IB DP Biology Unit 2 2 Water 2

IB DP Biology Unit 2. 2 Water

2. 2. U 1 Water molecules are polar and hydrogen bonds form between them.
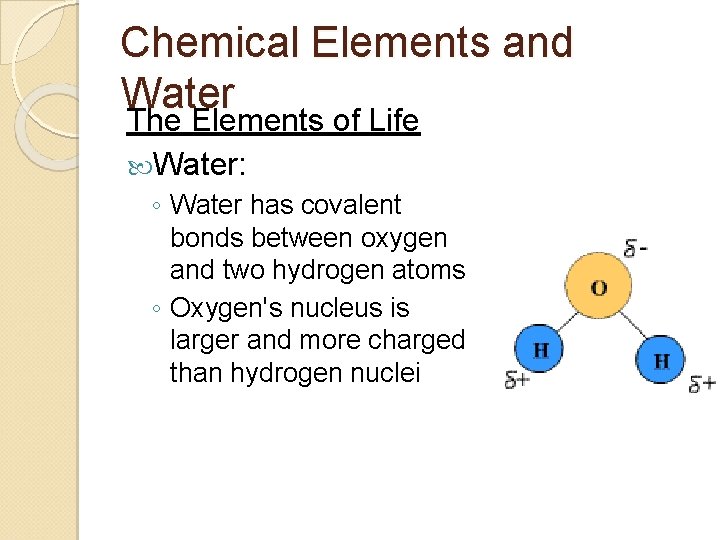
Chemical Elements and Water The Elements of Life Water: ◦ Water has covalent bonds between oxygen and two hydrogen atoms ◦ Oxygen's nucleus is larger and more charged than hydrogen nuclei

Chemical Elements and Water The Elements of Life Water: ◦ So, the electrons in the covalent bond are found 'closer' to the oxygen than the hydrogen ◦ Creates a polar molecule Negative dipole near oxygen Positive dipole near hydrogen

