IC T Surface Reactivity What determines the surface


- Slides: 53

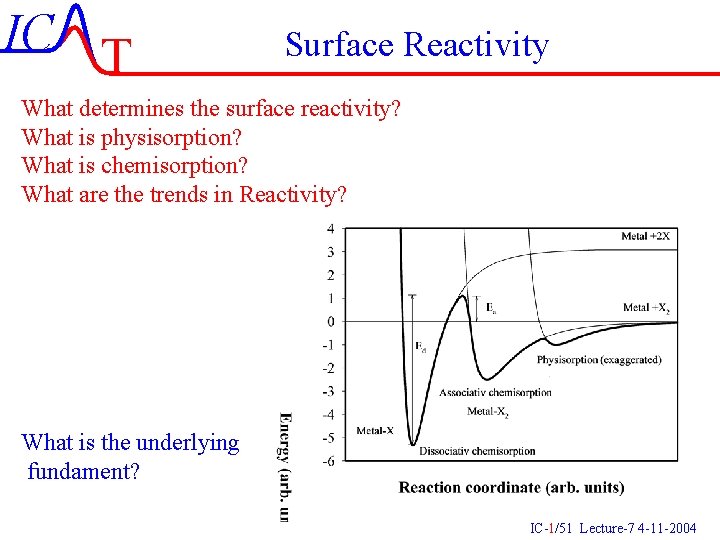
IC T Surface Reactivity What determines the surface reactivity? What is physisorption? What is chemisorption? What are the trends in Reactivity? What is the underlying fundament? IC-1/51 Lecture-7 4 -11 -2004

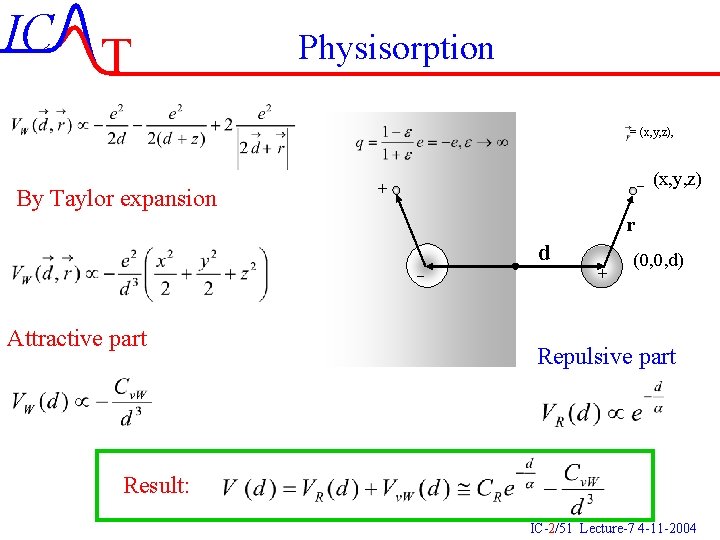
IC T Physisorption = (x, y, z), By Taylor expansion - (x, y, z) + r - Attractive part d + (0, 0, d) Repulsive part Result: IC-2/51 Lecture-7 4 -11 -2004

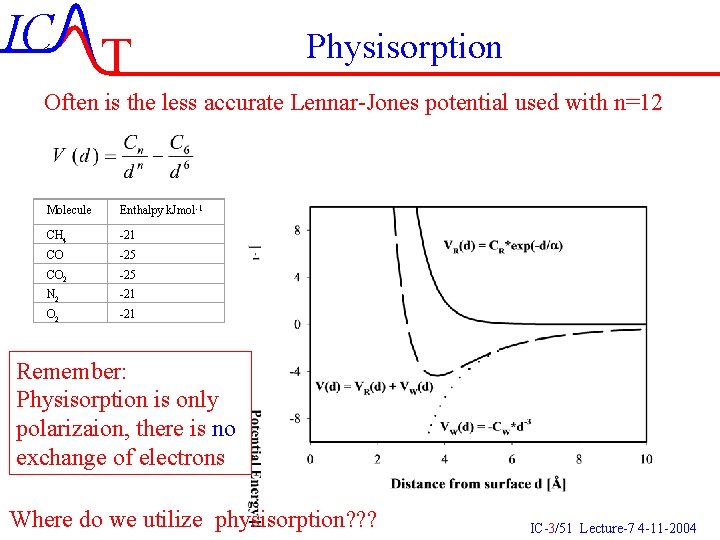
IC T Physisorption Often is the less accurate Lennar-Jones potential used with n=12 Molecule Enthalpy k. Jmol-1 CH 4 -21 CO -25 CO 2 -25 N 2 -21 O 2 -21 Remember: Physisorption is only polarizaion, there is no exchange of electrons Where do we utilize physisorption? ? ? IC-3/51 Lecture-7 4 -11 -2004

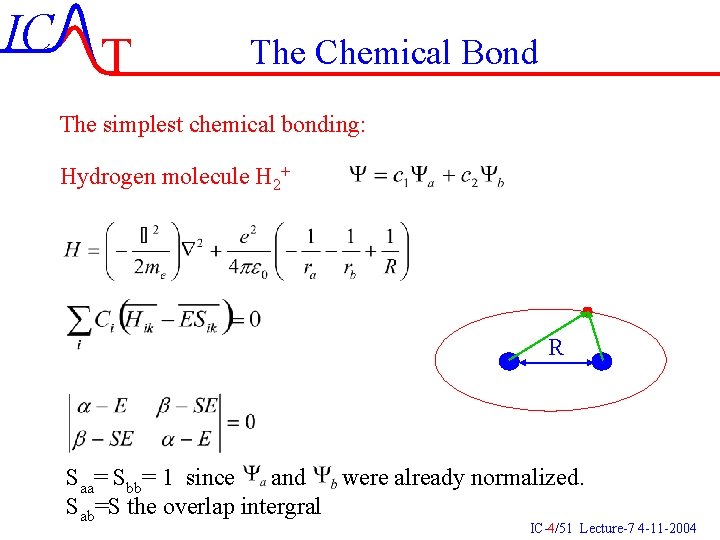
IC T The Chemical Bond The simplest chemical bonding: Hydrogen molecule H 2+ R Saa= Sbb= 1 since and were already normalized. Sab=S the overlap intergral IC-4/51 Lecture-7 4 -11 -2004

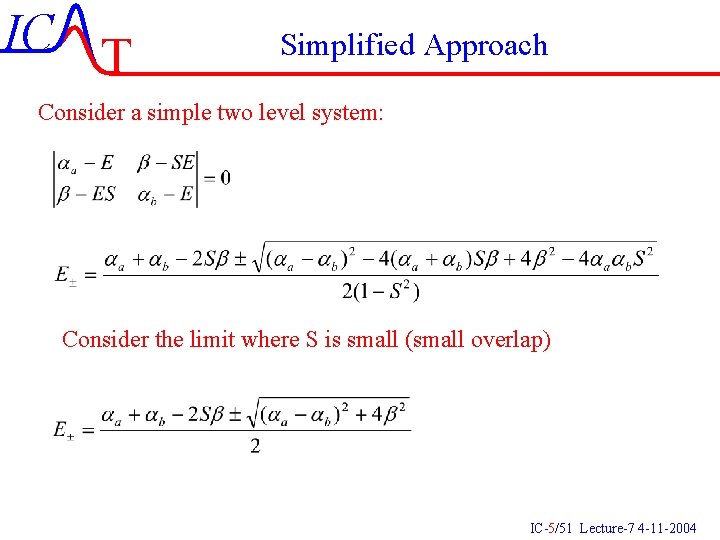
IC T Simplified Approach Consider a simple two level system: Consider the limit where S is small (small overlap) IC-5/51 Lecture-7 4 -11 -2004

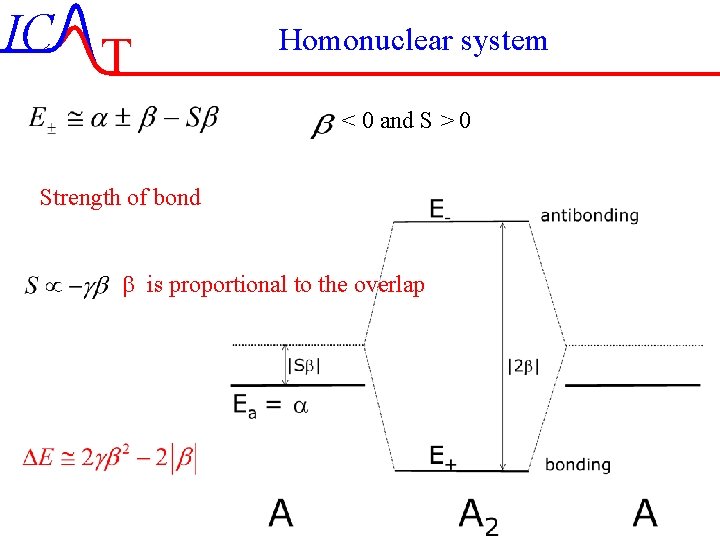
IC T Homonuclear system < 0 and S > 0 Strength of bond b is proportional to the overlap IC-6/51 Lecture-7 4 -11 -2004

