Iatrogenic Spinal Subdural Collections Lessons Learned ASNR 2015

Iatrogenic Spinal Subdural Collections: Lessons Learned ASNR 2015 e. Ed. E-221 -7047 Edwin Gulko MD, RL Zampolin MD, TS Miller MD; K Shifteh MD, J Burns MD, JA Bello, MD Division of Neuroradiology, Montefiore Medical Center Bronx, NY

The authors have no financial disclosures

OUTLINE • • • Spinal Meningeal Anatomy What is the Subdural Space? Imaged Guided Procedures Bed-Side Procedures Surgical Procedures Conclusions

GOALS • Understand spinal meningeal anatomy, and pathomechanisms for subdural collections. • Recognize imaging features of subdural injection during myelographic procedures. • Understand recognize imaging features of subdural collections on CT and MRI. ★= TAKE HOME POINT

INTRODUCTION • Iatrogenic spinal subdural collections pose a diagnostic challenge. • Making a clear distinction between subdural and epidural collections influences clinical management / surgical approaches, if required. • Firm understanding of spinal meningeal anatomy will allow the radiologist to confidently identify subdural collections when present. • Understanding the pathomechanism of subdural collections will aid in proper diagnosis, and prevent misdiagnoses such as metastatic disease.

Spinal Meningeal Anatomy • Dura Mater • Arachnoid Mater • Subarachnoid Space • Pia Mater Dorsal Septum Dentate ligament • Electron microscopy studies of human spinal cords by Nicholas et al 1, demonstrated that the arachnoid mater was closely opposed to the inner aspect of the dura. • They demonstrated the existence of a fenestrated intermediate leptomeningeal layer between the arachnoid and pia mater, which is reflected at intervals to form the dorsal septum. • At segmental levels, there are paired bilateral dentate ligaments (aka denticulate ligaments) composed of a collagen core and covered by a leptomeningeal layer. They fuse with dural and subpial collagen.

Spinal Meningeal Anatomy • The thecal sac is seen as a linear, T 2 hypointense structure (yellow arrows). • It is a combination of both dura and arachonoid mater, and helps delineate epidural fat from intradural contents.

What is the Subdural Space? • Prior uncertainty as to whether a potential subdural space truly existed at the dura-arachnoid junction within the brain and spinal cord meninges. • Haines et al. 2, 3 performed literature review on cranial meninges, citing evidence that a subdural space, with a potential cavity, does not exist. • The superficial/external dural layer is strong, composed of fibroblasts and extracellular intertwining collagen. The inner dura, or dural border cell layer (DBC) is composed of fibroblasts, lacks extracellular collagen, and contains few cell junctions. As a result, this layer of dura is weaker. • The accumulation of pathological fluid/hematoma within the meninges likely results from direct tissue damage within the dural border cell layer (DBC). • The arachnoid layer adjacent and deep to the DBC, called the arachnoid barrier cell layer, contains many cell junctions, and a basement membrane. Like the external dural cell layer, it is strong and resistant to passage of fluids. • ★As a result, the descriptions from Haines et al 2, 3 provided the following notions: 1. There is no biological space at the dura-arachnoid junction; (i. e. there is no naturally occurring subdural cavity or potential space). 2. The DBC is a structurally weak layer of the inner dura. In the setting of trauma/pathology, a cavity may develop within the DBC.

What is the Subdural Space? • An electron microscopy study by Vandenabeele et al 4 in human specimens confirmed that the spinal meninges were structurally similar to the cranial meninges. They also confirmed the absence of a naturally occurring subdural space within the spine. • They demonstrated the presence of a structurally weak DBC layer that was easily disrupted, with formation of an artificial subdural space. This work was substantiated by Reina et al 5. • Given the structural similarity of cranial and spinal meninges, the mechanism formation of cranial and spinal subdural hematomas/collections is likely similar 6. While the term ‘subdural’ is not an accurate descriptor of collections within the dural meninges, for this presentation, we will continue to use the term ‘subdural’ (SD) to remain consistent with current medical terminology. ★However the radiologist should realize, that anatomically what is considered a subdural collection is actually an inter-dural process.
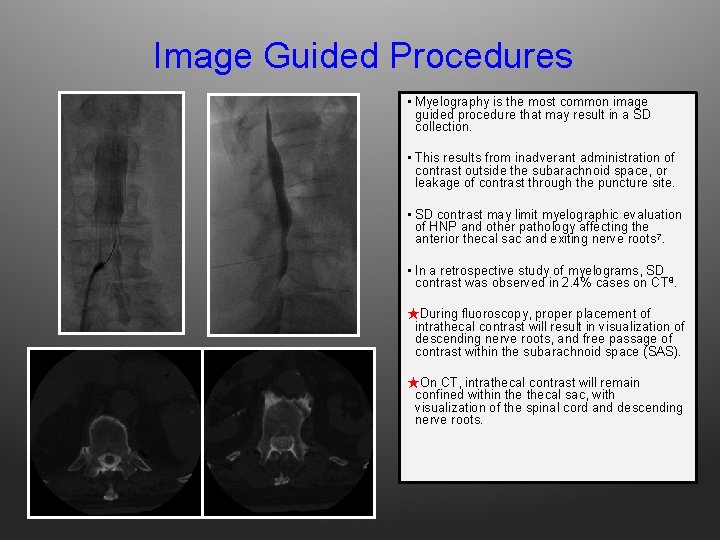
Image Guided Procedures • Myelography is the most common image guided procedure that may result in a SD collection. • This results from inadverant administration of contrast outside the subarachnoid space, or leakage of contrast through the puncture site. • SD contrast may limit myelographic evaluation of HNP and other pathology affecting the anterior thecal sac and exiting nerve roots 7. • In a retrospective study of myelograms, SD contrast was observed in 2. 4% cases on CT 8. ★During fluoroscopy, proper placement of intrathecal contrast will result in visualization of descending nerve roots, and free passage of contrast within the subarachnoid space (SAS). ★On CT, intrathecal contrast will remain confined within thecal sac, with visualization of the spinal cord and descending nerve roots.