Chemical Elements and Water Negative charge on oxygen is attracted to the hydrogen of other water molecules ◦ = Hydrogen bonds can occur between water and other polar molecules ◦ FON
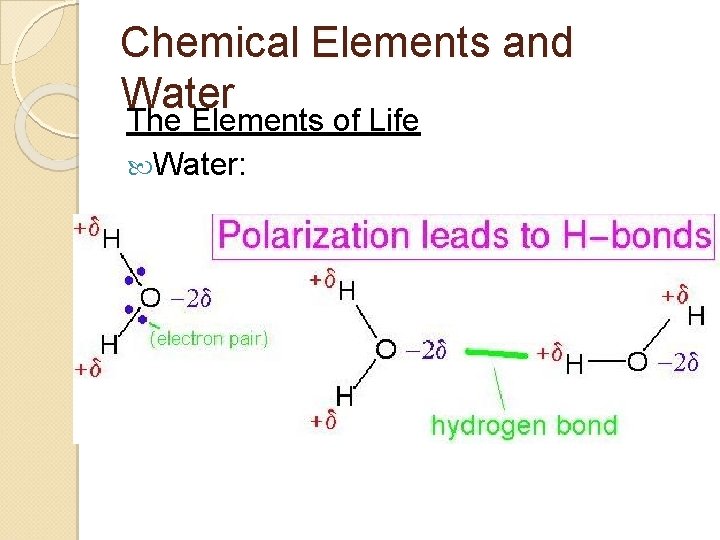
Chemical Elements and Water The Elements of Life Water:
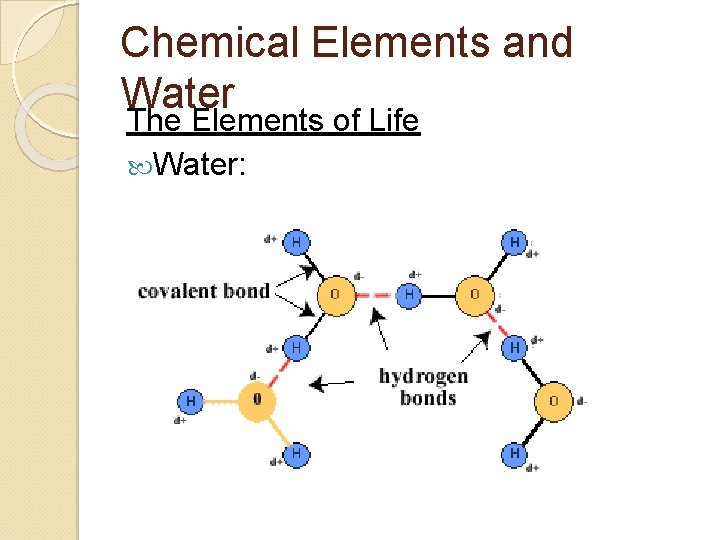
Chemical Elements and Water The Elements of Life Water:
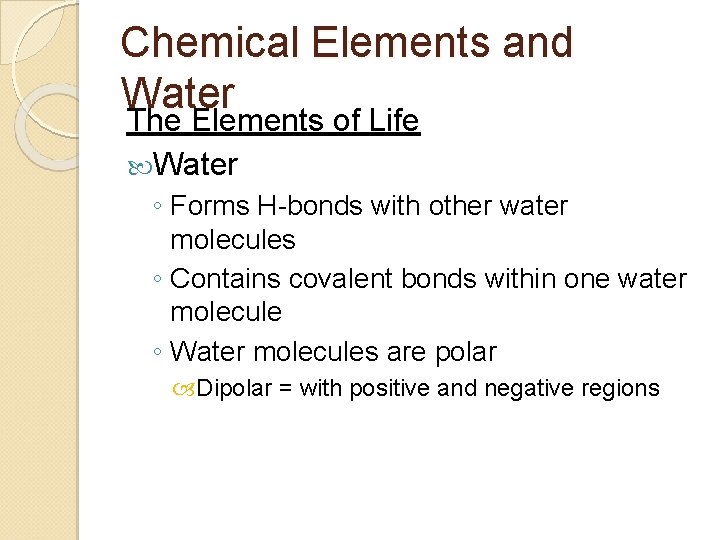
Chemical Elements and Water The Elements of Life Water ◦ Forms H-bonds with other water molecules ◦ Contains covalent bonds within one water molecule ◦ Water molecules are polar Dipolar = with positive and negative regions

2. 2. U 2 Hydrogen bonding and dipolarity explain the cohesive, adhesive, thermal and solvent properties of water.
Chemical Elements and Water Hydrogen bonds in water give it unique characteristics important to life, including: ◦ Thermal properties ◦ Cohesive properties ◦ Adhesive properties ◦ Solvent properties

Chemical Elements and Water Thermal properties ◦ Hydrogen bonds between polar water molecules cause water to resist change High specific heat (energy required to change water temperature) High heat of vaporization (energy required to boil water) High heat of fusion (loss of energy required to freeze water) ◦ Water produces a stable environment

Chemical Elements and Water Cohesion ◦ When “like substances” stick together ◦ Hydrogen bonds between polar water molecules cause them to stick together Plant transpiration moving water against gravity Water forms drops so that all molecules can be as close together as possible

Chemical Elements and Water Cohesion ◦ Surface tension between cohering water molecules Allowing for animals to walk on water Even though they are denser than water Such as water striders

Chemical Elements and Water Cohesion

Chemical Elements and Water Cohesion

Chemical Elements and Water Adhesion ◦ When "unlike" substances stick together ◦ Due to its polarity, water molecules will stick to surfaces Walls, Cloths, Xylem vessels, etc.
Chemical Elements and Water Capillary action ◦ Plants suck water from the roots to the leaves by capillary actions ◦ Cohesion Water molecules form H-bonds with each other to form a giant chain up the plant xylem ◦ Adhesion Water molecules are attracted by polarity to the surface walls (of the xylem) ◦ Same principle when you drink from a straw