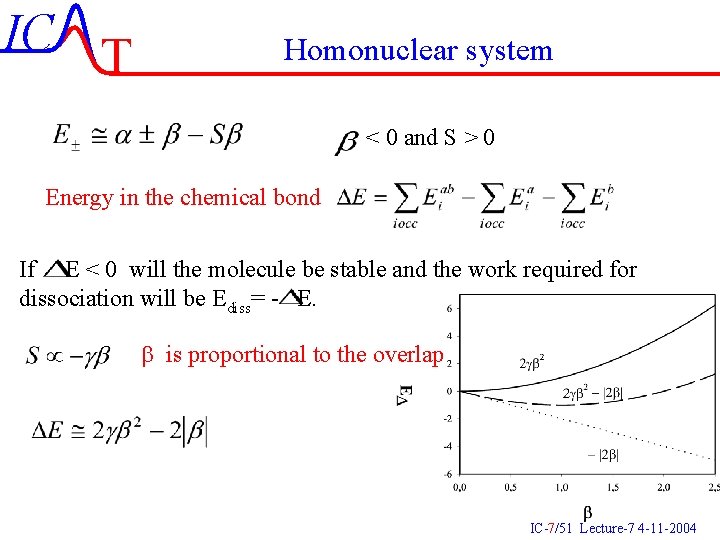
IC T Homonuclear system < 0 and S > 0 Energy in the chemical bond If E < 0 will the molecule be stable and the work required for dissociation will be Ediss= - E. b is proportional to the overlap IC-7/51 Lecture-7 4 -11 -2004

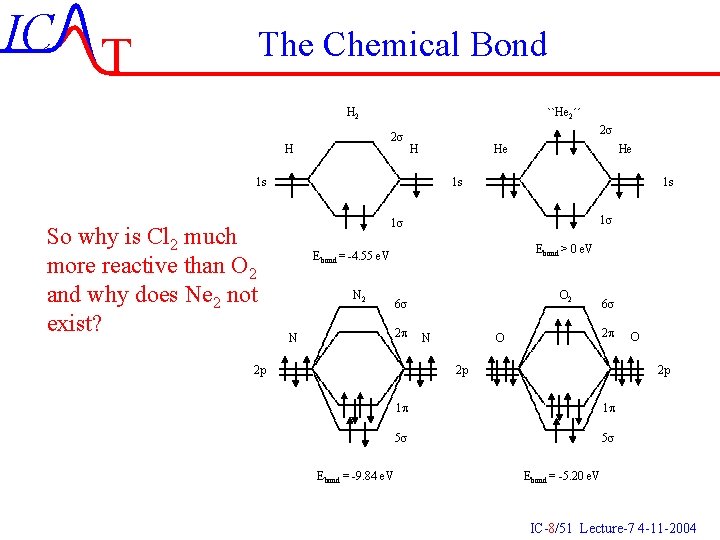
IC T The Chemical Bond H 2 ``He 2´´ 2 s He H 1 s So why is Cl 2 much more reactive than O 2 and why does Ne 2 not exist? He 1 s 1 s Ebond > 0 e. V Ebond = -4. 55 e. V N 2 2 p N O 2 6 s 2 p N 6 s 2 p O 2 p Ebond = -9. 84 e. V O 2 p 1 p 1 p 5 s 5 s Ebond = -5. 20 e. V IC-8/51 Lecture-7 4 -11 -2004

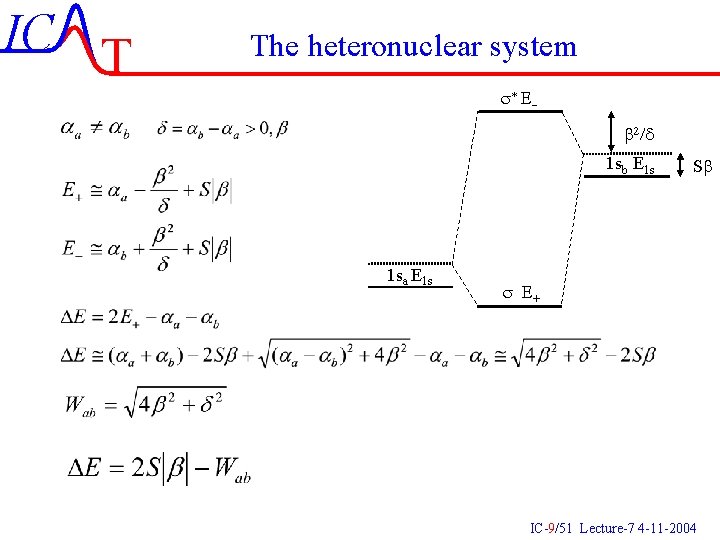
IC T The heteronuclear system s* E b 2/d 1 sb E 1 s 1 sa E 1 s Sb s E+ IC-9/51 Lecture-7 4 -11 -2004

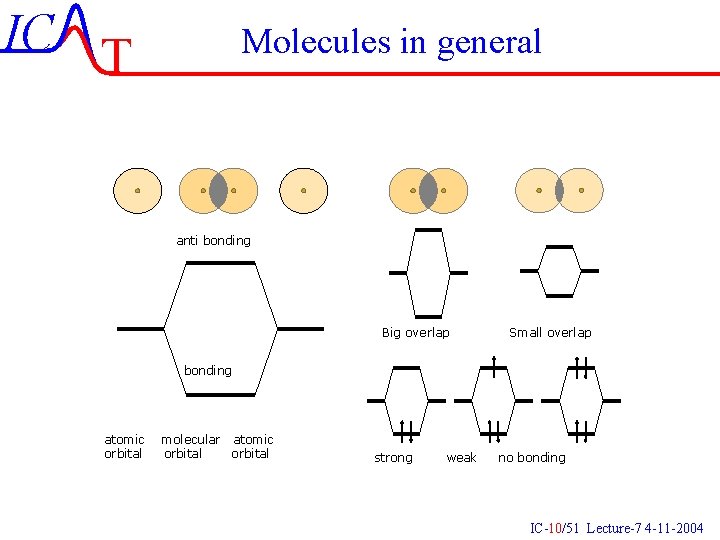
IC T Molecules in general anti bonding Big overlap Small overlap bonding atomic orbital molecular atomic orbital strong weak no bonding IC-10/51 Lecture-7 4 -11 -2004

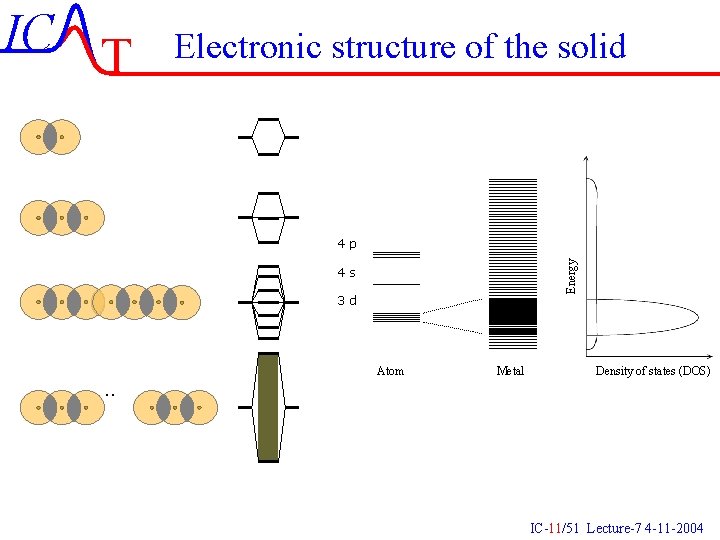
IC T Electronic structure of the solid Energy 4 p 4 s 3 d . . Atom Metal Density of states (DOS) IC-11/51 Lecture-7 4 -11 -2004

IC T Trends in the periodic system To the left the outer atomic orbital tends to be extended and will be more localized as we go to the right where they become filled. Thus the tendency forming bond will decrease when going to the left. From metallic to covalent to none. When going down the periodic system the outer orbital increases and becomes more extended and delocalized i. e. forms better bonding. Eventually even Xe can form covalent bonding and the highest melting point of metals have to be found in the third transition series where there is an additional bonding effect. gas Filling =localized=less strong bonding Increasing size= delocalization= increasing bonding metals IC-12/51 Lecture-7 4 -11 -2004

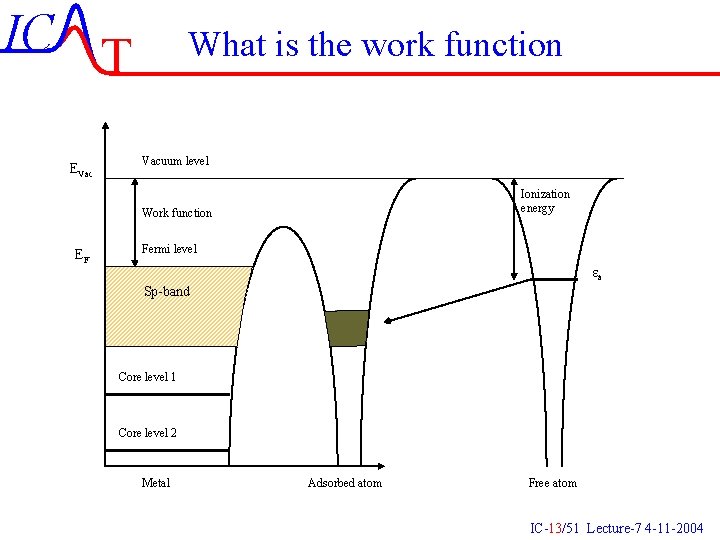
IC T EVac What is the work function Vacuum level Ionization energy Work function EF Fermi level ea Sp-band Core level 1 Core level 2 Metal Adsorbed atom Free atom IC-13/51 Lecture-7 4 -11 -2004

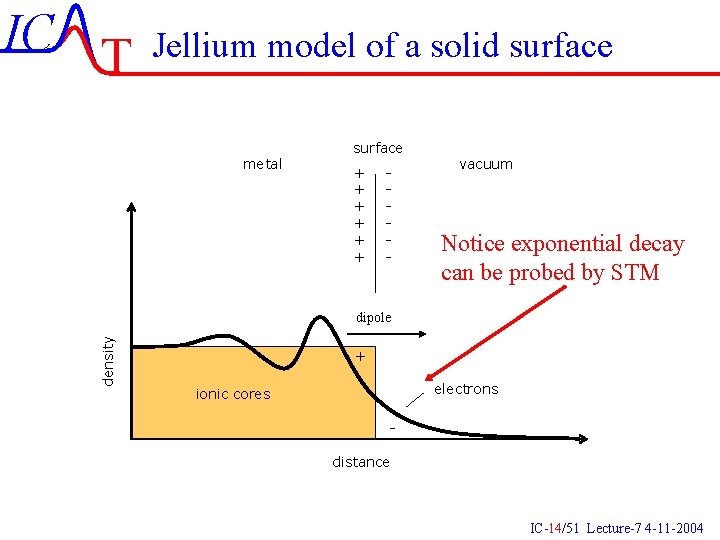
IC T Jellium model of a solid surface metal surface + + + - vacuum Notice exponential decay can be probed by STM density dipole + electrons ionic cores distance IC-14/51 Lecture-7 4 -11 -2004