Image Guided Procedures ★ On fluoroscopy and CT, SD contrast is more dense than contrast within the SAS 7. ★ On lateral fluoroscopic projections, SD contrast may be ventral or dorsal within the spinal canal. Here, the ventral margin is flat, bounded by the dura mater. The dorsal margin is irregular, as SD contrast circumferentially surrounds the arachnoid 7. A. In this example, ventral placement of the spinal needle accounts for the ventral subdural collection which is seen as a hyperdense collection (red arrows). The ventral margin is flat, bounded by outer dura. B. Further injection of contrast material in the same patient results in progressive enlargement of the SD collection.
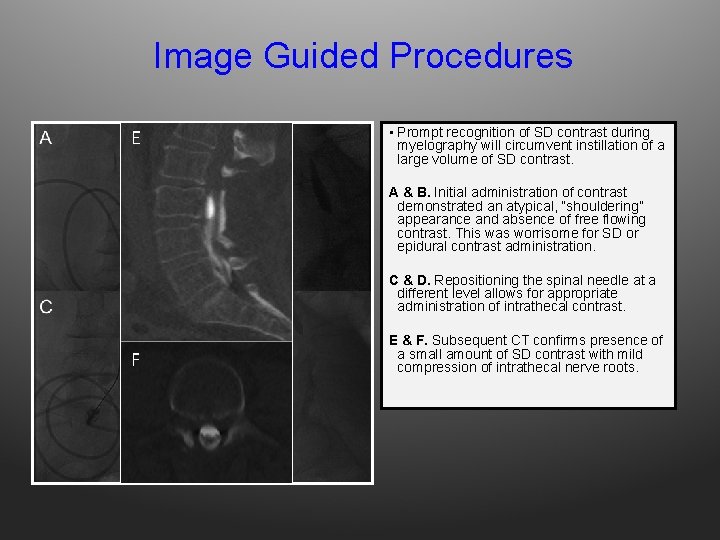
Image Guided Procedures E • Prompt recognition of SD contrast during myelography will circumvent instillation of a large volume of SD contrast. A & B. Initial administration of contrast demonstrated an atypical, “shouldering” appearance and absence of free flowing contrast. This was worrisome for SD or epidural contrast administration. C & D. Repositioning the spinal needle at a different level allows for appropriate administration of intrathecal contrast. F E & F. Subsequent CT confirms presence of a small amount of SD contrast with mild compression of intrathecal nerve roots.
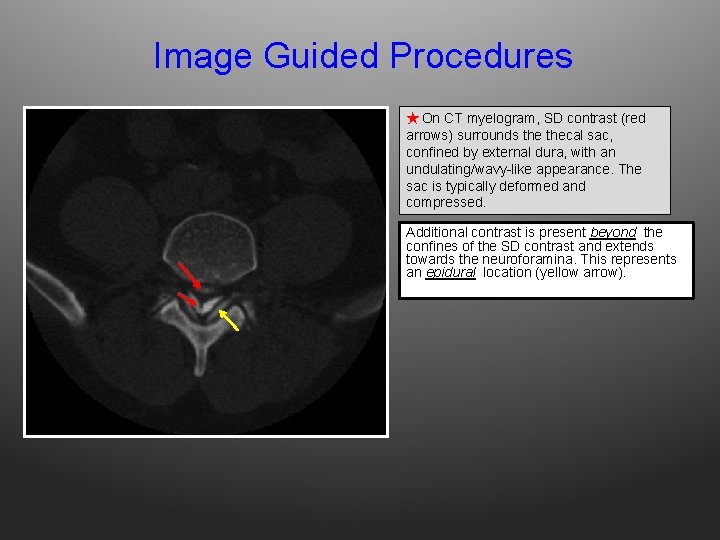
Image Guided Procedures ★ On CT myelogram, SD contrast (red arrows) surrounds thecal sac, confined by external dura, with an undulating/wavy-like appearance. The sac is typically deformed and compressed. Additional contrast is present beyond the confines of the SD contrast and extends towards the neuroforamina. This represents an epidural location (yellow arrow).
