ICE Tables and Equilibrium Concentrations Lesson Outline ICE

ICE Tables and Equilibrium Concentrations

Lesson Outline ICE Tables Calculating Equilibrium Concentrations using ICE Tables
![ICE Tables ICE tables are used to find [ ]’s at equilibrium Remember this ICE Tables ICE tables are used to find [ ]’s at equilibrium Remember this](http://slidetodoc.com/presentation_image_h2/356f8d8f4b9abac5bae0bd8afc6b5ab9/image-3.jpg)
ICE Tables ICE tables are used to find [ ]’s at equilibrium Remember this must be in a closed system I - Initial concentrations when reaction starts C- Change in concentrations as system proceeds to Eq E – Equilibrium Concentrations of the reaction Note Initial [products] = 0 (unless stated otherwise) Concentration is measured in mol/L
![ICE Tables Consider the following reaction [Initial] [Change] [Equilibrium] 5. 30 -1. 32 3. ICE Tables Consider the following reaction [Initial] [Change] [Equilibrium] 5. 30 -1. 32 3.](http://slidetodoc.com/presentation_image_h2/356f8d8f4b9abac5bae0bd8afc6b5ab9/image-4.jpg)
ICE Tables Consider the following reaction [Initial] [Change] [Equilibrium] 5. 30 -1. 32 3. 97 3. 53 -1. 32 2. 21 19. 04 +2. 64 21. 68 1. From what is given, calculate [change] for a species 2. Using stoichiometry, calculate [change] of the other species. 3. Calculate [equilibrium] of all remaining species [Change] can be + or – Whiteboard activity If reactants change is + then products change is – [Equilibrium] are always +
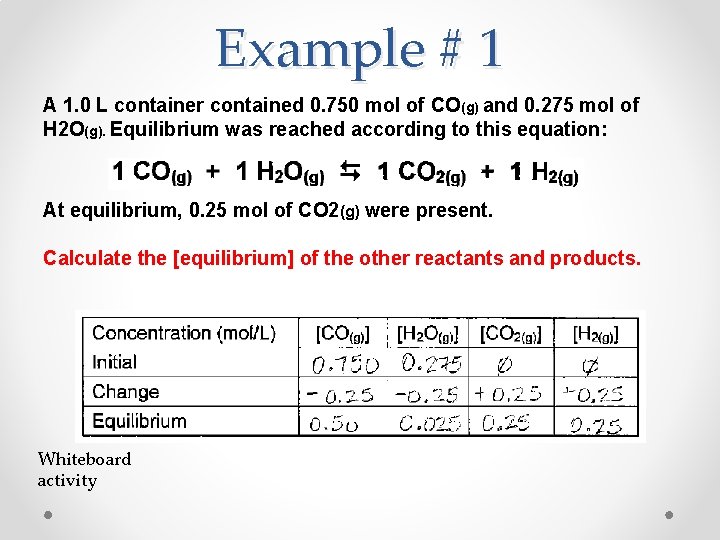
Example # 1 A 1. 0 L container contained 0. 750 mol of CO(g) and 0. 275 mol of H 2 O(g). Equilibrium was reached according to this equation: At equilibrium, 0. 25 mol of CO 2(g) were present. Calculate the [equilibrium] of the other reactants and products. Whiteboard activity
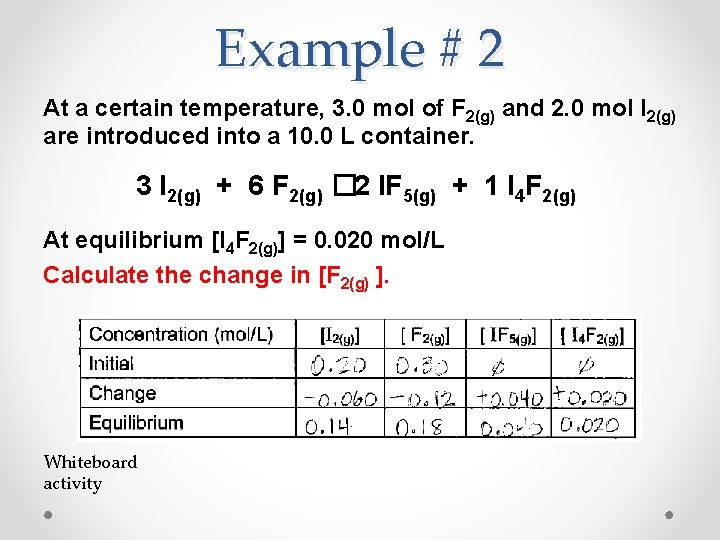
Example # 2 At a certain temperature, 3. 0 mol of F 2(g) and 2. 0 mol I 2(g) are introduced into a 10. 0 L container. 3 I 2(g) + 6 F 2(g) � 2 IF 5(g) + 1 I 4 F 2(g) At equilibrium [I 4 F 2(g)] = 0. 020 mol/L Calculate the change in [F 2(g) ]. Whiteboard activity
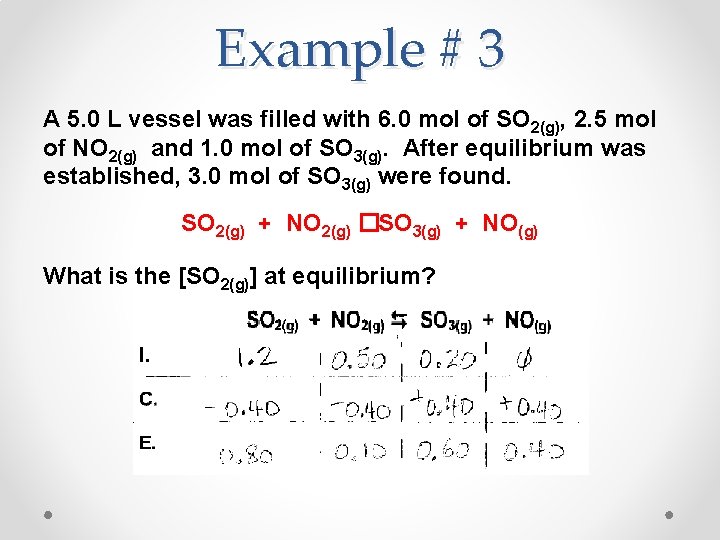
Example # 3 A 5. 0 L vessel was filled with 6. 0 mol of SO 2(g), 2. 5 mol of NO 2(g) and 1. 0 mol of SO 3(g). After equilibrium was established, 3. 0 mol of SO 3(g) were found. SO 2(g) + NO 2(g) �SO 3(g) + NO(g) What is the [SO 2(g)] at equilibrium?

Looking Forward Today E 4 questions Tomorrow The Equilibrium Constant Monday Quiz (E 4 -5)
- Slides: 8