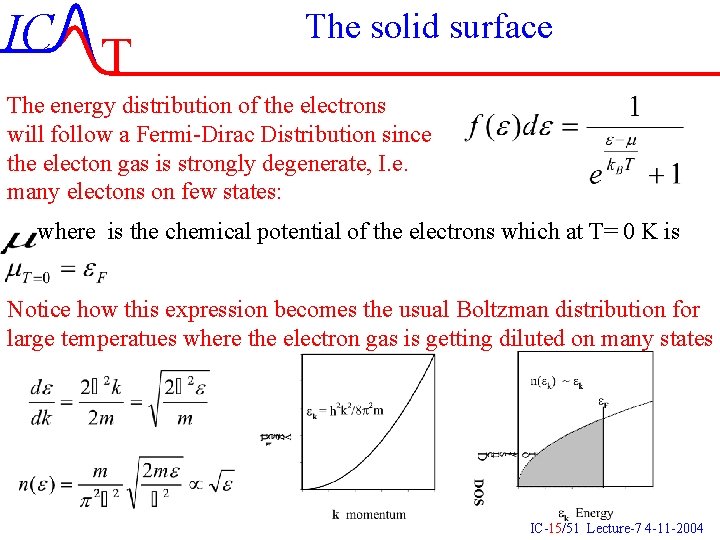
IC T The solid surface The energy distribution of the electrons will follow a Fermi-Dirac Distribution since the electon gas is strongly degenerate, I. e. many electons on few states: where is the chemical potential of the electrons which at T= 0 K is Notice how this expression becomes the usual Boltzman distribution for large temperatues where the electron gas is getting diluted on many states IC-15/51 Lecture-7 4 -11 -2004

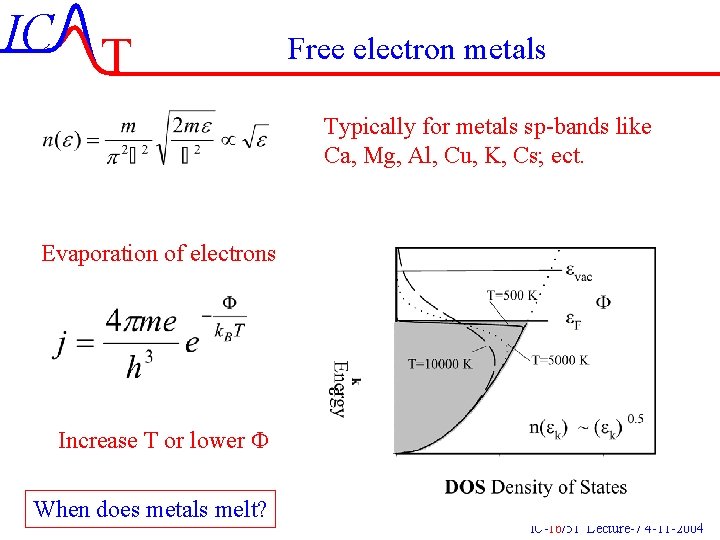
IC T Free electron metals Typically for metals sp-bands like Ca, Mg, Al, Cu, K, Cs; ect. Evaporation of electrons Increase T or lower F When does metals melt? IC-16/51 Lecture-7 4 -11 -2004

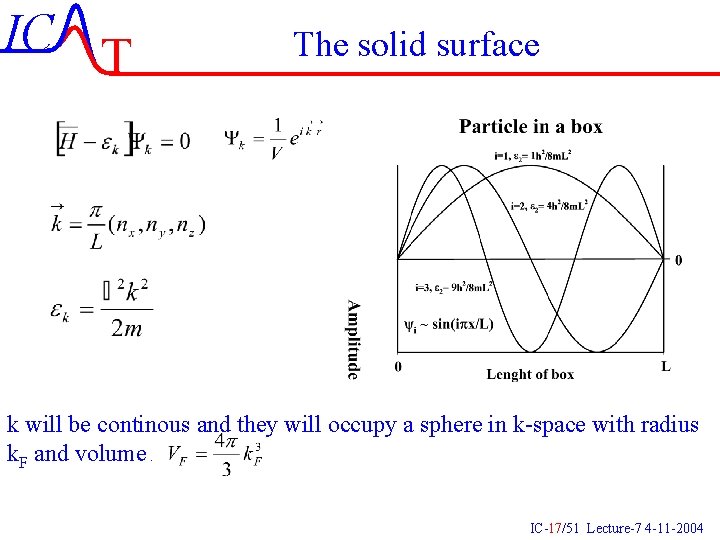
IC T The solid surface k will be continous and they will occupy a sphere in k-space with radius k. F and volume. IC-17/51 Lecture-7 4 -11 -2004

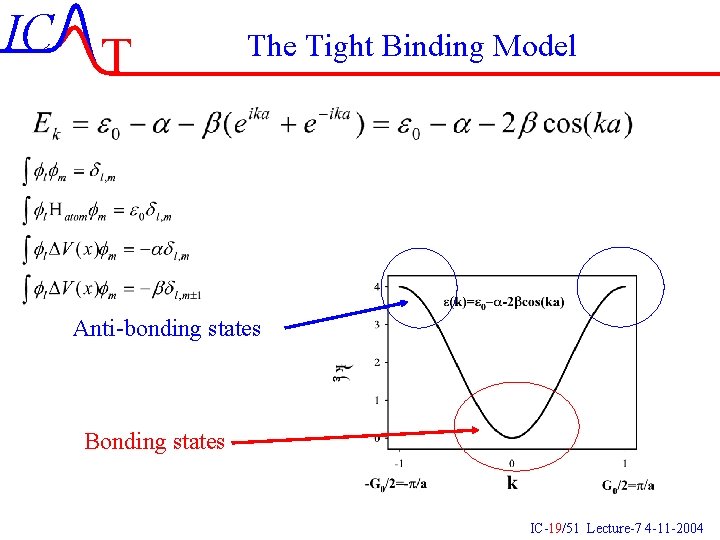
IC T The Tight Binding Model LCAO Where F are the individual atomic wave functions a e 0 Construct Block waves Core levels IC-18/51 Lecture-7 4 -11 -2004

IC T The Tight Binding Model Anti-bonding states Bonding states IC-19/51 Lecture-7 4 -11 -2004

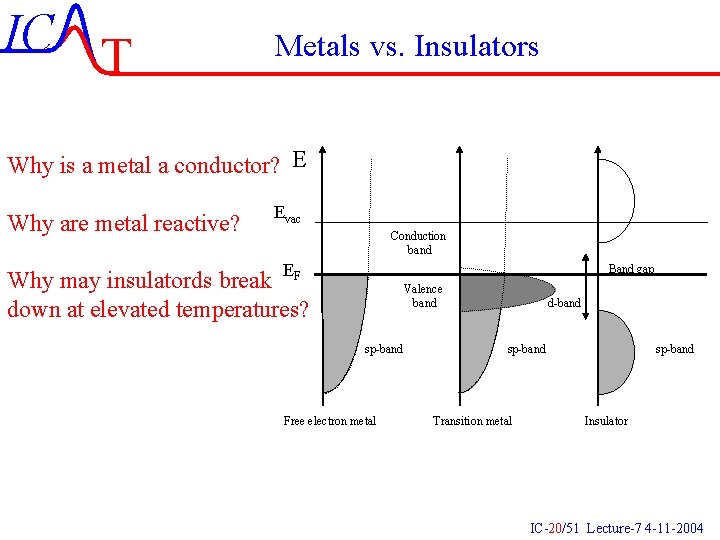
IC T Metals vs. Insulators Why is a metal a conductor? E Why are metal reactive? Evac Conduction band EF Band gap Why may insulatords break down at elevated temperatures? Valence band sp-band Free electron metal d-band sp-band Transition metal sp-band Insulator IC-20/51 Lecture-7 4 -11 -2004