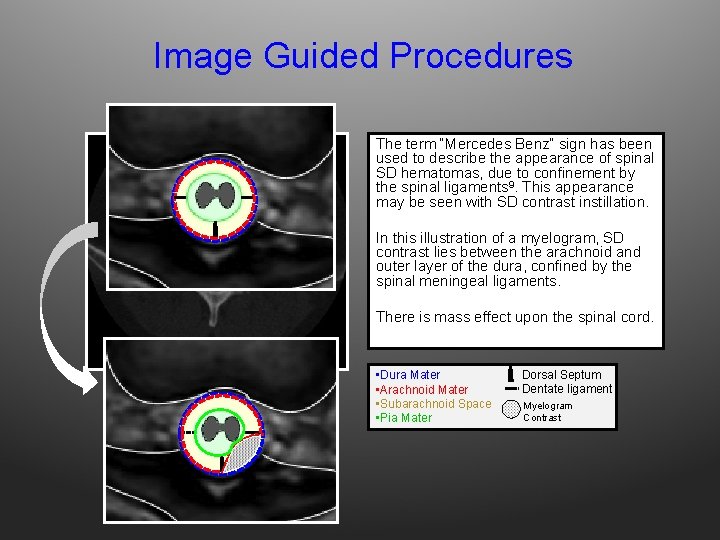
Image Guided Procedures The term “Mercedes Benz” sign has been used to describe the appearance of spinal SD hematomas, due to confinement by the spinal ligaments 9. This appearance may be seen with SD contrast instillation. In this illustration of a myelogram, SD contrast lies between the arachnoid and outer layer of the dura, confined by the spinal meningeal ligaments. There is mass effect upon the spinal cord. • Dura Mater • Arachnoid Mater • Subarachnoid Space • Pia Mater Dorsal Septum Dentate ligament Myelogram Contrast

Image Guided Procedures • In this example, the classic Mercedes Benz sign is not appreciated. However, hyperdense contrast is confined by the outer dural, confirming the presence of SD contrast deforming thecal sac.
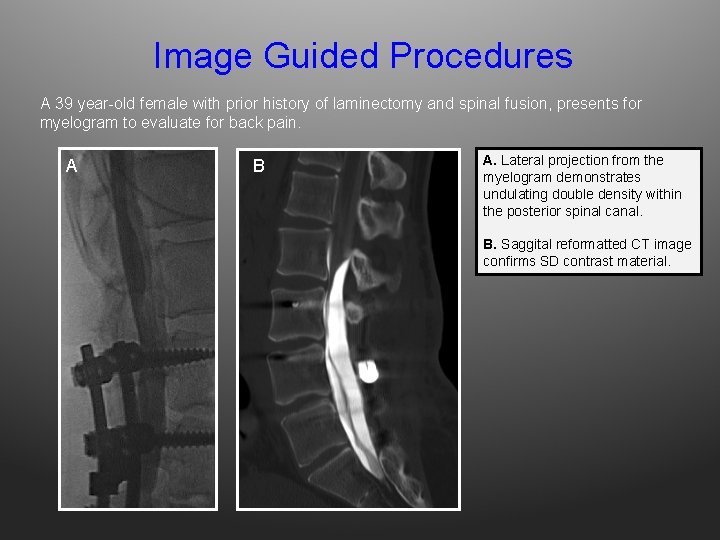
Image Guided Procedures A 39 year-old female with prior history of laminectomy and spinal fusion, presents for myelogram to evaluate for back pain. A B A. Lateral projection from the myelogram demonstrates undulating double density within the posterior spinal canal. B. Saggital reformatted CT image confirms SD contrast material.
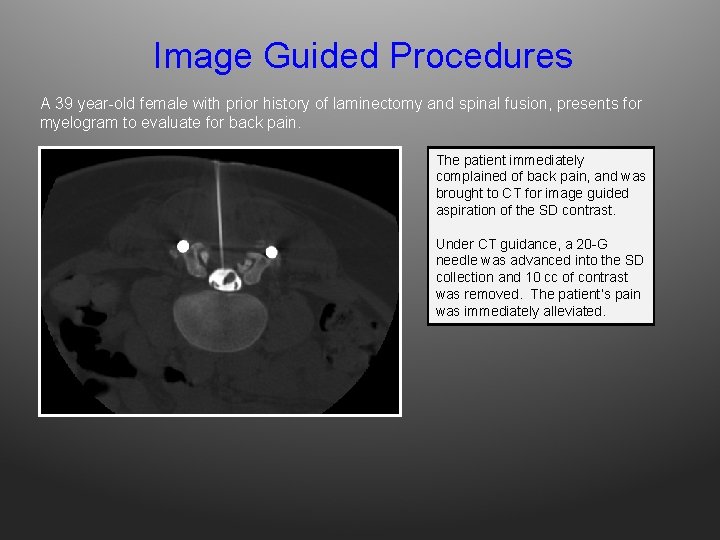
Image Guided Procedures A 39 year-old female with prior history of laminectomy and spinal fusion, presents for myelogram to evaluate for back pain. The patient immediately complained of back pain, and was brought to CT for image guided aspiration of the SD contrast. Under CT guidance, a 20 -G needle was advanced into the SD collection and 10 cc of contrast was removed. The patient’s pain was immediately alleviated.
