I POLAR VS NONPOLAR Polarity a separation of


- Slides: 9

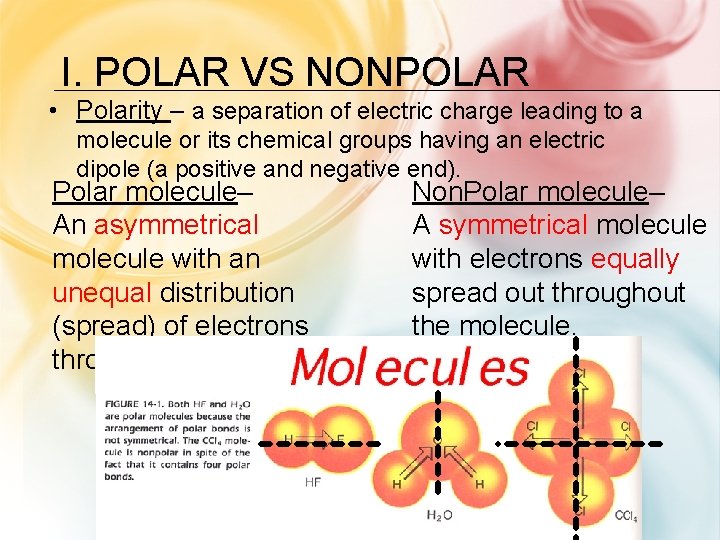
I. POLAR VS NONPOLAR • Polarity – a separation of electric charge leading to a molecule or its chemical groups having an electric dipole (a positive and negative end). Polar molecule– An asymmetrical molecule with an unequal distribution (spread) of electrons throughout. Non. Polar molecule– A symmetrical molecule with electrons equally spread out throughout the molecule.

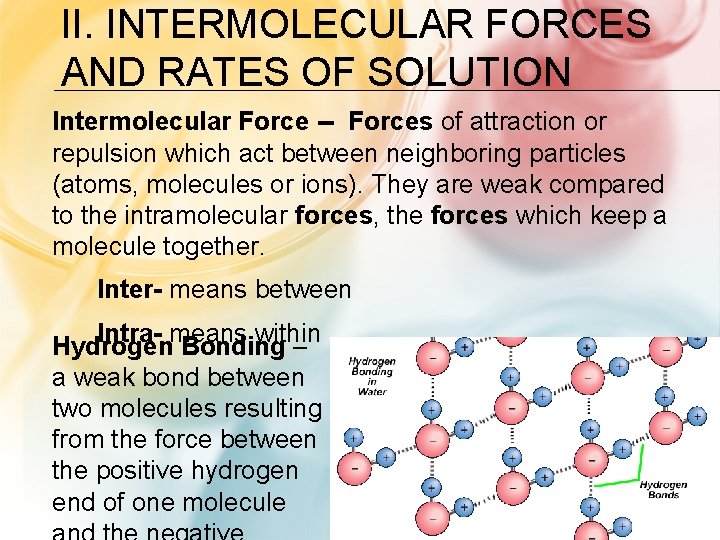
II. INTERMOLECULAR FORCES AND RATES OF SOLUTION Intermolecular Force -- Forces of attraction or repulsion which act between neighboring particles (atoms, molecules or ions). They are weak compared to the intramolecular forces, the forces which keep a molecule together. Inter- means between Intra- means within Hydrogen Bonding – a weak bond between two molecules resulting from the force between the positive hydrogen end of one molecule

II. INTERMOLECULAR FORCES AND RATES OF SOLUTION increase 1. Size of particles: (_______ surface area, ____ rate of solution) 2. Stirring – more surfacemore area brought into contact slower 3. Amount already dissolved: (_______ dissolved, ____ rate) 4. Temperature: increase • Solids and Liquids: _____ temperature, increase decrease _______ rate

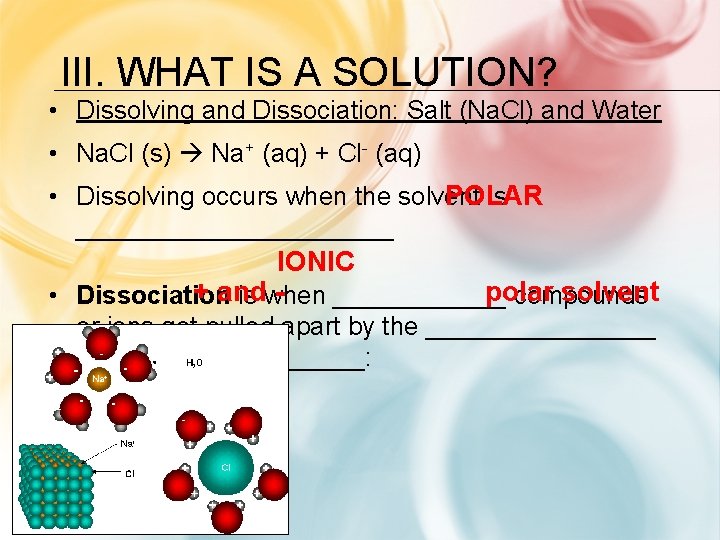
III. WHAT IS A SOLUTION? 1) What type of bonding is goingionic on in Na. Cl? 2) What type of compoundionic is Na. Cl? 3) What type of bonding is going Polar on in Hcovalent 2 O? 4) What type of compound is goingmolecular on in H 2 O? 5) What type of moleculepolar is H 2 O? 6) When Na. Cl is added to H 2 O, what happens to the Na+ and Cl- ions with respect to the H 2 O molecules? Positive Na ions are attracted to the negative pole (O) in the water molecule Negative Cl ions are attracted to the positive pole (H’s) in the water molecule