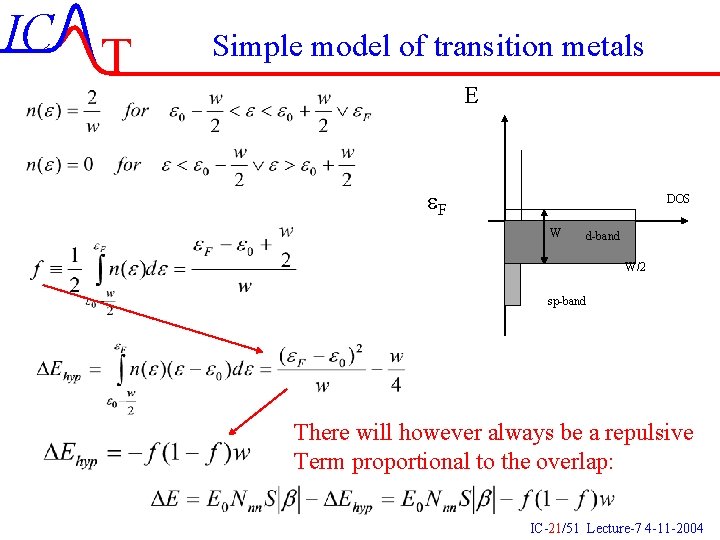
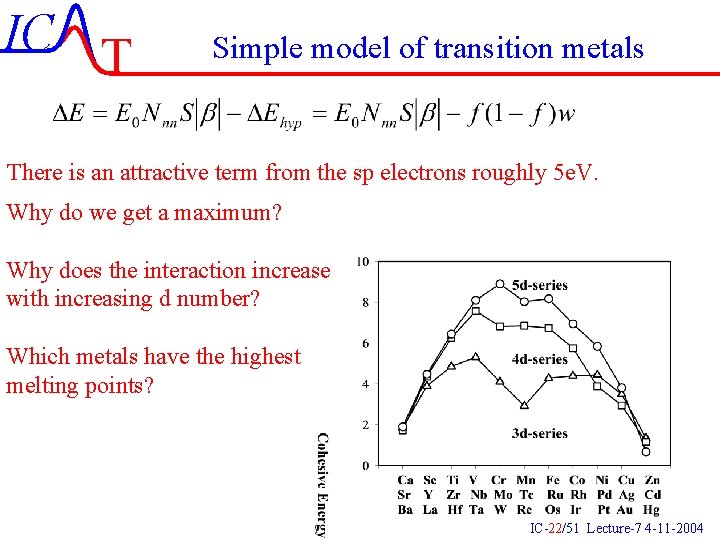
IC T Simple model of transition metals E e. F DOS W d-band W/2 sp-band There will however always be a repulsive Term proportional to the overlap: IC-21/51 Lecture-7 4 -11 -2004

IC T Simple model of transition metals There is an attractive term from the sp electrons roughly 5 e. V. Why do we get a maximum? Why does the interaction increase with increasing d number? Which metals have the highest melting points? IC-22/51 Lecture-7 4 -11 -2004

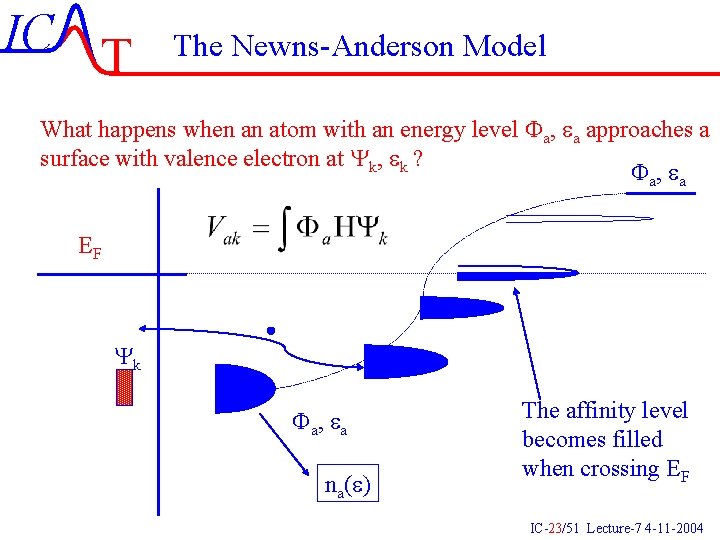
IC T The Newns-Anderson Model What happens when an atom with an energy level Fa, ea approaches a surface with valence electron at Yk, ek ? Fa , e a EF Yk Fa , e a na(e) The affinity level becomes filled when crossing EF IC-23/51 Lecture-7 4 -11 -2004

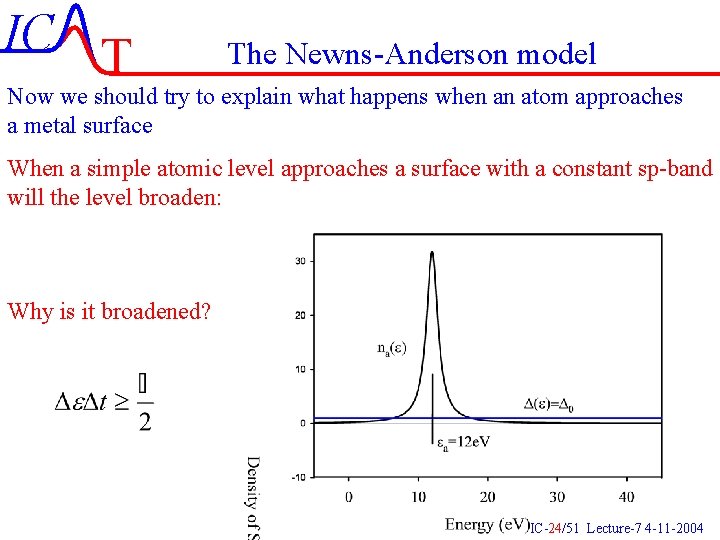
IC T The Newns-Anderson model Now we should try to explain what happens when an atom approaches a metal surface When a simple atomic level approaches a surface with a constant sp-band will the level broaden: Why is it broadened? IC-24/51 Lecture-7 4 -11 -2004

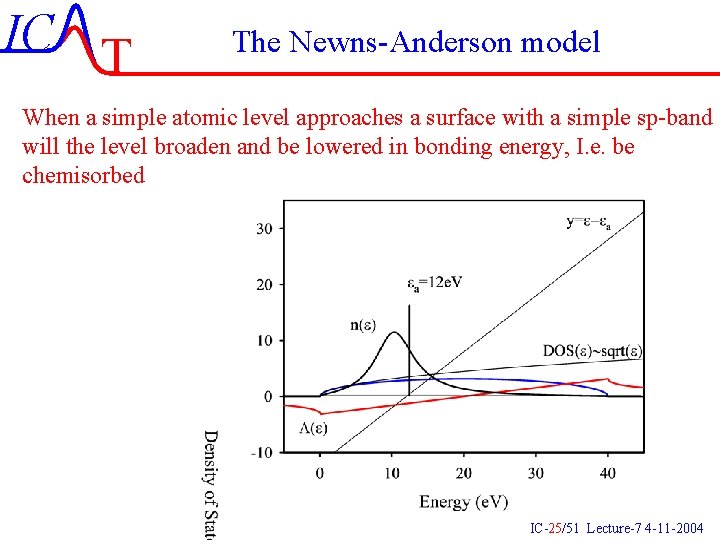
IC T The Newns-Anderson model When a simple atomic level approaches a surface with a simple sp-band will the level broaden and be lowered in bonding energy, I. e. be chemisorbed IC-25/51 Lecture-7 4 -11 -2004

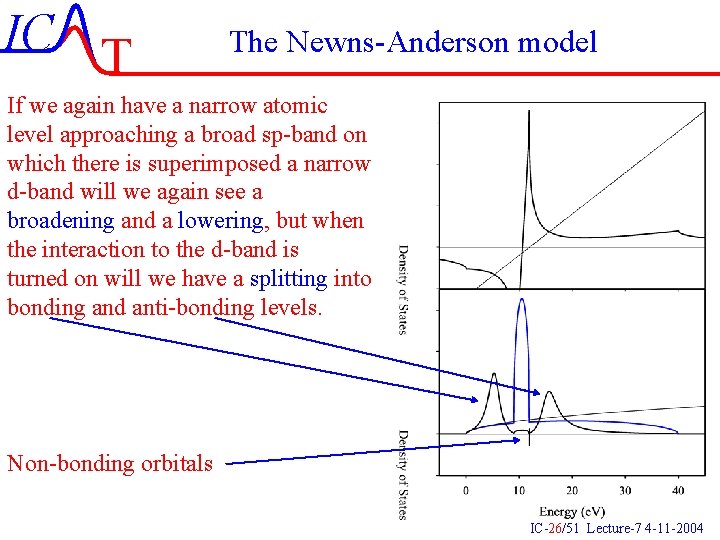
IC T The Newns-Anderson model If we again have a narrow atomic level approaching a broad sp-band on which there is superimposed a narrow d-band will we again see a broadening and a lowering, but when the interaction to the d-band is turned on will we have a splitting into bonding and anti-bonding levels. Non-bonding orbitals IC-26/51 Lecture-7 4 -11 -2004

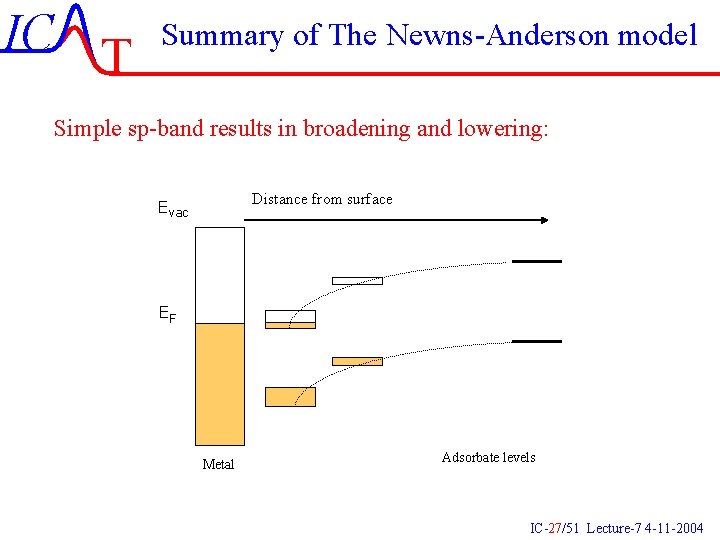
IC T Summary of The Newns-Anderson model Simple sp-band results in broadening and lowering: Distance from surface Evac EF Metal Adsorbate levels IC-27/51 Lecture-7 4 -11 -2004

