ICE Tables ICE tables To do equilibrium problems

ICE Tables

ICE tables • To do equilibrium problems set up an ICE table. • Write the balanced equation. Underneath it label three rows. • Initial Concentrations (pressure) • Change • Equilibrium concentration (pressure)
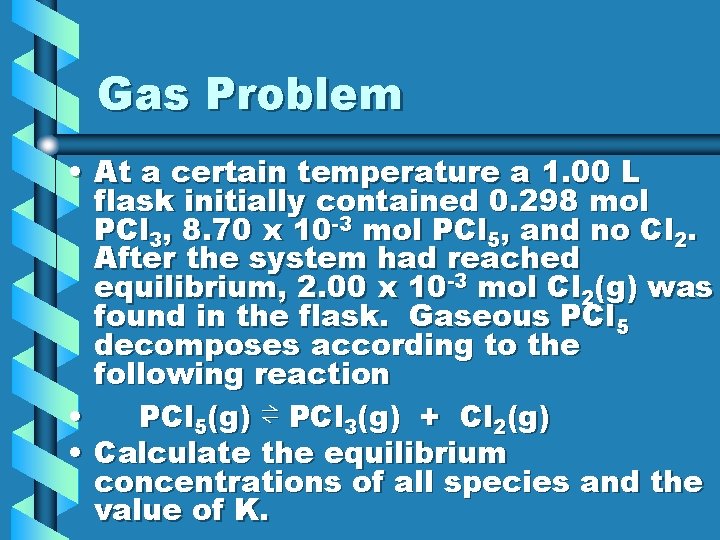
Gas Problem • At a certain temperature a 1. 00 L flask initially contained 0. 298 mol PCl 3, 8. 70 x 10 -3 mol PCl 5, and no Cl 2. After the system had reached equilibrium, 2. 00 x 10 -3 mol Cl 2(g) was found in the flask. Gaseous PCl 5 decomposes according to the following reaction • PCl 5(g) ⇌ PCl 3(g) + Cl 2(g) • Calculate the equilibrium concentrations of all species and the value of K.

Answer • PCl 5(g) PCl 3(g) + Cl 2(g) • I 8. 7 x 10 -3 M. 298 M 0 • C -x +x +x. 300 M 2. 0 x 10 -3 M • E. 0067 M • It must have shifted to the right because chlorine increased. • x must = 2. 0 x 10 -3

K • K =. 3 (. 002) /. 0067 • =. 0896

Concentration Problem • Carbon monoxide reacts with steam to produce carbon dioxide and hydrogen. At 700 K the equilibrium constant is 5. 10. Calculate the equilibrium concentrations of all species if 1. 000 mol of each component is mixed in a 1. 000 -L flask.
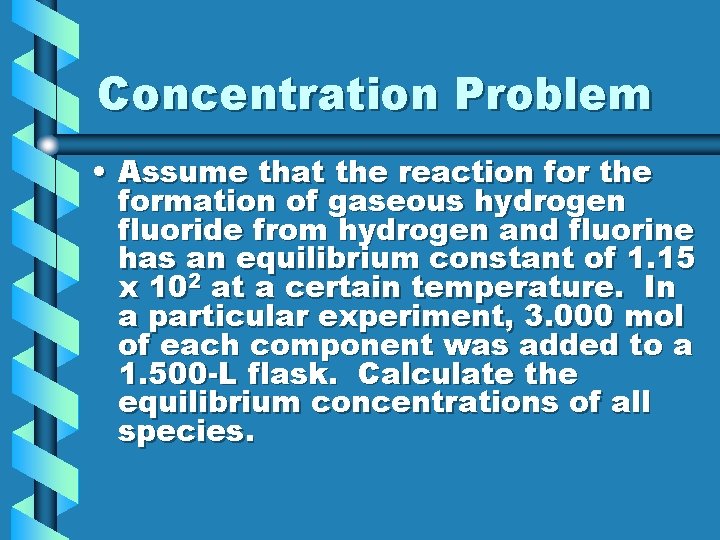
Concentration Problem • Assume that the reaction for the formation of gaseous hydrogen fluoride from hydrogen and fluorine has an equilibrium constant of 1. 15 x 102 at a certain temperature. In a particular experiment, 3. 000 mol of each component was added to a 1. 500 -L flask. Calculate the equilibrium concentrations of all species.

Quadratic Equation • To solve some problems you need to use the quadratic equation • or solver function on a calculator • For a x 2 + bx + c = 0 • x = -b b 2 – 4 ac • 2 a

Solver Function, Quadratic program • Graphing calculators (required for this course) come with a solver function to solve equations. • You can also have a quadratic equation program. • All of this is legal for the AP or any of my tests. • *except on multiple choice.

Simplified Assumptions • If you are using a solver function this is unnecessary. • You do have to understand the concept for a possible multiple choice • For some reactions the change will be very small compared to the initial amount. • You always have to check!