III. WHAT IS A SOLUTION? • Dissolving and Dissociation: Salt (Na. Cl) and Water • Na. Cl (s) Na+ (aq) + Cl- (aq) POLAR • Dissolving occurs when the solvent is ___________ IONIC polar solvent + and • Dissociation is when ______ compounds or ions get pulled apart by the ________ end of the______:

III. WHAT IS A SOLUTION? • Types of Solutions: - Gas with Atmosphere gas: (O 2, N 2, CO 2) Antifreeze - Liquid with liquid: - Gas with Liquid: Soda (CO 2 (g) in H 2 O (l)) - Solid with liquid: Salt water (Na. Cl (s) in H 2 O (l)) - Sold with Solid: Alloys: Mixture of metals (brass – coppe

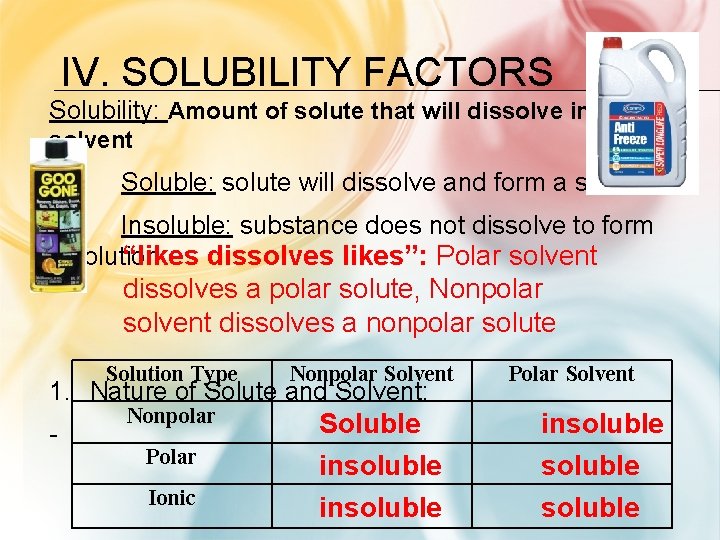
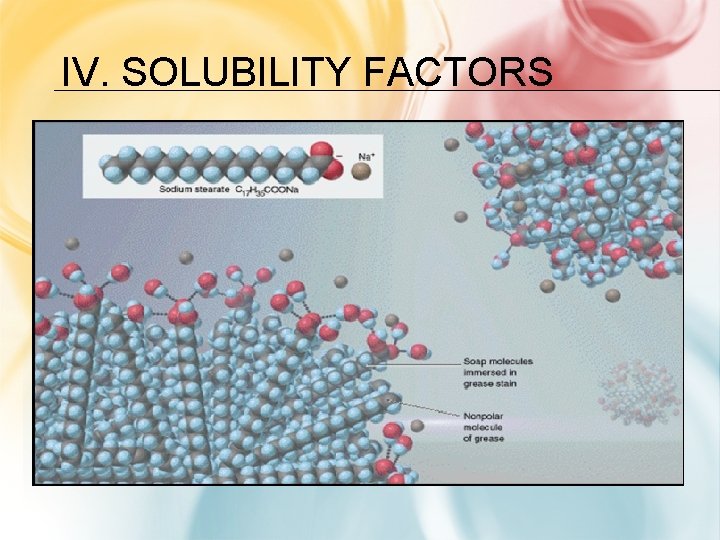
IV. SOLUBILITY FACTORS Solubility: Amount of solute that will dissolve in solvent Soluble: solute will dissolve and form a solution Insoluble: substance does not dissolve to form “likes dissolves likes”: Polar solvent a solution dissolves a polar solute, Nonpolar solvent dissolves a nonpolar solute Solution Type Nonpolar Solvent 1. Nature of Solute and Solvent: - Nonpolar Polar Ionic Soluble insoluble Polar Solvent insoluble

IV. SOLUBILITY FACTORS

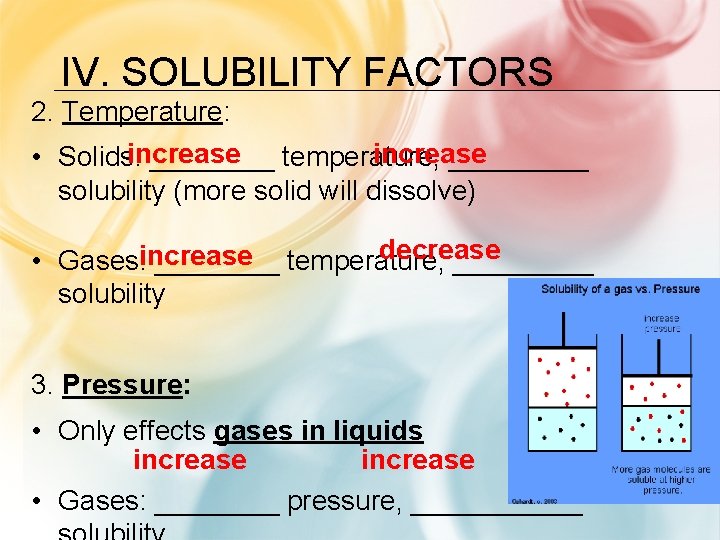
IV. SOLUBILITY FACTORS 2. Temperature: increase • Solids: ____ temperature, _____ solubility (more solid will dissolve) decrease • Gases: increase ____ temperature, _____ solubility 3. Pressure: • Only effects gases in liquids increase • Gases: ____ pressure, ______