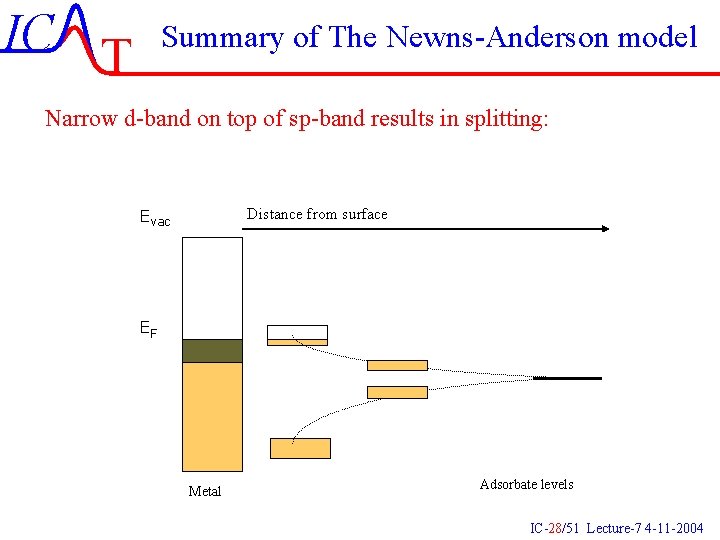
IC T Summary of The Newns-Anderson model Narrow d-band on top of sp-band results in splitting: Distance from surface Evac EF Metal Adsorbate levels IC-28/51 Lecture-7 4 -11 -2004

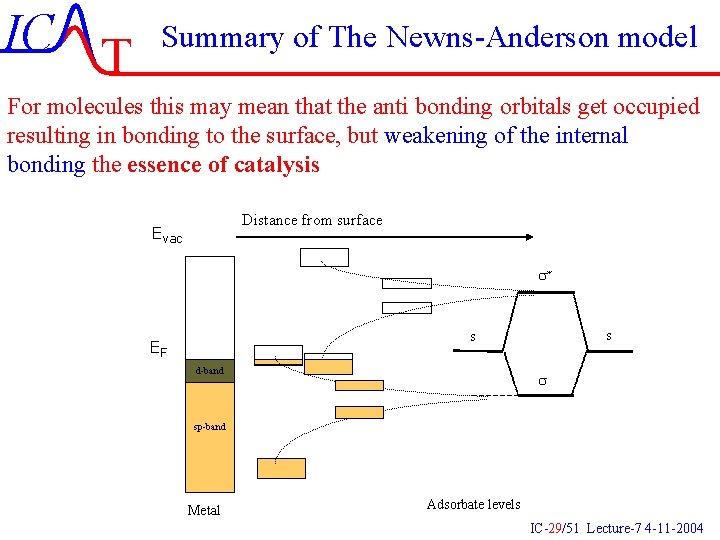
IC T Summary of The Newns-Anderson model For molecules this may mean that the anti bonding orbitals get occupied resulting in bonding to the surface, but weakening of the internal bonding the essence of catalysis Distance from surface Evac s* s s EF d-band s sp-band Metal Adsorbate levels IC-29/51 Lecture-7 4 -11 -2004

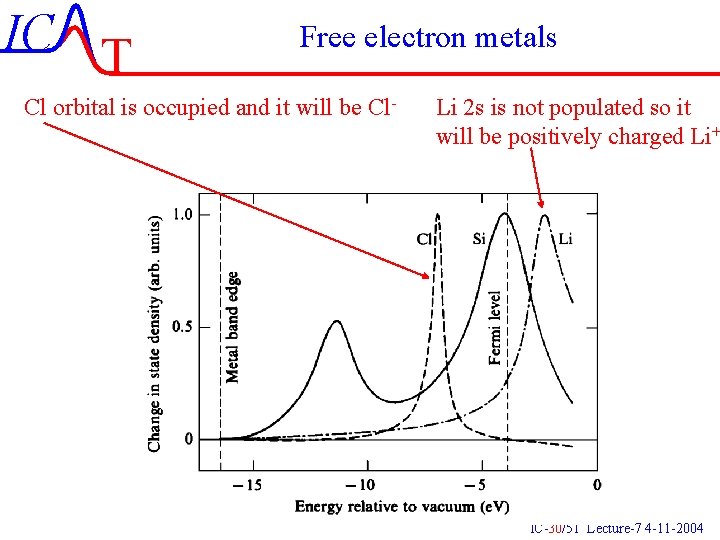
IC T Free electron metals Cl orbital is occupied and it will be Cl- Li 2 s is not populated so it will be positively charged Li+ IC-30/51 Lecture-7 4 -11 -2004

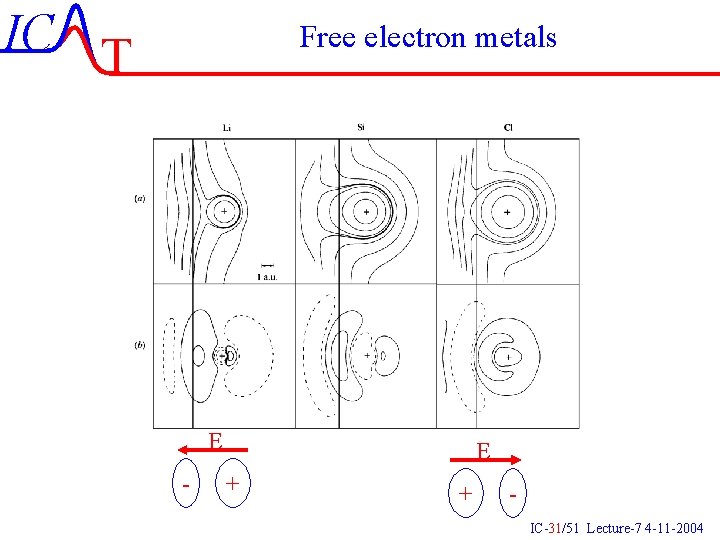
IC T Free electron metals E - E + + IC-31/51 Lecture-7 4 -11 -2004

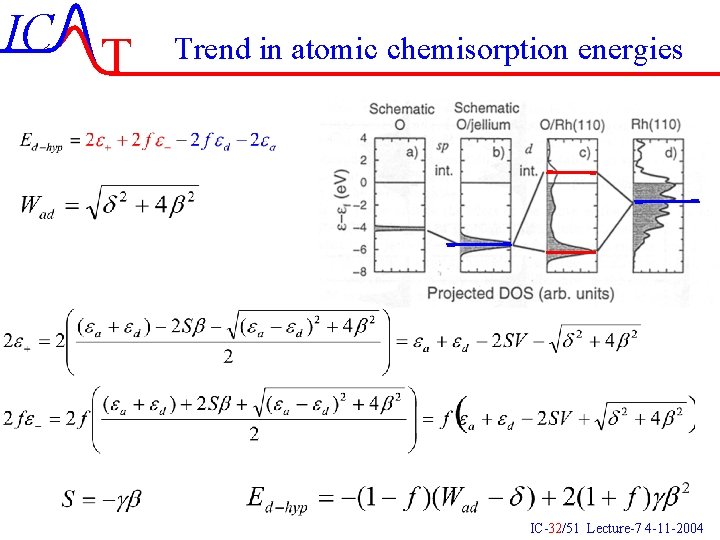
IC T Trend in atomic chemisorption energies IC-32/51 Lecture-7 4 -11 -2004

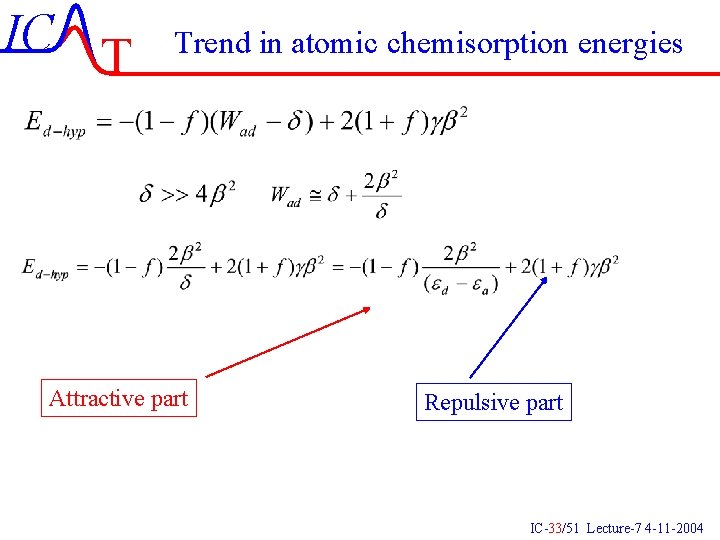
IC T Trend in atomic chemisorption energies Attractive part Repulsive part IC-33/51 Lecture-7 4 -11 -2004

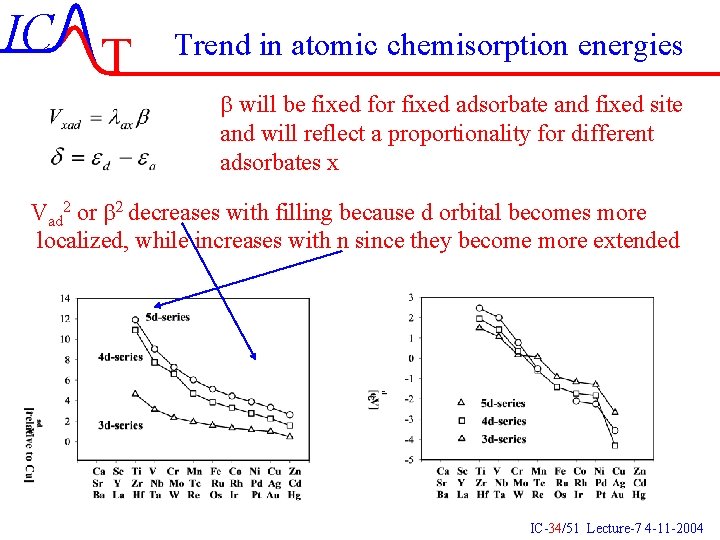
IC T Trend in atomic chemisorption energies b will be fixed for fixed adsorbate and fixed site and will reflect a proportionality for different adsorbates x Vad 2 or b 2 decreases with filling because d orbital becomes more localized, while increases with n since they become more extended IC-34/51 Lecture-7 4 -11 -2004