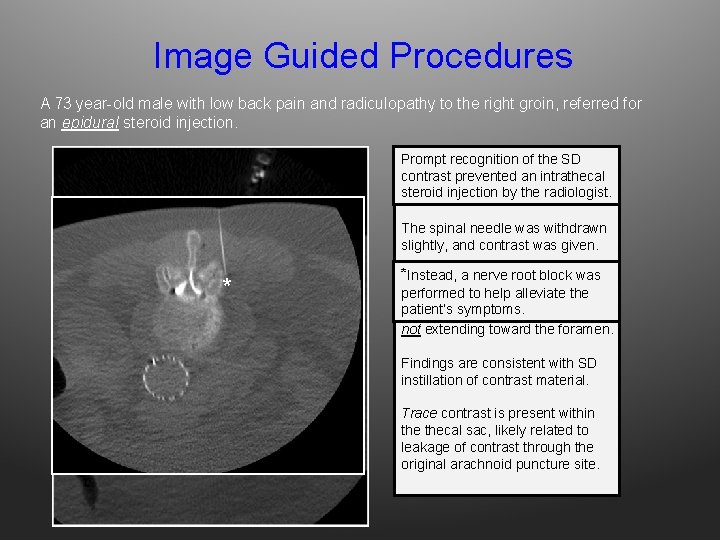
Image Guided Procedures A 73 year-old male with low back pain and radiculopathy to the right groin, referred for an epidural steroid injection. Prompt recognition the SD A 22 G spinal needleofwas contrast prevented an intrathecal inadvertently advanced into the steroid injection the radiologist. SAS (image not by shown). The spinal needle was withdrawn slightly, and contrast was given. * *AInstead, a nerve block was ‘Mercedes Benz’root sign is seen, performed to confined help alleviate the with contrast by outer patient’s symptoms. dura, deforming thecal sac but not extending toward the foramen. Findings are consistent with SD instillation of contrast material. Trace contrast is present within thecal sac, likely related to leakage of contrast through the original arachnoid puncture site.
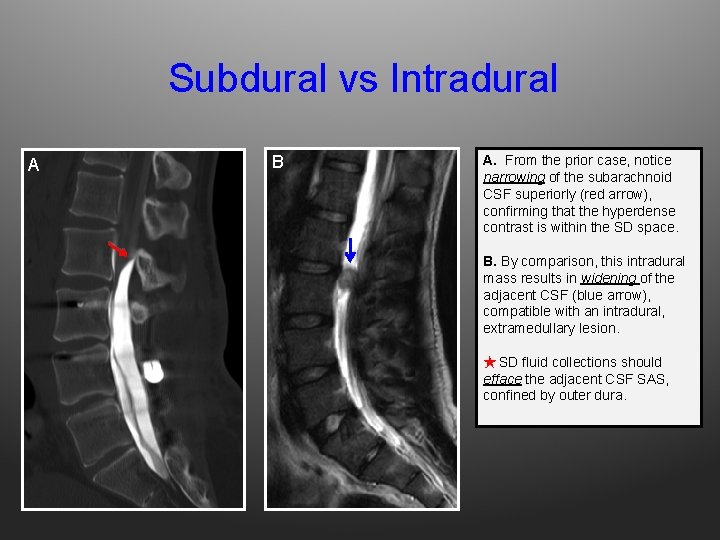
Subdural vs Intradural A B A. From the prior case, notice narrowing of the subarachnoid CSF superiorly (red arrow), confirming that the hyperdense contrast is within the SD space. B. By comparison, this intradural mass results in widening of the adjacent CSF (blue arrow), compatible with an intradural, extramedullary lesion. ★ SD fluid collections should efface the adjacent CSF SAS, confined by outer dura.
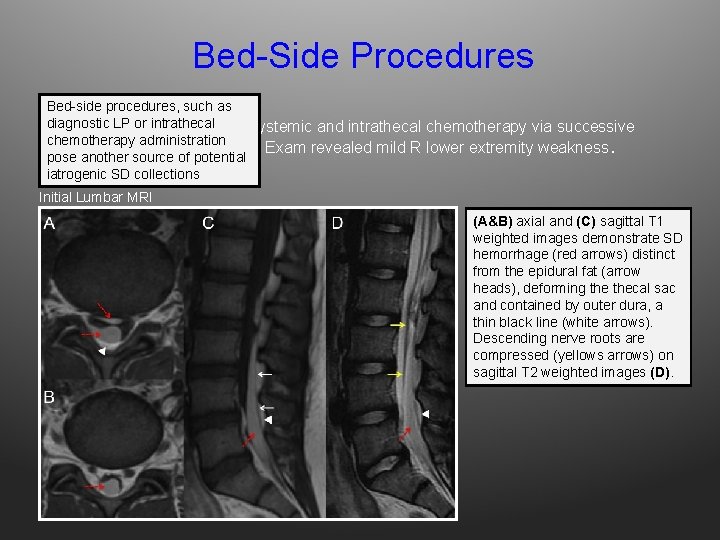
Bed-Side Procedures Bed-side procedures, such as or ALL intrathecal Adiagnostic 16 yo M LP with receiving systemic and intrathecal chemotherapy via successive chemotherapy administration LPs, developed new back pain. Exam revealed mild R lower extremity weakness. pose another source of potential iatrogenic SD collections Initial Lumbar MRI (A&B) axial and (C) sagittal T 1 weighted images demonstrate SD hemorrhage (red arrows) distinct from the epidural fat (arrow heads), deforming thecal sac and contained by outer dura, a thin black line (white arrows). Descending nerve roots are compressed (yellows arrows) on sagittal T 2 weighted images (D).