Chemical Elements and Water Solvent properties ◦ Polarity of water attracts, or dissolves, any other polar or charged particles By forming hydrogen bonds with them ◦ Proteins, glucose or ions (such as sodium or calcium) are all soluble

Chemical Elements and Water Properties allow water to be by life as a: ◦ Coolant ◦ Medium for metabolic reactions ◦ Transport medium
Chemical Elements and Water Coolant ◦ Water has a high heat capacity A measure of energy input needed for a certain rise in temperature ◦ Water absorbs a lot of heat before there is a change in temperature ◦ Useful for living things Both physiologically and ecologically

Chemical Elements and Water Coolant ◦ Water has a high heat of vaporization High energy needed to change liquid water to gas Because hydrogen bonds must be broken ◦ Water absorbed a great deal of energy before entering the vapor phase Good at removing heat Maintaining of body temperature

Chemical Elements and Water Coolant ◦ Water absorbs a lot of energy (heat) before changing from liquid gas This energy makes the molecules move fast enough to break the H-bonds ◦ Fastest/hottest molecules leave first Making average temperature of water cooler This is how sweating works!

Chemical Elements and Water Coolant ◦ Heat generated by body needs to be removed To prevent denaturation of enzymes Evaporation of water from plant leaves (transpiration) or from human skin (sweat) Blood can absorb and carry heat away from hot parts of the body to the cooler parts
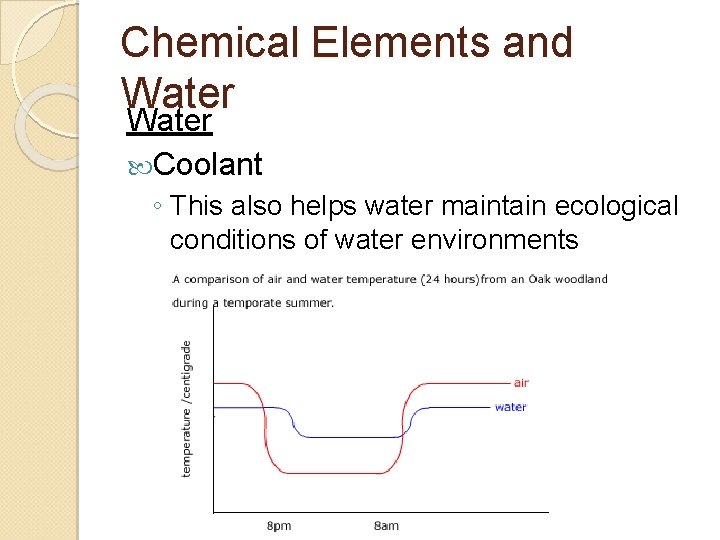
Chemical Elements and Water Coolant ◦ This also helps water maintain ecological conditions of water environments

Chemical Elements and Water Medium for metabolic reactions ◦ Cytoplasm is primarily water Providing a polar medium in which other polar or charged molecules dissolve ◦ Many enzymes are globular proteins that are water soluble They dissolve in cytoplasm where they control metabolic reactions
Chemical Elements and Water Transport medium ◦ Water can move things dissolved in it ◦ As water is lost by transpiration from plants, hydrogen bonds between water molecules pull water up against gravity Up to 100 meters

Chemical Elements and Water

Chemical Elements and Water What kind of bears dissolve in water?

TOK QUESTION: Smart water? There have been many claims (some scientifically proven) regarding the ‘memory of water’, wherein it retains properties of previously dissolved properties that have been removed. By what criteria can a claim be judged to be pseudoscientific?

2. 2. U 3 Substances can be hydrophilic or hydrophobic.

Chemical Elements and Water Hydrophilic ◦ Polar substances (with a charge) are attracted to water Either to the positive or negative side ◦ This allows them to be dissolved in water ◦ Examples: Salt, Carbon dioxide, Sugar, etc.