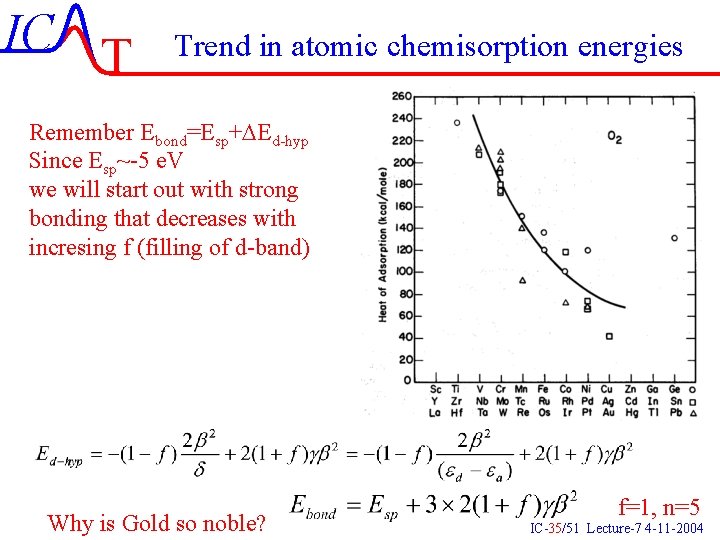
IC T Trend in atomic chemisorption energies Remember Ebond=Esp+DEd-hyp Since Esp~-5 e. V we will start out with strong bonding that decreases with incresing f (filling of d-band) Why is Gold so noble? f=1, n=5 IC-35/51 Lecture-7 4 -11 -2004

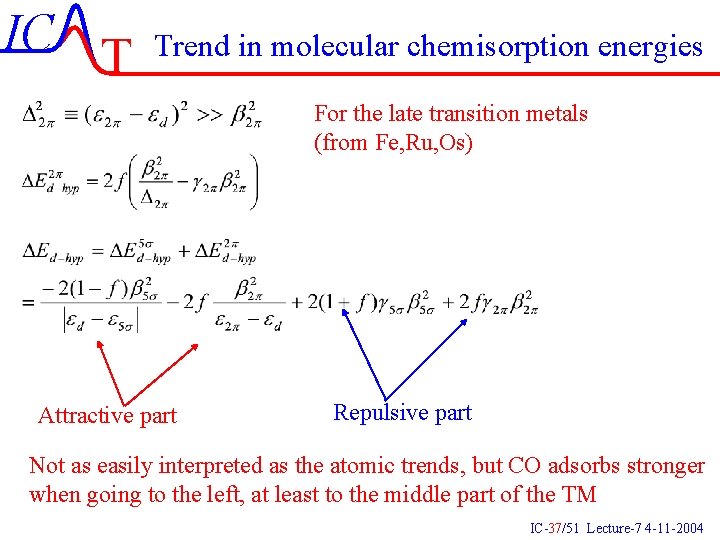
IC T Trend in molecular chemisorption energies IC-36/51 Lecture-7 4 -11 -2004

IC T Trend in molecular chemisorption energies For the late transition metals (from Fe, Ru, Os) Attractive part Repulsive part Not as easily interpreted as the atomic trends, but CO adsorbs stronger when going to the left, at least to the middle part of the TM IC-37/51 Lecture-7 4 -11 -2004

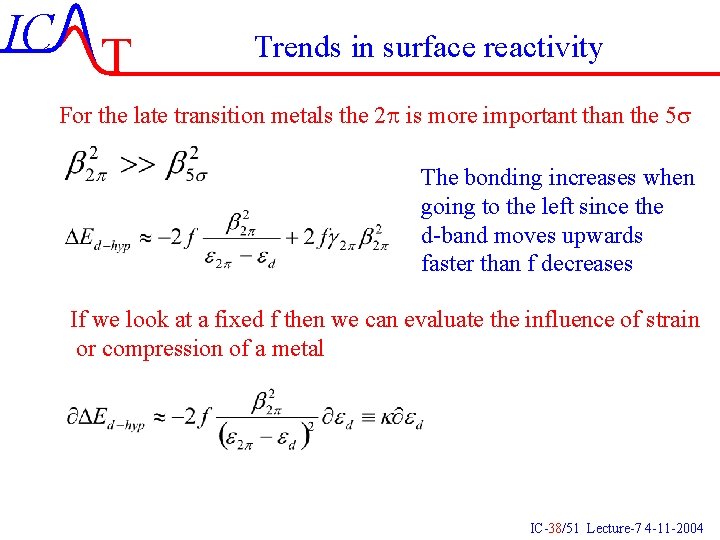
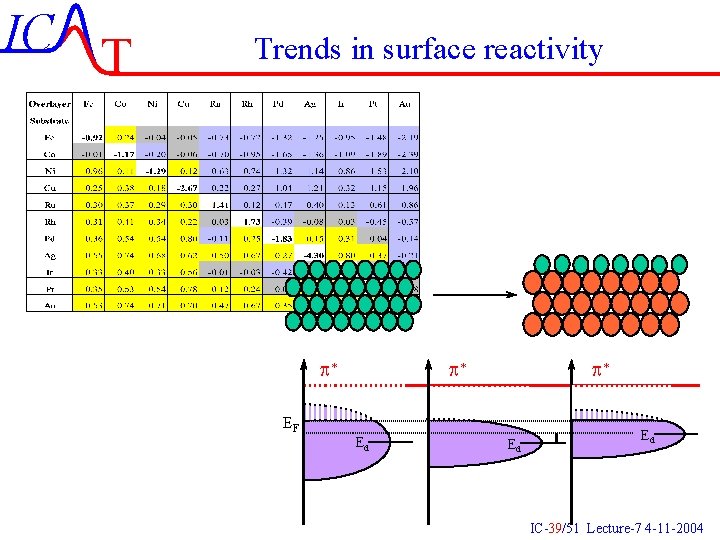
IC T Trends in surface reactivity For the late transition metals the 2 p is more important than the 5 s The bonding increases when going to the left since the d-band moves upwards faster than f decreases If we look at a fixed f then we can evaluate the influence of strain or compression of a metal IC-38/51 Lecture-7 4 -11 -2004

IC T Trends in surface reactivity p* p* p* EF Ed Ed Ed IC-39/51 Lecture-7 4 -11 -2004

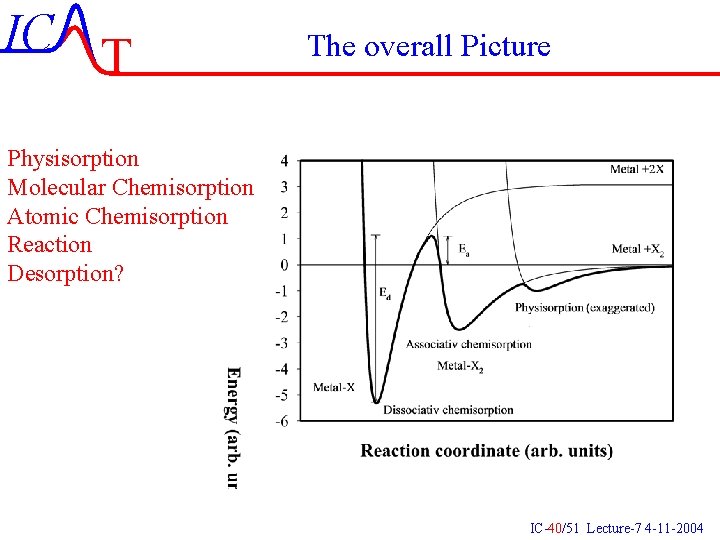
IC T The overall Picture Physisorption Molecular Chemisorption Atomic Chemisorption Reaction Desorption? IC-40/51 Lecture-7 4 -11 -2004

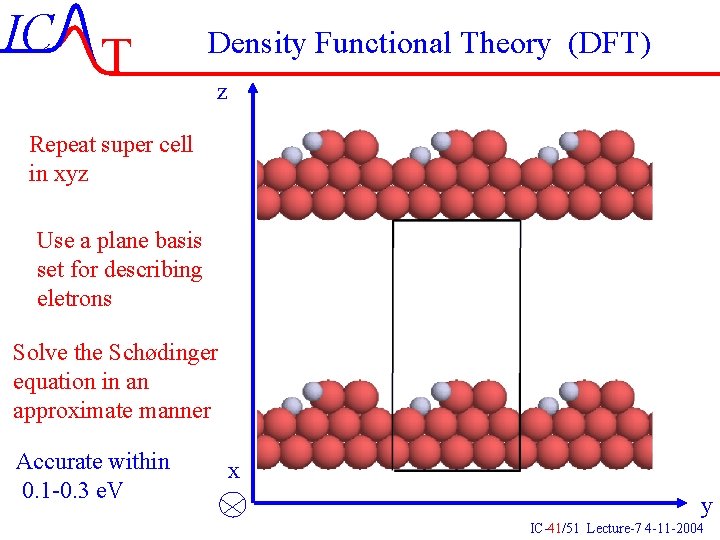
IC T Density Functional Theory (DFT) z Repeat super cell in xyz Use a plane basis set for describing eletrons Solve the Schødinger equation in an approximate manner Accurate within 0. 1 -0. 3 e. V x y IC-41/51 Lecture-7 4 -11 -2004

