 21 $14, 286 Myobloc 23 $11, 133 Xeomin 3 $647 525 $431, 681 61

Botulinumtoxin Products Will review profiles of patients with paid claims on the medical side to assess what the botulinumtoxin is most likely being used for (e. g. cervical dystonia, migraines). Even though Botox does not require prior authorization at this time, the department has been receiving prior authorization requests for Botox for migraines. Need to develop criteria for Botox’s place in therapy as it is not first -line therapy. FDA approved for chronic migraines for patients with at least 15 days of migraines per month with each migraine lasting at least four hours. 62

Testosterone Products Testosterone enanthate Testosterone cypionate 63

Antipsychotic Indication Evaluation- Hold for Future 64

AAP and DVTs- Hold for future 65

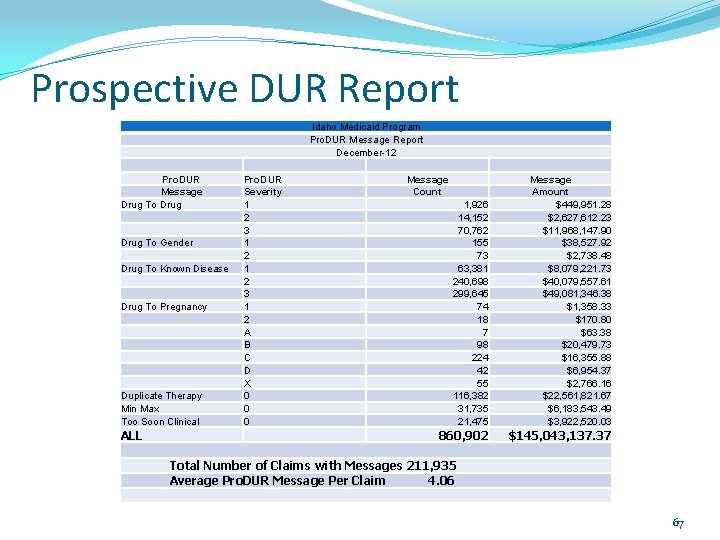
Prospective DUR Report History Errors: Non-History Errors: • DD – drug-to-drug • PA – drug-to-age • PG – drug to pregnancy • HD – high dose • TD – therapeutic duplication • LD – low dose • ER – early refill • SX – drug-to-gender • MC – drug-to-disease 66

Prospective DUR Report Idaho Medicaid Program Pro. DUR Message Report December-12 Pro. DUR Message Drug To Gender Drug To Known Disease Drug To Pregnancy Duplicate Therapy Min Max Too Soon Clinical Pro. DUR Severity 1 2 3 1 2 A B C D X 0 0 0 Message Count 1, 926 14, 152 70, 762 155 73 63, 381 240, 698 299, 645 74 18 7 98 224 42 55 116, 382 31, 735 21, 475 ALL 860, 902 Total Number of Claims with Messages 211, 935 Average Pro. DUR Message Per Claim 4. 06 Message Amount $449, 951. 28 $2, 627, 612. 23 $11, 968, 147. 90 $38, 527. 92 $2, 738. 48 $8, 079, 221. 73 $40, 079, 557. 61 $49, 081, 346. 38 $1, 358. 33 $170. 80 $63. 38 $20, 479. 73 $16, 355. 88 $6, 954. 37 $2, 766. 16 $22, 561, 821. 67 $6, 183, 543. 49 $3, 922, 520. 03 $145, 043, 137. 37 67

DUR Winter Newsletter Copy of Fall Newsletter in packet Brainstorm for new topics 68

Medicaid Update 69
 Drug utilization evaluation template
Drug utilization evaluation template Deliberate adulteration examples
Deliberate adulteration examples Logisticare dallas
Logisticare dallas Ny medicaid ehr incentive program
Ny medicaid ehr incentive program Drug review
Drug review Drug review
Drug review Texas drug offender education program test answers
Texas drug offender education program test answers Tadra is an acronym for georgia’s
Tadra is an acronym for georgia’s Who program for international drug monitoring
Who program for international drug monitoring Alabama prescription drug monitoring program
Alabama prescription drug monitoring program Residential drug abuse program (rdap)
Residential drug abuse program (rdap) Texas pmp aware
Texas pmp aware Warehouse goals
Warehouse goals Utilization law
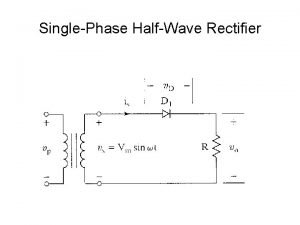
Utilization law Half wave rectifier battery charger
Half wave rectifier battery charger Ecss pus
Ecss pus Cube utilization formula
Cube utilization formula Link utilization
Link utilization Citrate utilization test
Citrate utilization test Citrate results
Citrate results Tsi test: principle
Tsi test: principle Cascade utilization
Cascade utilization Data utilization strategies
Data utilization strategies Asset utilization formula
Asset utilization formula Load distance method
Load distance method Chapter 6 process selection and facility layout
Chapter 6 process selection and facility layout Design capacity formula
Design capacity formula Litmus milk
Litmus milk Asset utilization analysis
Asset utilization analysis Basic unit of cpu utilization
Basic unit of cpu utilization Resource utilization definition in project management
Resource utilization definition in project management Plant utilization factor
Plant utilization factor Throughput flow rate
Throughput flow rate Processor utilization
Processor utilization Unit hour utilization formula
Unit hour utilization formula Bandwidth utilization multiplexing and spreading
Bandwidth utilization multiplexing and spreading Bandwidth utilization multiplexing and spreading
Bandwidth utilization multiplexing and spreading Medication utilization evaluation
Medication utilization evaluation Oats amino acid profile
Oats amino acid profile Healthcare utilization project
Healthcare utilization project Research utilization process steps
Research utilization process steps Factors affecting chronaxie
Factors affecting chronaxie Jadi
Jadi Isolux diagram for finding luminance on road surface is a
Isolux diagram for finding luminance on road surface is a Dd form 2406
Dd form 2406 Apc violation sophos
Apc violation sophos Farooq ghani
Farooq ghani Healthcare utilization project
Healthcare utilization project P vulgaris nitrate reduction test
P vulgaris nitrate reduction test Va office of small and disadvantaged business utilization
Va office of small and disadvantaged business utilization Define research utilization
Define research utilization Utilization rate operations management
Utilization rate operations management What is multiplexer
What is multiplexer Assets utilization
Assets utilization Cisco bandwidth utilization calculation
Cisco bandwidth utilization calculation Snake river teacup diagram
Snake river teacup diagram Nrf idaho
Nrf idaho Bishops storehouse
Bishops storehouse Idaho travel council
Idaho travel council Csi office on aging
Csi office on aging Vocational rehabilitation idaho
Vocational rehabilitation idaho Idaho state police forensics
Idaho state police forensics Idaho state communications
Idaho state communications Idaho core teaching standards
Idaho core teaching standards Idaho core teaching standards
Idaho core teaching standards Idaho dbe directory
Idaho dbe directory Cya idaho falls
Cya idaho falls Idaho heart institute
Idaho heart institute Idaho refugee conference
Idaho refugee conference Idaho legal aid services
Idaho legal aid services