Chemical Elements and Water Hydrophobic ◦ Non-polar substance (no charge) are repelled by water Water is attracted to itself, and thus ‘squeezes’ the hydrophobic material away ◦ Can not dissolve in water ◦ Examples Oils and Fats

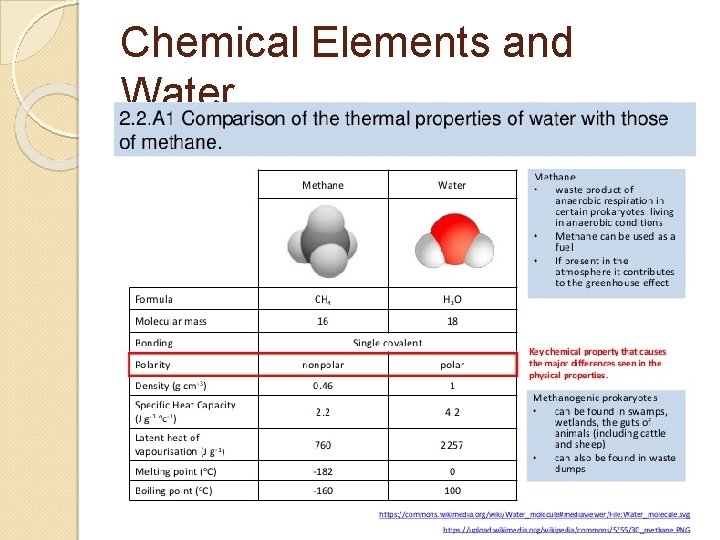
2. 2. A 1 Comparison of thermal properties of water with those of methane.

Chemical Elements and Water Methane ◦ Can be used as a fuel ◦ Present in the atmosphere and contributes to the greenhouse effect ◦ Made by prokaryotes Special type of anaerobic respiration Can be found in swamps, landfills, wetlands, and guts of animals (cattle and sheep)
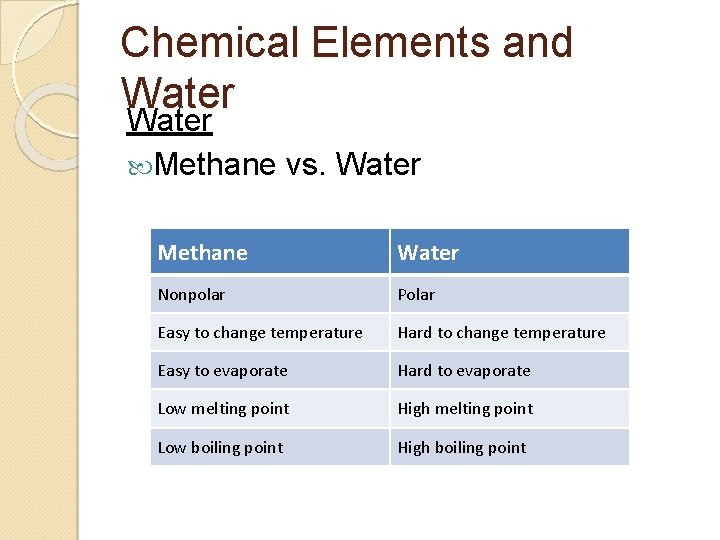
Chemical Elements and Water Methane vs. Water Methane Water Nonpolar Polar Easy to change temperature Hard to change temperature Easy to evaporate Hard to evaporate Low melting point High melting point Low boiling point High boiling point
Chemical Elements and Water
2. 2. A 2 Use of water as a coolant in sweat.

Chemical Elements and Water Coolant in plants and animals ◦ High temperatures denature proteins Cause enzymes to stop working ◦ Water absorbs a lot of energy before changing temperature Thanks to H-bonds holding molecules together Keeps temperature of water stable Good as a habitat

Chemical Elements and Water ◦ Water absorbs a lot of energy to change from liquid to gas Because of H-bonds holding molecules together ◦ Body releases water on your skin Heat moves from your body and blood vessels to the water Molecules absorb heat and move faster Faster molecules leave first, cooling your skin Cooler blood can circulate from skin to organs

2. 2. A 3 Modes of transport of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in blood in relation to their solubility in water.

