ICH Update Developments and Future Directions IV Pan




































- Slides: 39

ICH Update: Developments and Future Directions IV Pan American Conference on Drug Regulatory Harmonization Boca Chica, Dominican Republic 2 -4 March, 2005 Mike Ward Health Canada

A Unique Approach International Conference on Harmonisation (ICH) was created in 1990 l Agreement between the EU, Japan and the USA to harmonize different regional requirements for registration of pharmaceutical drug products l Unique because joint effort by regulators and associated pharmaceutical industry trade associations l

ICH Objectives Identification and elimination of the need to duplicate studies to meet different regulatory requirements l More efficient use of resources in the R&D process, as a consequence l Quicker access for patients to safe and effective new medicines l

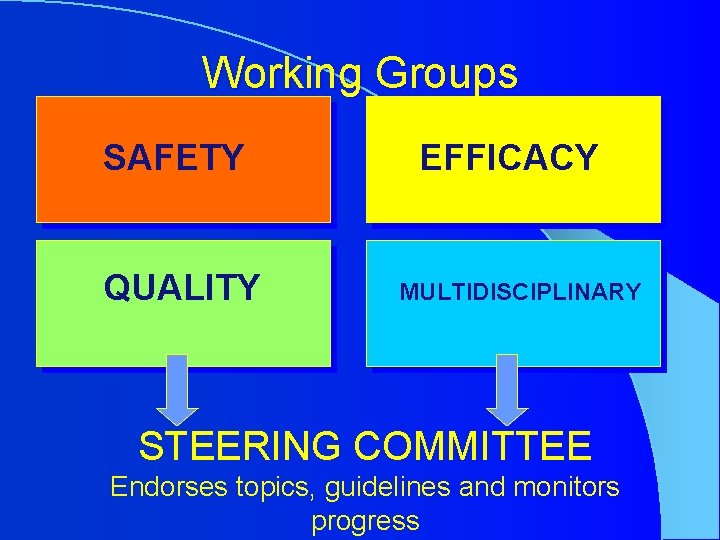
Working Groups SAFETY QUALITY EFFICACY MULTIDISCIPLINARY STEERING COMMITTEE Endorses topics, guidelines and monitors progress

Retrospective ICH – a ‘mature’ harmonization initiative Over 50 technical guidelines, a dictionary of medical terms, electronic standards and a common format for drug registration l Six ICH conferences to date, including ICH 6 in Osaka, Japan, November 2003 l Witnessing increased interest in and use of ICH guidelines globally l Formation and evolution of Global Cooperation Group (GCG) l l

Time to Reflect Following over a decade of significant achievements in the harmonization of quality, safety and efficacy requirements for the registration of new drugs in the three ICH regions, is the work of ICH mostly done? Will ICH become a maintenance-only organization?

No, however… Appropriate time to evaluate the future of ICH to ensure: – Proper balance between maintenance and new activities – New topics are of high value and objectives are achievable – Most efficient use and management of resources – Flexible enough to deal with evolving science and technologies – More transparent

ICH Output since 3 rd Pan-Am Conference, April 02 (1) New products: l Five technical guidelines: – 3 Quality: Stability (Q 1 E, Q 1 F), Biotech comparability (Q 5 E) – 2 Efficacy: post-approval safety (E 2 D) and pharmacovigilance planning (E 2 E) Specifications established for electronic submissions: e. CTD l Four ‘Considerations’ documents: 3 on gene therapy, 1 on gender and clinical trials l

ICH Output since 3 rd Pan-Am Conference (2) l Responding to user needs and new data: maintenance, improvement, clarification activities: – Introduction of question and answers documents (M 4 - CTD/e. CTD, E 5 -ethnic factors, E 2 B(M)-transmission of safety case reports) – Revision of Quality guidelines on stability (Q 1 A) and impurities (Q 3 B) – Maintenance of limits for residual solvents (Q 3 C) – New addendum to periodic safety update reports (E 2 C); `granularity` annex to Common Technical Document (CTD) guidance

ICH Output since 3 rd Pan-Am Conference (3) l Med. DRA: – Continual improvement: Med. DRA version 8. 0 scheduled for release March 2005 – Now available in 8 languages, including Spanish and Portuguese – New draft ‘Points to Consider’ documents on term selection and data retrieval/presentation – Release of first Standardized Med. DRA Queries (SMQs) – collaboration with Council for International Organization of Medical Services (CIOMS)

ICH: Developments and Future Directions `Themes`: – New topics: facilitating innovation – New topics: promoting safety/public health – Regulatory communication – Maintenance/implementation – Mechanics of ICH – Transparency and communication