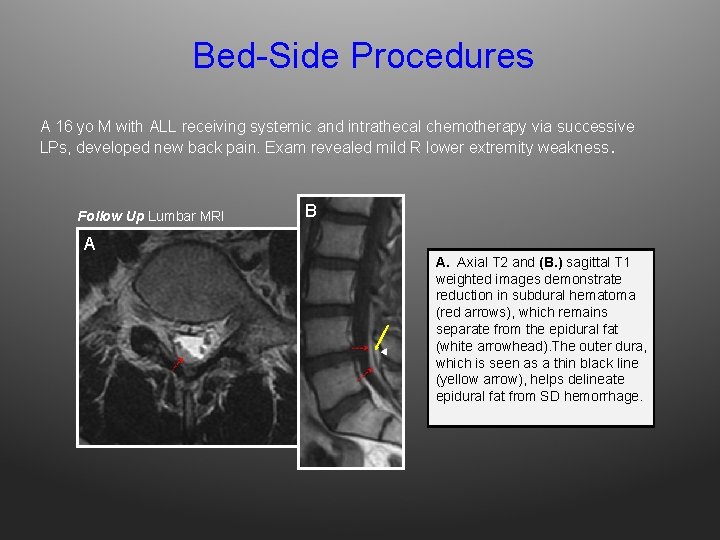
Bed-Side Procedures A 16 yo M with ALL receiving systemic and intrathecal chemotherapy via successive LPs, developed new back pain. Exam revealed mild R lower extremity weakness. Follow Up Lumbar MRI B A A. Axial T 2 and (B. ) sagittal T 1 weighted images demonstrate reduction in subdural hematoma (red arrows), which remains separate from the epidural fat (white arrowhead). The outer dura, which is seen as a thin black line (yellow arrow), helps delineate epidural fat from SD hemorrhage.
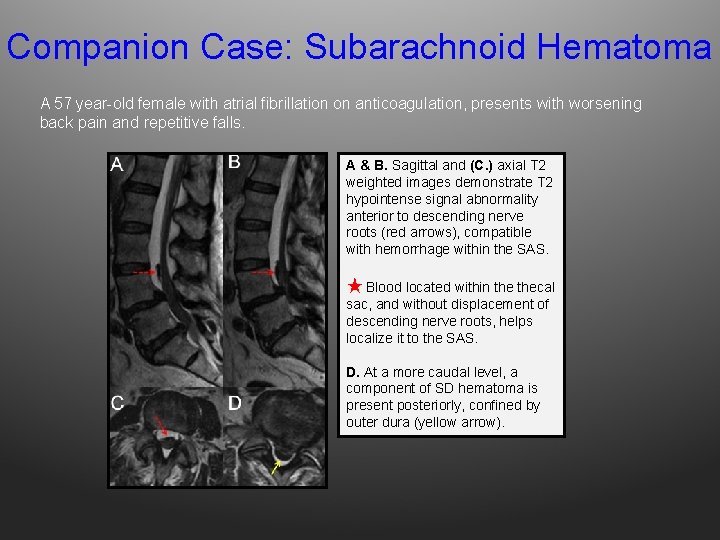
Companion Case: Subarachnoid Hematoma A 57 year-old female with atrial fibrillation on anticoagulation, presents with worsening back pain and repetitive falls. A & B. Sagittal and (C. ) axial T 2 weighted images demonstrate T 2 hypointense signal abnormality anterior to descending nerve roots (red arrows), compatible with hemorrhage within the SAS. ★ Blood located within thecal sac, and without displacement of descending nerve roots, helps localize it to the SAS. D. At a more caudal level, a component of SD hematoma is present posteriorly, confined by outer dura (yellow arrow).
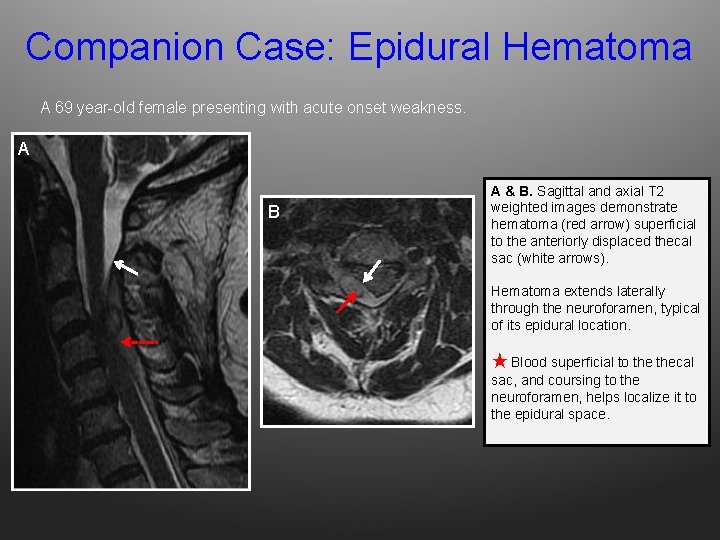
Companion Case: Epidural Hematoma A 69 year-old female presenting with acute onset weakness. A B A & B. Sagittal and axial T 2 weighted images demonstrate hematoma (red arrow) superficial to the anteriorly displaced thecal sac (white arrows). Hematoma extends laterally through the neuroforamen, typical of its epidural location. ★ Blood superficial to thecal sac, and coursing to the neuroforamen, helps localize it to the epidural space.