Chemical Elements and Water Blood plasma is 95% water ◦ Rest is the dissolved material it transports
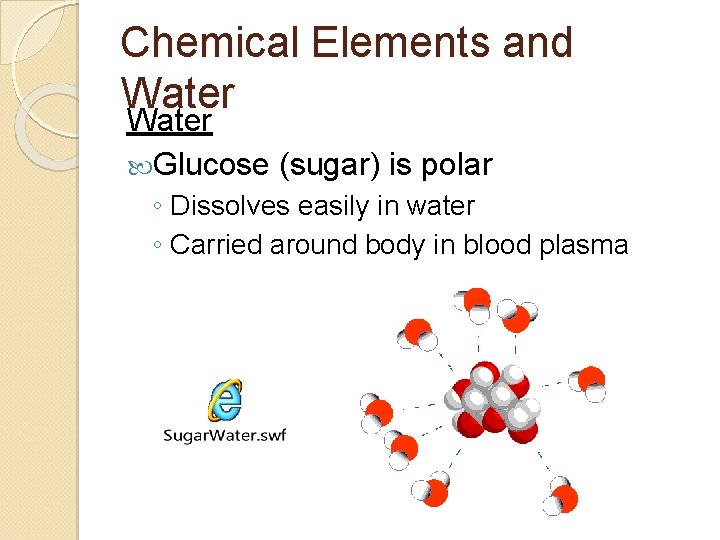
Chemical Elements and Water Glucose (sugar) is polar ◦ Dissolves easily in water ◦ Carried around body in blood plasma
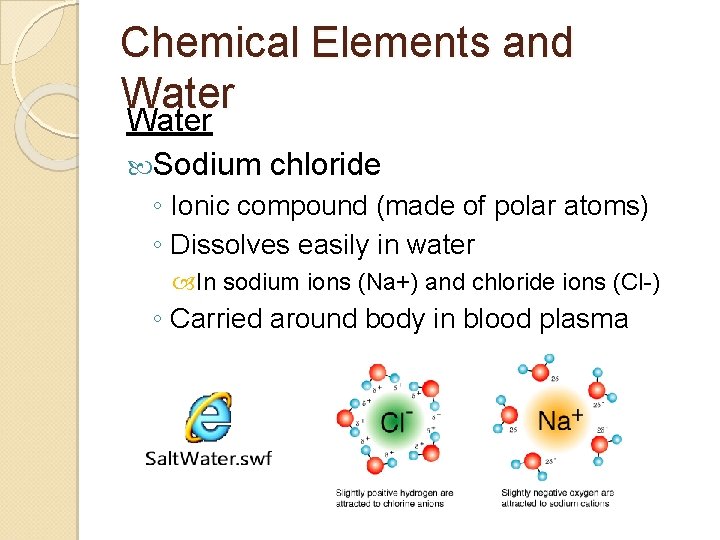
Chemical Elements and Water Sodium chloride ◦ Ionic compound (made of polar atoms) ◦ Dissolves easily in water In sodium ions (Na+) and chloride ions (Cl-) ◦ Carried around body in blood plasma
Chemical Elements and Water Amino acids (proteins) are generally polar ◦ Soluble in water ◦ Carried around body in blood plasma Amino acid R-groups are variable ◦ Can be polar or non-polar ◦ Can be big or small ◦ R-group determines solubility

Chemical Elements and Water Oxygen (O 2) is non-polar ◦ So small that it is soluble anyway (barely) ◦ Water becomes saturated with O 2 quickly ◦ As temperature increases, O 2 solubility decreases Too little O 2 can be carried by blood O 2 is mostly carried by hemoglobin in RBCs ◦ Has 4 -binding sites for O 2 around Iron