New Topics: Facilitating Innovation l Examples: – Q 5 E: comparability of biotech/biological products subject to manufacturing changes – Q 8: pharmaceutical development – Q 9: risk management – application to quality requirements and practices Serve to facilitate innovation and improvement l More informed, risked-based approach to regulation l Consistent with FDA’s GMPs for the 21 st Century initiative l

New Topics: Promoting Safety/Public Health l Examples: – E 2 E: pharmacovigilance planning (completed) – E 14/S 7 B: QT prolongation (draft) Topics identified by regulators l Considered important in contributing to drug safety and public health l Potential new pharmacovigilance topics to be considered by ICH following gap analysis of current regional guidelines l

New Topics: Other projects l Q 4 B: regulatory acceptance of (Japanese, European and US) pharmacopeial interchangeability – Process established – Piloting l S 8: immunotoxicology – Nonclinical test methods and decision tree for assessing immunosuppression caused by non-biologic pharmaceuticals

Regulatory Communication (1) l ICH has sought to enhance regulatory communication through the development of: – – Standardized data elements, example: E 2 B Controlled vocabularies, example: Med. DRA Common format for drug registration: CTD Electronic specifications and standards for the transmission of such information, example: ESTRI and e. CTD

Regulatory Communication (2) l Benefits: – Speak same language: promotes communication – Quality, accuracy and consistency of information – Improve timeliness of communications (electronic transmission) – CTD/e. CTD reduces delays and costs associated with registration of drug applications

Regulatory Communication (3) l Status of CTD/e. CTD implementation: – As of July 2003 CTD mandatory in EU and Japan, ‘highly recommended’ in US and Canada – Integration of CTD in existing regulatory frameworks – No significant difficulties encountered – New era: e. CTDs now being submitted in all ICH regions

Regulatory Communication (4) l New: M 5: Data elements and standards for drug dictionaries: – For use in describing medicinal products and active ingredients – Controlled vocabulary includes units, routes of administration, pharmaceutical forms – Electronic message specifications to be developed

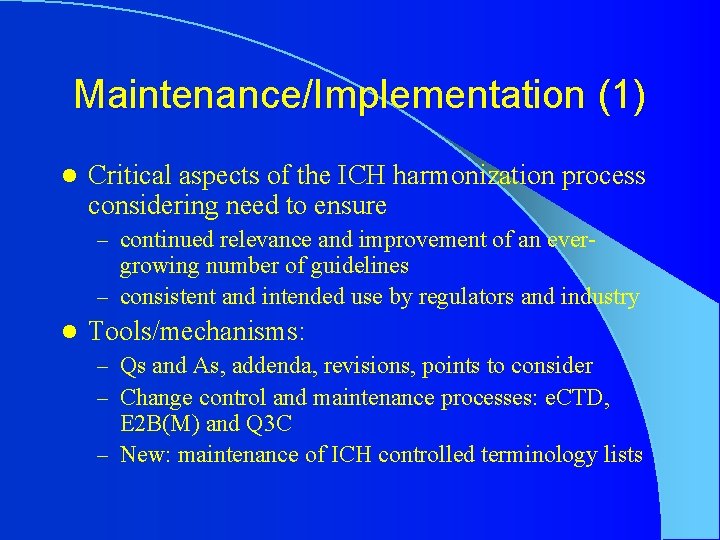
Maintenance/Implementation (1) l Critical aspects of the ICH harmonization process considering need to ensure – continued relevance and improvement of an ever- growing number of guidelines – consistent and intended use by regulators and industry l Tools/mechanisms: – Qs and As, addenda, revisions, points to consider – Change control and maintenance processes: e. CTD, E 2 B(M) and Q 3 C – New: maintenance of ICH controlled terminology lists

Maintenance/Implementation (2) l Tools/mechanisms: – Med. DRA maintenance and support service organization (MSSO) – Creation of implementation working groups (e. g. , for CTD/e. CTD, E 2 B(M)) and implementation tool (new) – Implementation standing item on ICH Steering Committee agenda – General procedures and roles/responsibilities under development

Mechanics of ICH Given costs involved in harmonization activities, it is important that effective structure and procedures are in place to ensure maximum benefit is derived l Periodic reassessment of mandate, procedures within context of ‘Future of ICH’ l Most recent review in progress: June 03 – May 2005; focus includes: l – – – selection of new topics: business case now required streamlining of procedures clarifying roles and responsibilities means of assessing implementation of guidelines Transparency and communication

Transparency and Communication l Reviewing current practices at regional and ICH level l Options to be considered to strengthen transparency and communication l GCG seen as increasingly important mechanism for communication and engagement with non-ICH regions and parties

Global Cooperation Group (GCG) Established March 1999 as sub-committee of ICH Steering Committee • Formed to respond to this growing interest in ICH guidelines • Name reflective of desire to establish links with non-ICH regions • Membership : • – 6 ICH parties – 2 Observers (WHO and Health Canada) – ICH secretariat

