I Transmutation Transmutation The conversion of one element



- Slides: 14


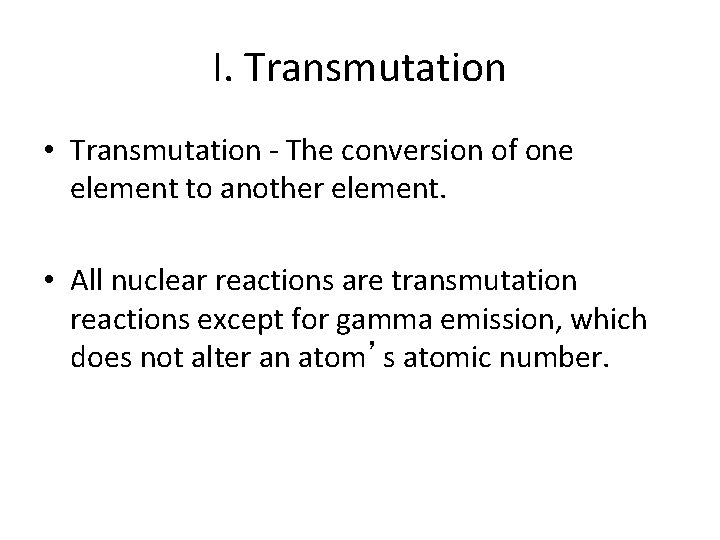
I. Transmutation • Transmutation - The conversion of one element to another element. • All nuclear reactions are transmutation reactions except for gamma emission, which does not alter an atom’s atomic number.

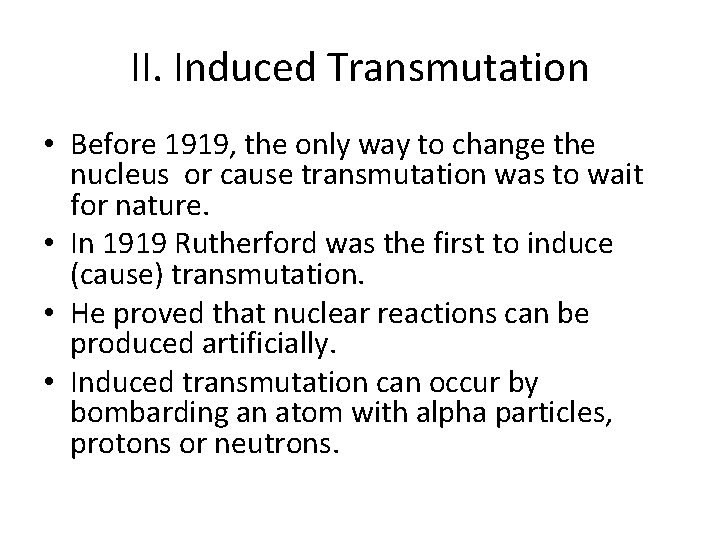
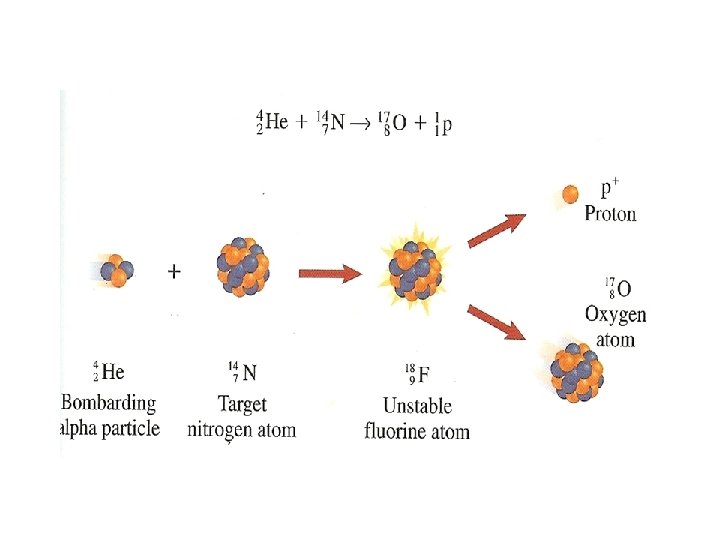
II. Induced Transmutation • Before 1919, the only way to change the nucleus or cause transmutation was to wait for nature. • In 1919 Rutherford was the first to induce (cause) transmutation. • He proved that nuclear reactions can be produced artificially. • Induced transmutation can occur by bombarding an atom with alpha particles, protons or neutrons.