Chemical Elements and Water Fats are large and non-polar ◦ Insoluble in water ◦ Carried inside vesicle-like bags in blood plasma Called ‘lipoprotein complexes’

Chemical Elements and Water Cholesterol is mostly non-polar ◦ Small hydrophilic region at one end ◦ Insoluble in water ◦ Carried inside vesicle-like bags in blood plasma Called ‘lipoprotein complexes’
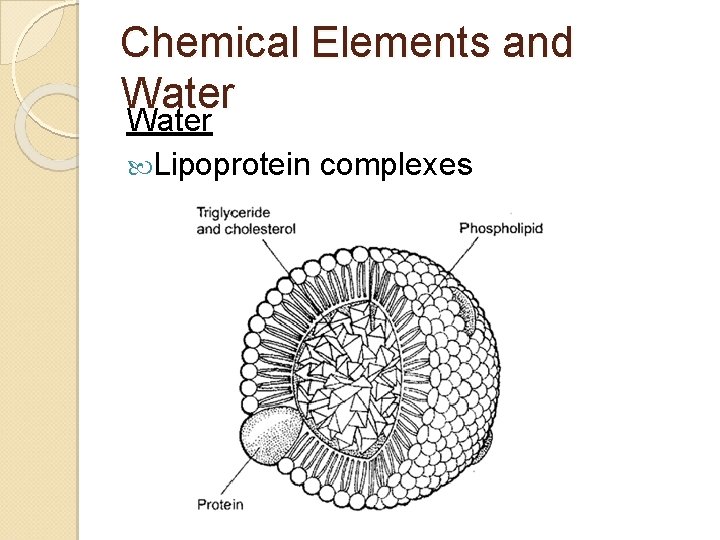
Chemical Elements and Water Lipoprotein complexes

Chemical Elements and Water Lipoprotein complexes ◦ Single layer of phospholipids Hydrophilic heads face out – touching water Hydrophobic tails face in – touching fats Cholesterol and proteins in the membrane
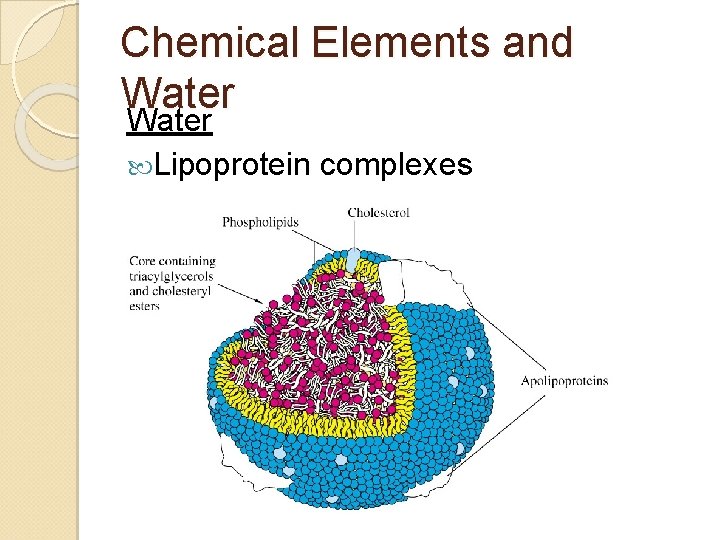
Chemical Elements and Water Lipoprotein complexes

MAJOR SOURCES Thank you to my favorite sources of information when making these lectures! John Burrell (Bangkok, TH) www. click 4 biology. info Dave Ferguson (Kobe, JA) http: //canada. canacad. ac. jp/High/49 Stephen Taylor (Bandung, IN) www. i-biology. net Andrew Allott – Biology for the IB Diploma C. J. Clegg – Biology for the IB Diploma Weem, Talbot, Mayrhofer – Biology for the International Baccalaureate Howard Hugh’s Medical Institute – www. hhmi. org/biointeractive Mr. Hoye’s TOK Website – http: //mrhoyestokwebsite. com And all the contributors at www. You. Tube. com
- Slides: 51