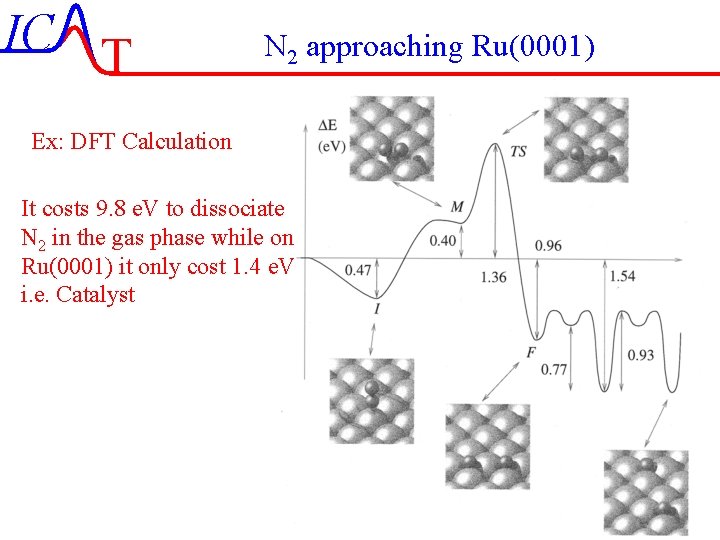
IC T N 2 approaching Ru(0001) Ex: DFT Calculation It costs 9. 8 e. V to dissociate N 2 in the gas phase while on Ru(0001) it only cost 1. 4 e. V i. e. Catalyst IC-42/51 Lecture-7 4 -11 -2004

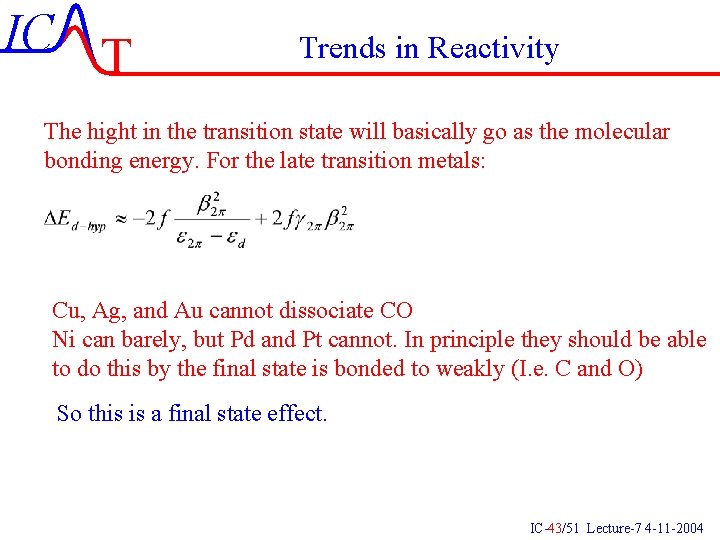
IC T Trends in Reactivity The hight in the transition state will basically go as the molecular bonding energy. For the late transition metals: Cu, Ag, and Au cannot dissociate CO Ni can barely, but Pd and Pt cannot. In principle they should be able to do this by the final state is bonded to weakly (I. e. C and O) So this is a final state effect. IC-43/51 Lecture-7 4 -11 -2004

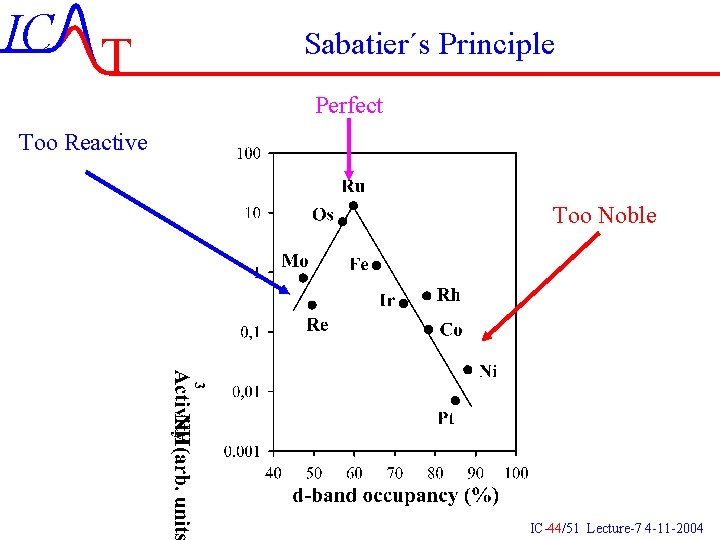
IC T Sabatier´s Principle Perfect Too Reactive Too Noble IC-44/51 Lecture-7 4 -11 -2004

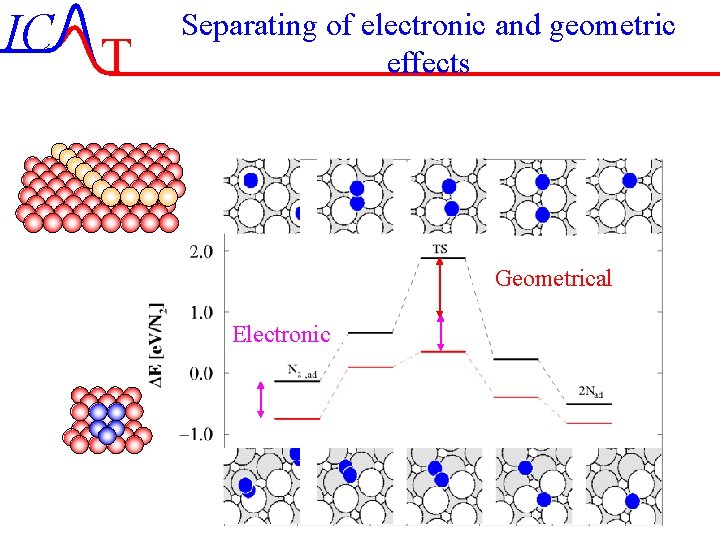
IC T Separating of electronic and geometric effects Geometrical Electronic IC-45/51 Lecture-7 4 -11 -2004

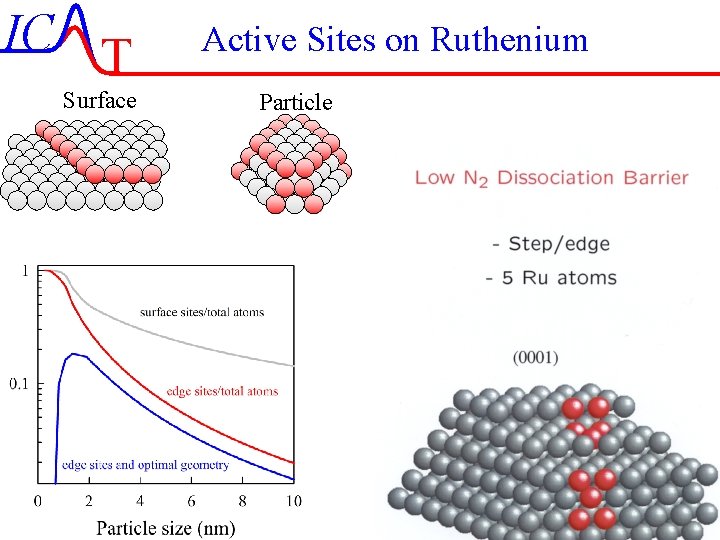
IC T Surface Active Sites on Ruthenium Particle IC-46/51 Lecture-7 4 -11 -2004

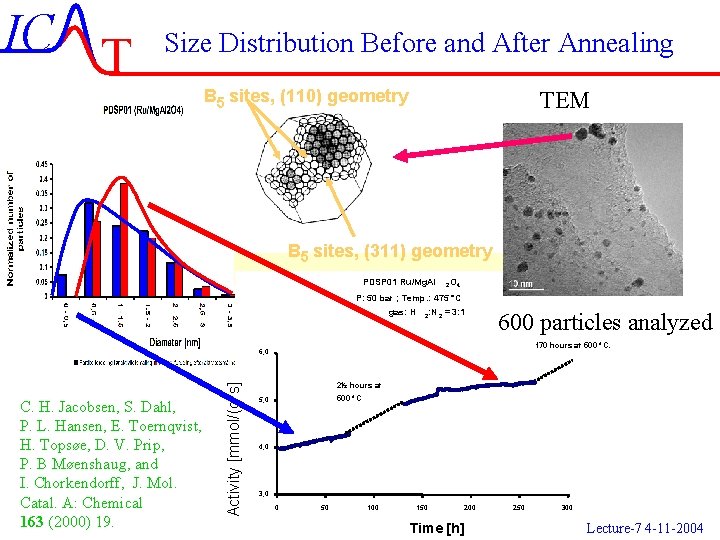
IC T Size Distribution Before and After Annealing B 5 sites, (110) geometry TEM B 5 sites, (311) geometry PDSP 01 Ru/Mg. Al 2 O 4 P: 50 bar ; Temp. : 475 °C gas: H 2 : N 2 = 3: 1 600 particles analyzed 170 hours at 500 °C. C. H. Jacobsen, S. Dahl, P. L. Hansen, E. Toernqvist, H. Topsøe, D. V. Prip, P. B Møenshaug, and I. Chorkendorff, J. Mol. Catal. A: Chemical 163 (2000) 19. Activity [mmol/(g*s] 6, 0 2½ hours at 500 °C 5, 0 4, 0 3, 0 0 50 100 150 200 Time [h] 250 300 IC-47/51 Lecture-7 4 -11 -2004