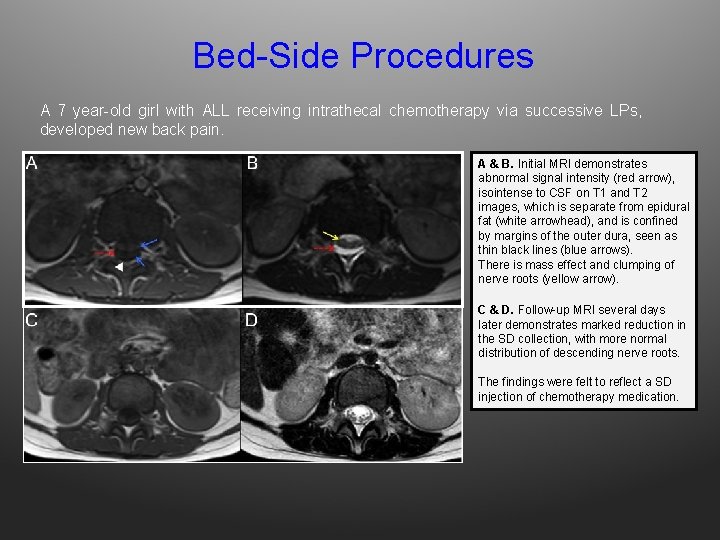
Bed-Side Procedures A 7 year-old girl with ALL receiving intrathecal chemotherapy via successive LPs, developed new back pain. A & B. Initial MRI demonstrates abnormal signal intensity (red arrow), isointense to CSF on T 1 and T 2 images, which is separate from epidural fat (white arrowhead), and is confined by margins of the outer dura, seen as thin black lines (blue arrows). There is mass effect and clumping of nerve roots (yellow arrow). C & D. Follow-up MRI several days later demonstrates marked reduction in the SD collection, with more normal distribution of descending nerve roots. The findings were felt to reflect a SD injection of chemotherapy medication.
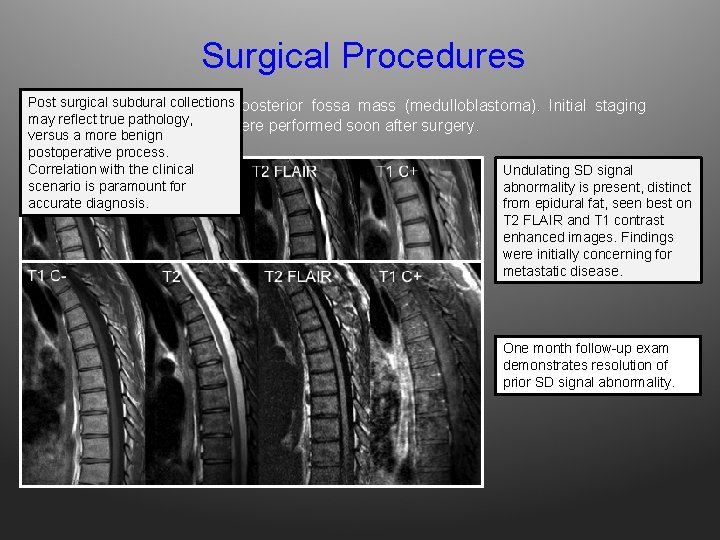
Surgical Procedures Post collections 32 surgical yo M subdural s/p resection of posterior fossa mass (medulloblastoma). Initial staging may reflect true pathology, examinations of the spine were performed soon after surgery. versus a more benign postoperative process. Correlation with the clinical Undulating SD signal scenario is paramount for abnormality is present, distinct accurate diagnosis. from epidural fat, seen best on T 2 FLAIR and T 1 contrast enhanced images. Findings were initially concerning for metastatic disease. One month follow-up exam demonstrates resolution of prior SD signal abnormality.

Surgical Procedures ★ Spinal Subdural Enhancement following resection of posterior fossa tumors: • Several reports have described spinal SD collections following resection of posterior fossa tumors, with resolution on followup examinations 10, 11, 12. • The precise mechanism is unknown. Several hypotheses include introduction of blood into thecal sac with resultant meningeal irritation and enhancement 12, CSF pressure changes during surgery in patients with obstructive hydrocephalus 11. • Consequently, spinal imaging for staging of posterior fossa tumors, should be performed prior to surgery to prevent confounding imaging findings. When such findings are encountered, follow-up exam is important.