III. Transuranium Elements • Elements with atomic number above 92. • All transuranium elements undergo transmutation • None of the transuranium elements occur in nature and have been produced through induced transmutation.

IV. Half-life • The time required for one-half of a radioisotope’s nuclei to decay into its products • After each half-life, half of the existing radioactive atoms have decayed into atoms of a new element • Amount remaining at time T = (initial amt)(1/2)n where n= number of half-lives • n = total time ÷ time of one half-life

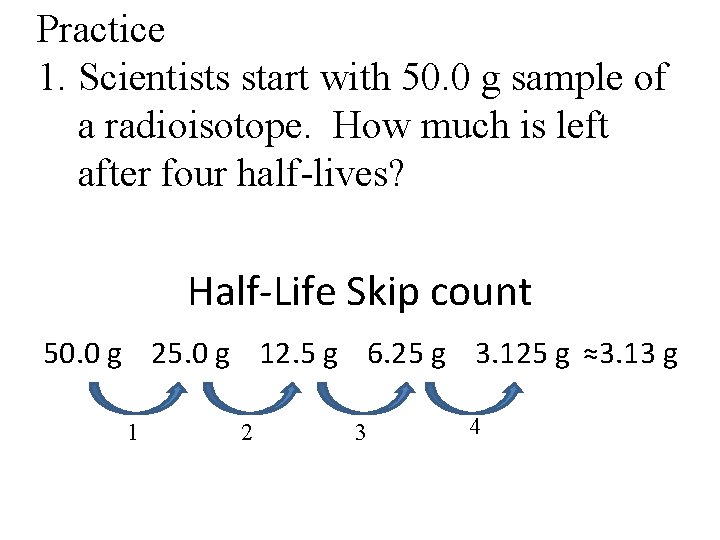
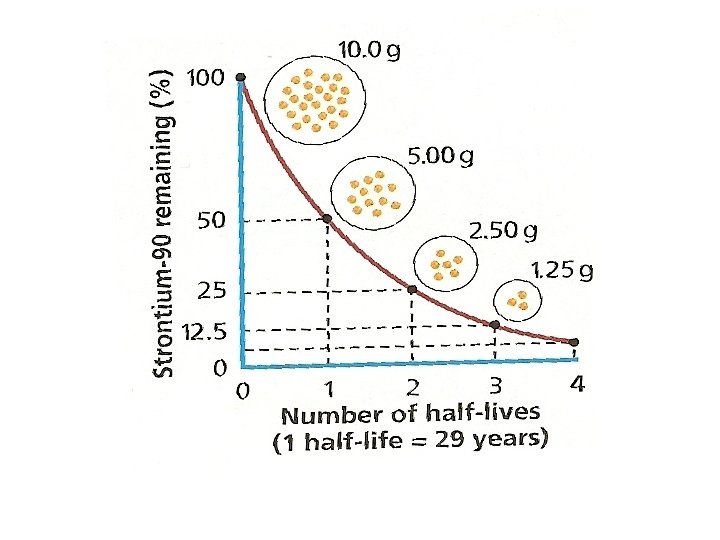
Practice 1. Scientists start with 50. 0 g sample of a radioisotope. How much is left after four half-lives? Half-Life Skip count 50. 0 g 25. 0 g 12. 5 g 6. 25 g 3. 125 g ≈3. 13 g 1 2 3 4