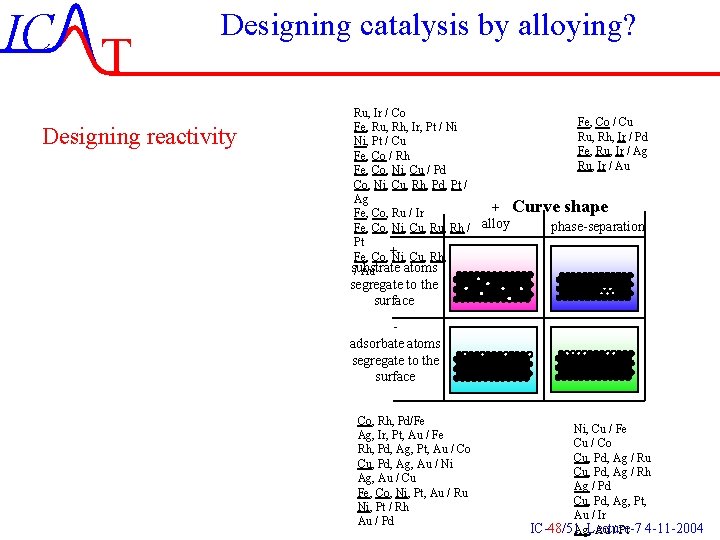
IC T Designing catalysis by alloying? Designing reactivity Ru, Ir / Co Fe, Co / Cu Fe, Ru, Rh, Ir, Pt / Ni Ru, Rh, Ir / Pd Ni, Pt / Cu Fe, Ru, Ir / Ag Fe, Co / Rh Ru, Ir / Au Fe, Co, Ni, Cu / Pd Co, Ni, Cu, Rh, Pd, Pt / Ag + Curve shape Fe, Co, Ru / Ir phase-separation Fe, Co, Ni, Cu, Rh / alloy Pt Fe, Co, +Ni, Cu, Rh, Pd, Pt substrate atoms / Au segregate to the surface adsorbate atoms segregate to the surface Co, Rh, Pd/Fe Ag, Ir, Pt, Au / Fe Rh, Pd, Ag, Pt, Au / Co Cu, Pd, Ag, Au / Ni Ag, Au / Cu Fe, Co, Ni, Pt, Au / Ru Ni, Pt / Rh Au / Pd Ni, Cu / Fe Cu / Co Cu, Pd, Ag / Ru Cu, Pd, Ag / Rh Ag / Pd Cu, Pd, Ag, Pt, Au / Ir IC-48/51 Ag, Lecture-7 Au / Pt 4 -11 -2004

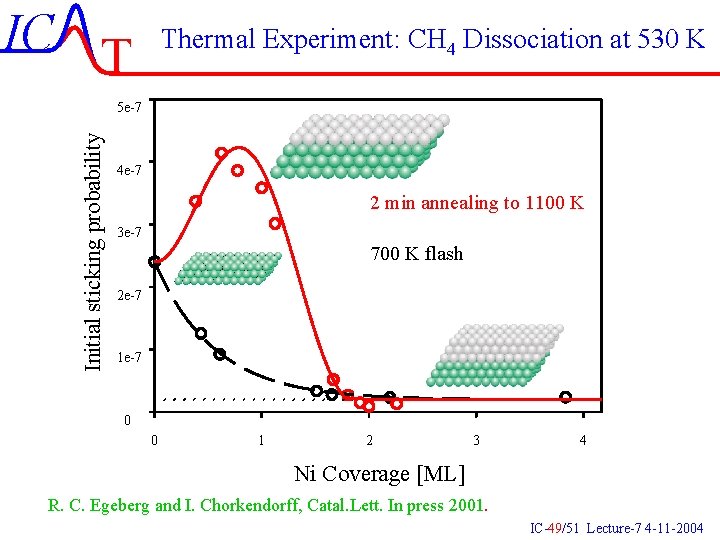
IC T Thermal Experiment: CH 4 Dissociation at 530 K Initial sticking probability 5 e-7 4 e-7 2 min annealing to 1100 K 3 e-7 700 K flash 2 e-7 1 e-7 0 0 1 2 3 4 Ni Coverage [ML] R. C. Egeberg and I. Chorkendorff, Catal. Lett. In press 2001. IC-49/51 Lecture-7 4 -11 -2004

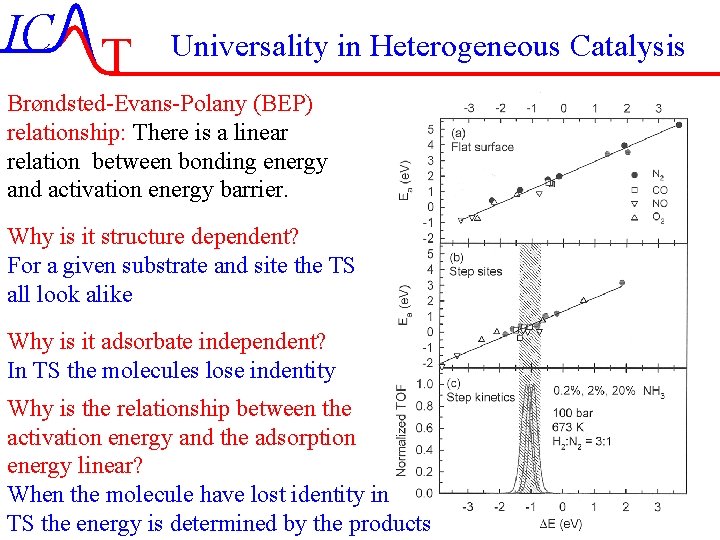
IC T Universality in Heterogeneous Catalysis Brøndsted-Evans-Polany (BEP) relationship: There is a linear relation between bonding energy and activation energy barrier. Why is it structure dependent? For a given substrate and site the TS all look alike Why is it adsorbate independent? In TS the molecules lose indentity Why is the relationship between the activation energy and the adsorption energy linear? When the molecule have lost identity in TS the energy is determined by the products IC-50/51 Lecture-7 4 -11 -2004

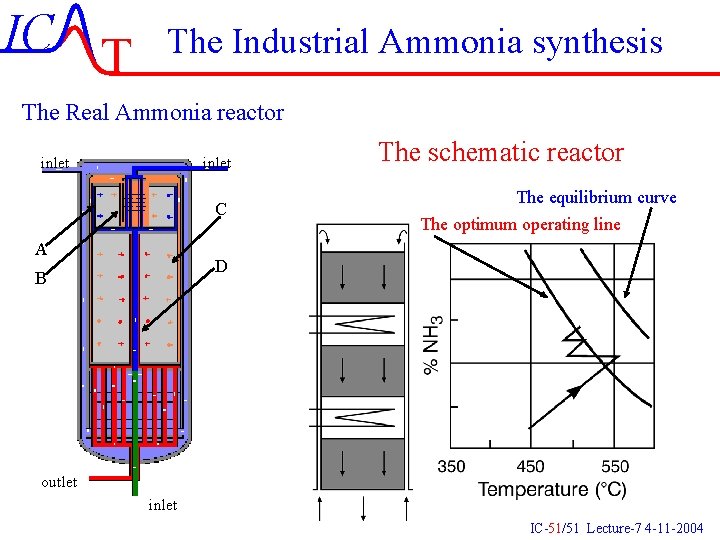
IC T The Industrial Ammonia synthesis The Real Ammonia reactor inlet C A The schematic reactor The equilibrium curve The optimum operating line D B outlet inlet IC-51/51 Lecture-7 4 -11 -2004

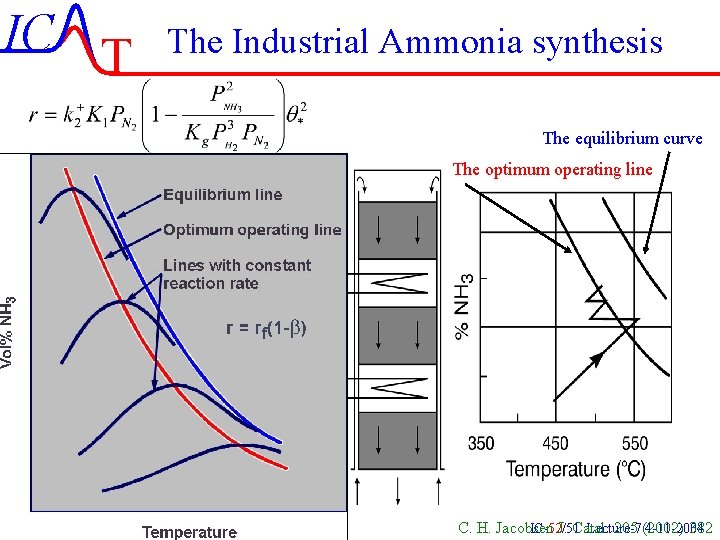
IC T The Industrial Ammonia synthesis The equilibrium curve The optimum operating line C. H. Jacobsen J. Catal. 205 (2002) 382 IC-52/51 Lecture-7 4 -11 -2004

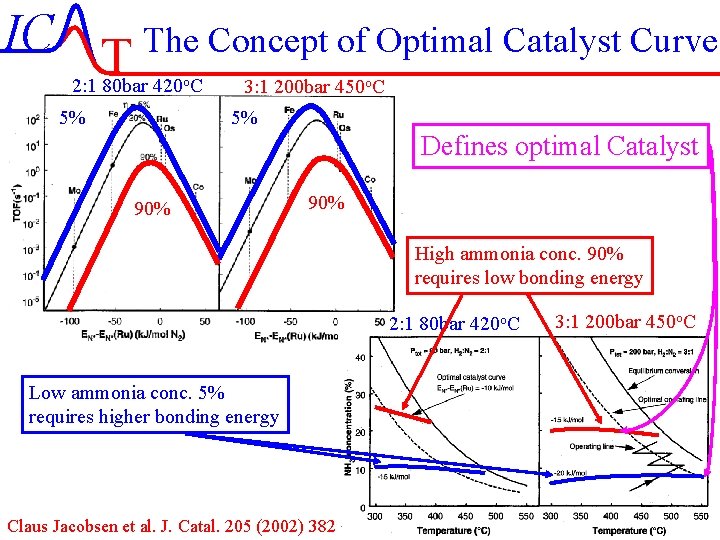
IC T The Concept of Optimal Catalyst Curve 2: 1 80 bar 420 o. C 5% 3: 1 200 bar 450 o. C 5% Defines optimal Catalyst 90% High ammonia conc. 90% requires low bonding energy 2: 1 80 bar 420 o. C 3: 1 200 bar 450 o. C Low ammonia conc. 5% requires higher bonding energy Claus Jacobsen et al. J. Catal. 205 (2002) 382 IC-53/51 Lecture-7 4 -11 -2004