Example • Gaseous NOCl decomposes to form the gases NO and Cl 2. At 35 o C, the K = 1. 6 x 10 -5. The initial conc. Of NOCl is. 5 M. What are the equilibrium concentrations? • 2 NOCl(g) ⇌ 2 NO(g) + Cl 2(g) • The small K value means this will favor the reactant side so there wouldn’t be a large shift to the right.
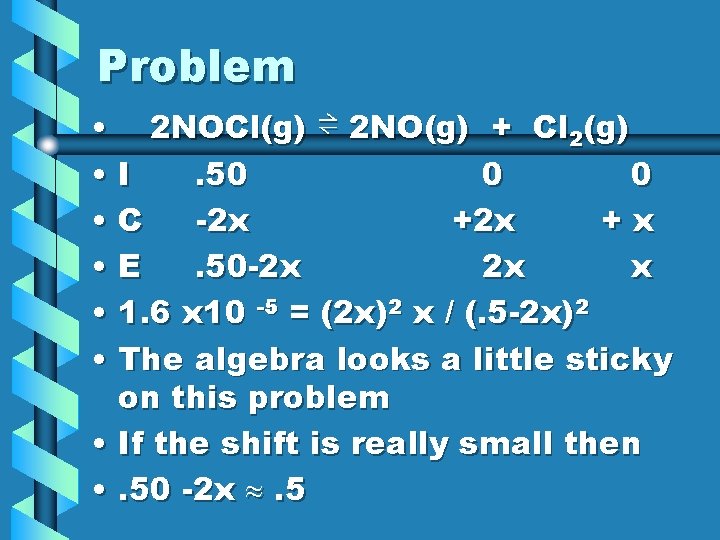
Problem • 2 NOCl(g) ⇌ 2 NO(g) + Cl 2(g) • I. 50 0 0 • C -2 x +x • E. 50 -2 x 2 x x • 1. 6 x 10 -5 = (2 x)2 x / (. 5 -2 x)2 • The algebra looks a little sticky on this problem • If the shift is really small then • . 50 -2 x . 5

Solve now • 1. 6 x 10 -5 = (2 x)2 x / (. 5)2 • x =. 01 • Of course this is ONLY ACCEPTABLE IF. 50 -2 x . 5 • . 50 – 2 (. 01) =. 48 • If the change is less than 5% it is considered small enough to ignore • . 48/. 5 = 96% or a 4% change.
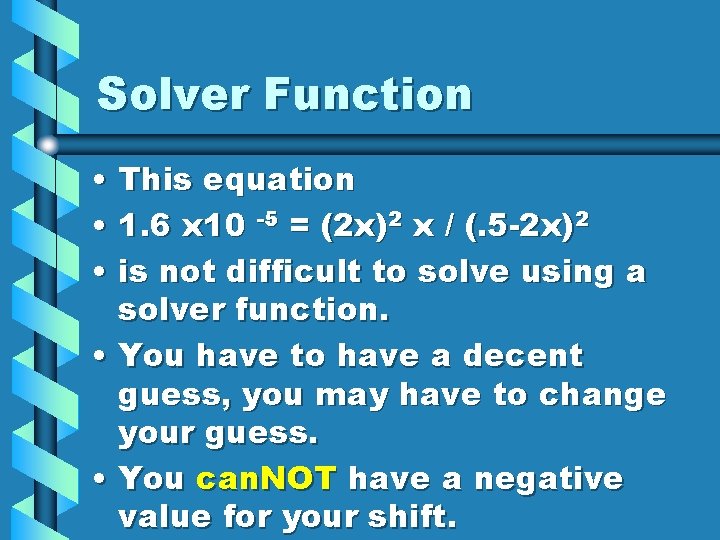
Solver Function • This equation • 1. 6 x 10 -5 = (2 x)2 x / (. 5 -2 x)2 • is not difficult to solve using a solver function. • You have to have a decent guess, you may have to change your guess. • You can. NOT have a negative value for your shift.

Simplified Assumptions • In a study of halogen bond strengths, 0. 50 mol I 2 was heated in a 2. 5 L vessel, and the following reaction occurred: • I 2(g) ⇌ 2 I(g). • Calculate [I 2]eq and [I]eq at 600 K; Kc = 2. 94 x 10 -10. • Calculate [I 2]eq and [I]eq at 2000 K; Kc = 0. 209.
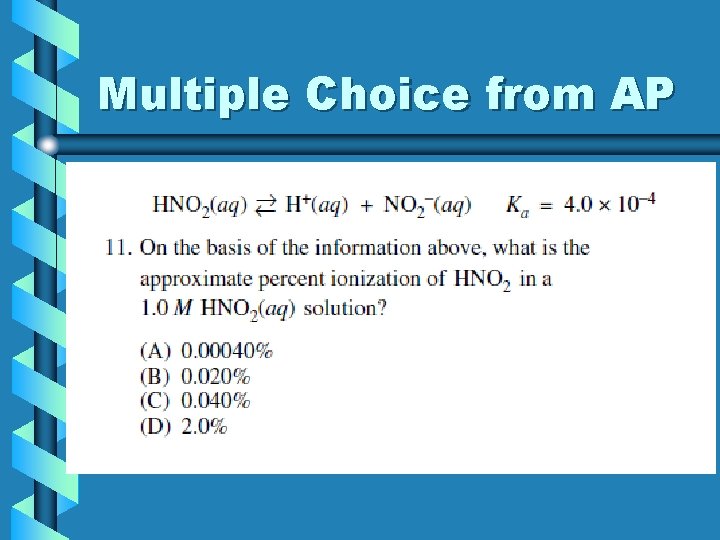
Multiple Choice from AP
- Slides: 16