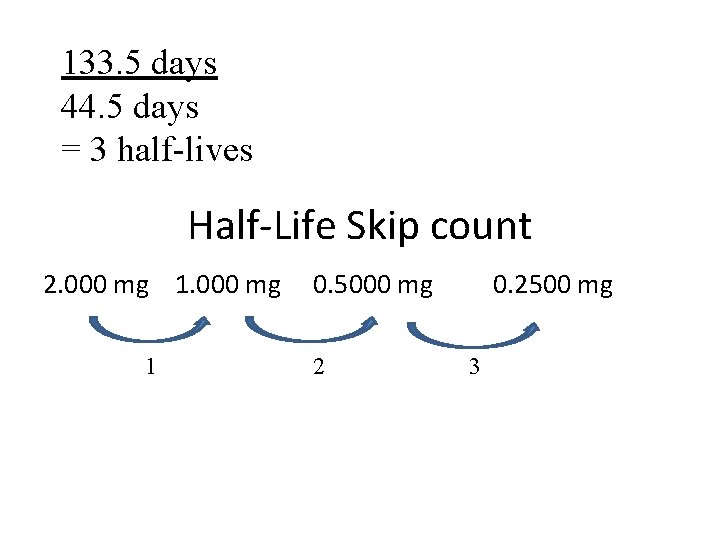
2. Iron-59 is used in medicine to diagnose blood circulation disorders. The half-life of iron 59 is 44. 5 days. How much of a 2. 000 mg sample will remain after 133. 5 days? (first find out how many half-lives, then skip count)

133. 5 days 44. 5 days = 3 half-lives Half-Life Skip count 2. 000 mg 1 0. 5000 mg 2 0. 2500 mg 3

V. Carbon-14 Dating • Carbon 14 dating is the process of determining the age of artifacts that were once part of a living organism by measuring the amount of 14 C remaining in that artifact • Carbon-14 is radioactive and undergoes beta decay. It has a half-life of 5730 years.

Carbon-14 • 14 C evenly spread in the Earth’s biosphere • Plants incorporate 14 C into their structure that matches the level in the atmosphere. • When an organism dies, 14 C declines at a known rate. (Half-life of C-14 = 5730 years) • Comparing the remaining 14 C fraction of a sample to that expected from atmospheric 14 C allows the age of the sample to be estimated. • Dates carbon-bearing materials up to 62, 000 years.

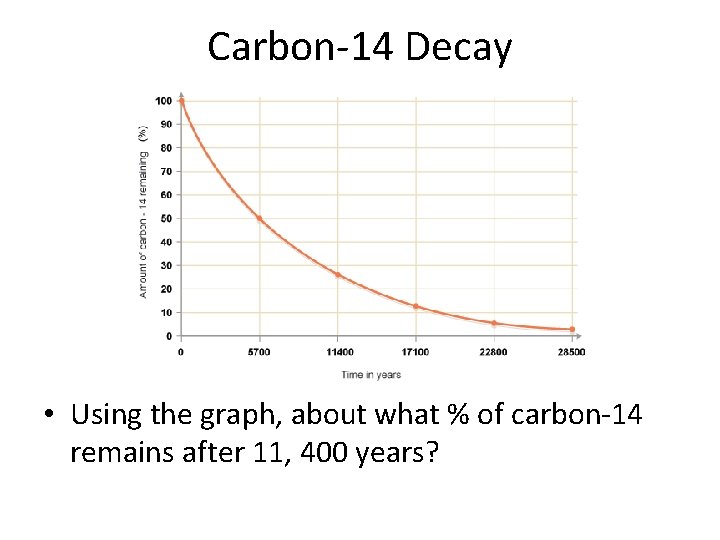
Carbon-14 Decay • Using the graph, about what % of carbon-14 remains after 11, 400 years?