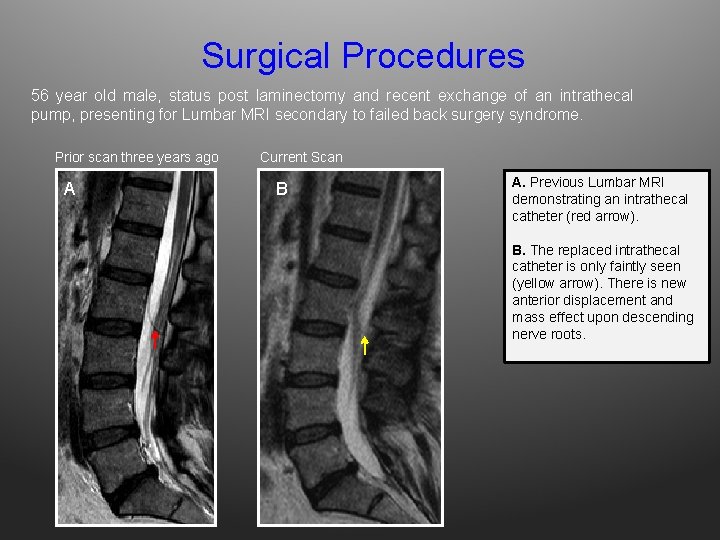
Surgical Procedures 56 year old male, status post laminectomy and recent exchange of an intrathecal pump, presenting for Lumbar MRI secondary to failed back surgery syndrome. Prior scan three years ago A Current Scan B A. Previous Lumbar MRI demonstrating an intrathecal catheter (red arrow). B. The replaced intrathecal catheter is only faintly seen (yellow arrow). There is new anterior displacement and mass effect upon descending nerve roots.
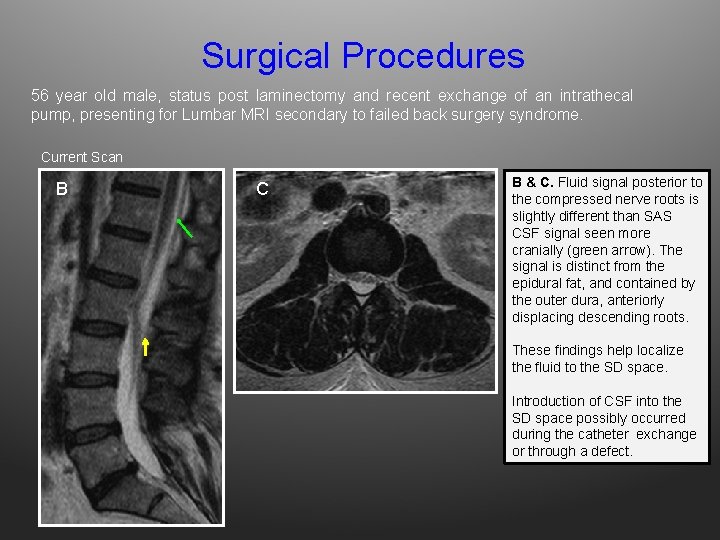
Surgical Procedures 56 year old male, status post laminectomy and recent exchange of an intrathecal pump, presenting for Lumbar MRI secondary to failed back surgery syndrome. Current Scan B C B & C. Fluid signal posterior to the compressed nerve roots is slightly different than SAS CSF signal seen more cranially (green arrow). The signal is distinct from the epidural fat, and contained by the outer dura, anteriorly displacing descending roots. These findings help localize the fluid to the SD space. Introduction of CSF into the SD space possibly occurred during the catheter exchange or through a defect.
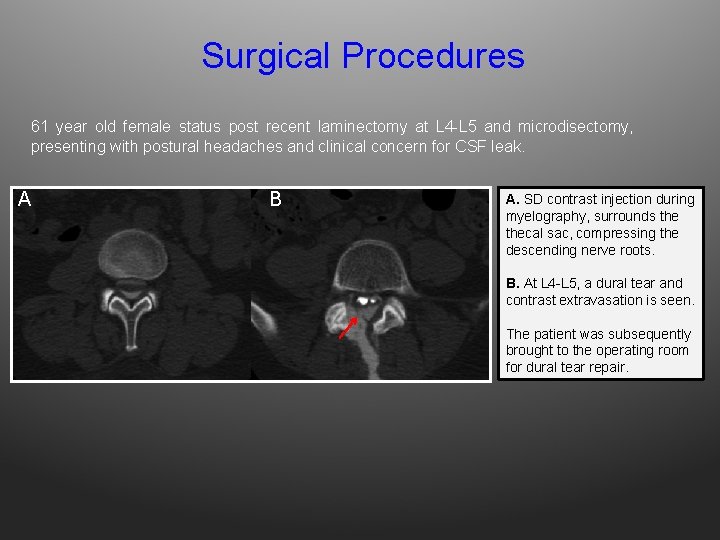
Surgical Procedures 61 year old female status post recent laminectomy at L 4 -L 5 and microdisectomy, presenting with postural headaches and clinical concern for CSF leak. A B A. SD contrast injection during myelography, surrounds thecal sac, compressing the descending nerve roots. B. At L 4 -L 5, a dural tear and contrast extravasation is seen. The patient was subsequently brought to the operating room for dural tear repair.