Initial Mandate Initial focus on information-sharing • Soon became clear that more active engagement necessary to respond to increasing interest in ICH and ICH guidelines •

Evolution In GCG Activities and Thinking l Series of joint meetings with regional representatives in preparation for ICH 6 satellite session l Parallel discussions within GCG on need to be more relevant through establishing closer ties with regional harmonisation initiatives

Osaka, Nov. 2003: An Important Milestone • Endorsement by ICH SC of new Mandate and Terms of Reference that call for • the ongoing participation of regional harmonization initiatives • Greater transparency

Regional Harmonization Initiatives l APEC – Asia-Pacific Economic Cooperation l ASEAN – Association of the Southeast Asian Nations l GCC – Gulf Cooperation Council l PANDRH – Pan American Network for Drug Regulatory Harmonization l SADC – Southern African Development Community

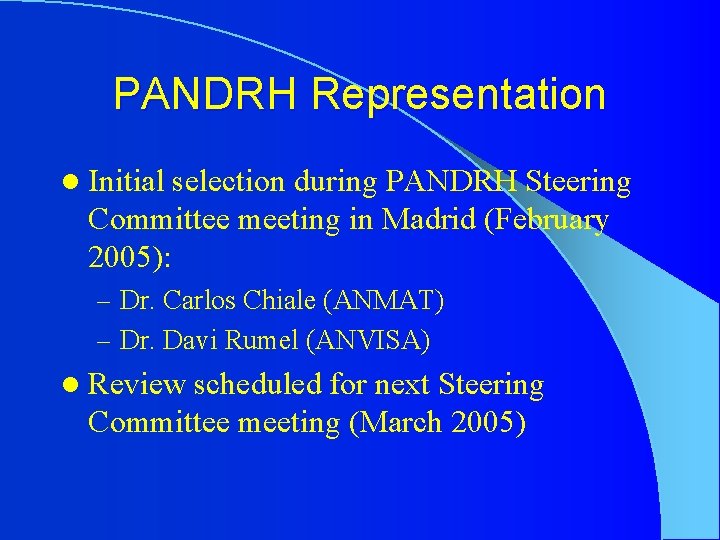
PANDRH Representation l Initial selection during PANDRH Steering Committee meeting in Madrid (February 2005): – Dr. Carlos Chiale (ANMAT) – Dr. Davi Rumel (ANVISA) l Review scheduled for next Steering Committee meeting (March 2005)

GCG Meeting 16 November 2004, Yokohama, Japan GCG Co-chairs: Dr. Yves Juillet (European Industry) Mr. Mike Ward (Health Canada)

GCG Role and Procedures l Definition of GCG membership – Maximum of 2 permanent representatives per regional harmonization initiative – Representatives should solicit/present views of regional initiatives l Criteria for harmonization initiative to participate in GCG l GCG principles: openness and transparency

GCG Reflection’s Paper: Mission, Activities and Operations of GCG l Issue: While new terms of reference provide authority, tools and rules (the means), they do not define the mission of the new GCG (the ends) l Mission/vision necessary to guide and validate future activities

Considerations (1) l Moving beyond bounds of ICH l Serve as unique forum for harmonization initiatives to discuss – Best practices, lessons learned and innovative approaches to harmonization and regulation – ICH topics of interest – Technical guidelines beyond scope of ICH

Considerations (2) l Managing expectations: resources, realistic objectives – GCG not a technical body – Funding issues l Stepwise approach – build on successes

Draft Mission Statement “To promote a mutual understanding of regional initiatives in order to facilitate harmonisation processes related to ICH guidelines regionally and globally; and to strengthen the capacity of drug regulatory authorities and industry to implement them. ”

Areas of work l ICH technical guidelines l Harmonization/regulation l Training and capacity building

Identified Topics of Interest • • CTD implementation and e-submission GCP implementation E 5 implementation Pharmacovigilance guidelines and Med. DRA implementation Interest also expressed in: - technical assistance expressed - review process and data assessment - joint GCP inspections

Regional Meetings Identified l SADC January 05: implementation of ICH guidelines, including CTD l ASEAN February 05: pharmacovigilance

Next Steps: Regional Input l l l Comments on draft ICH guidelines (Q 8, S 8, then Q 9) Proposals for pharmacovigilance plenary session (May 05) Identification and presentation of guideline implementation and technical difficulties (formal presentations at GCG Brussels meeting) GCG teleconference organized for April 7, 2005 Next GCG meeting May 10, 2005 in Brussels

Summary ICH continues to balance development of important new topics with maintenance and implementation activities l New procedures and business case templates meant to improve efficiency and value of ICH process l Transparency, communication and engagement through GCG seen as increasingly important l