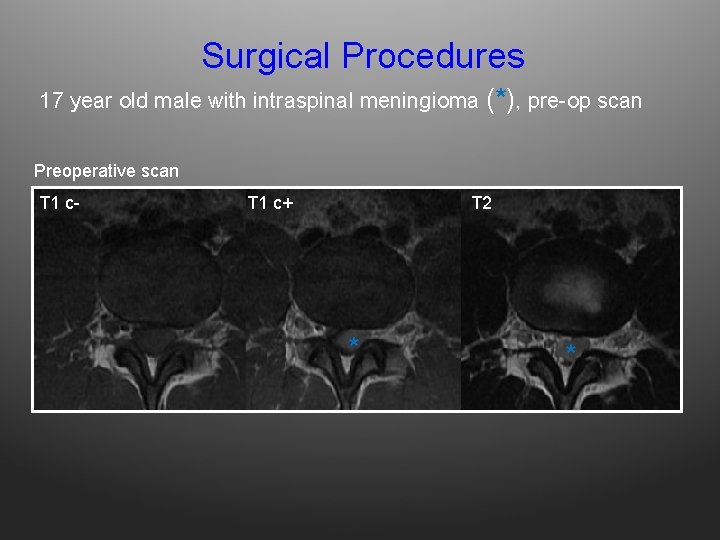
Surgical Procedures 17 year old male with intraspinal meningioma (*), pre-op scan Preoperative scan T 1 c- T 1 c+ T 2 * *
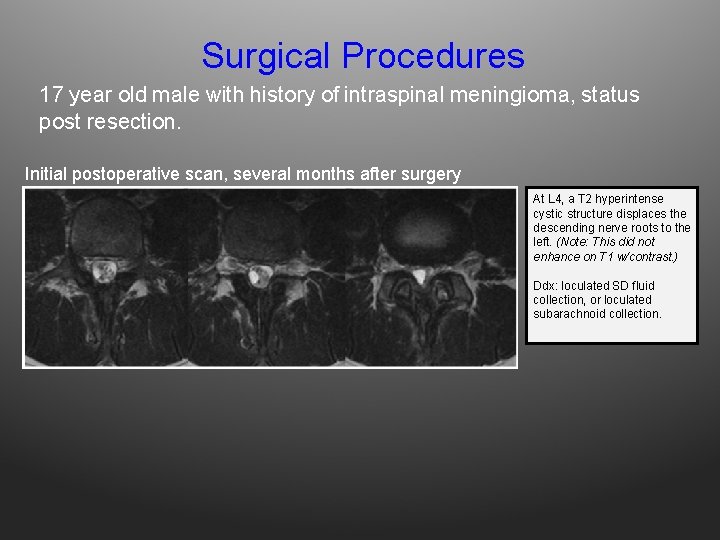
Surgical Procedures 17 year old male with history of intraspinal meningioma, status post resection. Initial postoperative scan, several months after surgery At L 4, a T 2 hyperintense cystic structure displaces the descending nerve roots to the left. (Note: This did not enhance on T 1 w/contrast. ) Ddx: loculated SD fluid collection, or loculated subarachnoid collection.

Conclusions • A true potential cavity does not exist within the dural meninges; however, injury to the inner dural border cell layer may result in accumulation of contrast, blood or fluid, confined by the outer dura, resulting in compression of the neuraxis within thecal sac. • SD collections (i. e. inter-dural collections) have a distinct imaging appearance, distinct from subarachnoid fluid or epidural collections, and should be properly identified. • Prompt recognition of SD injection during myelography prevents instillation of a large volume of contrast outside of the SAS. • In the setting of posterior fossa tumor resection, SD enhancement may reflect a benign process that will resolve, warranting follow-up imaging 2 – 4 weeks later.

References 1. Nicholas DS, Weller RO. The fine anatomy of the human spinal meninges. A light and scanning electron microscopy study. J Neurosurg. 1988; 69(2): 276 -282. 2. Haines DE. On the question of a subdural space. Anat Rec. 1991; 230(1): 3 -21. 3. Haines DE, Harkey HL, al-Mefty O. The "subdural" space: a new look at an outdated concept. Neurosurgery. 1993; 32(1): 111 -120. 4. Vandenabeele F, Creemers J, Lambrichts I. Ultrastructure of the human spinal arachnoid mater and dura mater. J Anat. 1996; 189 ( Pt 2): 417 -430. 5. Reina MA, De Leon Casasola O, Lopez A, De Andres JA, Mora M, Fernandez A. The origin of the spinal subdural space: ultrastructure findings. Anesth Analg. 2002; 94(4): 991 -995, table of contents. 6. Post MJ, Becerra JL, Madsen PW, et al. Acute spinal subdural hematoma: MR and CT findings with pathologic correlates. AJNR Am J Neuroradiol. 1994; 15(10): 1895 -1905. 7. Ajar AH, Rathmell JP, Mukherji SK. The subdural compartment. Reg Anesth Pain Med. 2002; 27(1): 72 -76. 8. Milants WP, Parizel PM, de Moor J, Tobback IG, De Schepper AM. Epidural and subdural contrast in myelography and CT myelography. Eur J Radiol. 1993; 16(2): 147 -150. 9. Castillo M. Neuroradiology companion : methods, guidelines, and imaging fundamentals. 3 rd ed. Philadelphia: Lippincott Williams & Willkins; 2006. 10. Shaw DW, Weinberger E, Brewer DK, Geyer JR, Berger MS, Blaser SI. Spinal subdural enhancement after suboccipital craniectomy. AJNR Am J Neuroradiol. 1996; 17(7): 1373 -1377. 11. Warmuth-Metz M, Kuhl J, Krauss J, Solymosi L. Subdural enhancement on postoperative spinal MRI after resection of posterior cranial fossa tumours. Neuroradiology. 2004; 46(3): 219 -223. 12. Wiener MD, Boyko OB, Friedman HS, Hockenberger B, Oakes WJ. False-positive spinal MR findings for subarachnoid spread of primary CNS tumor in postoperative pediatric patients. AJNR Am J Neuroradiol. 1990; 11(6): 1100 -1103.
- Slides: 33