I How to Use Pipette Balance II Pipetting

I. How to Use Pipette & Balance II. Pipetting & Reagent Preparation Winuthayanon Lab

Outline: How to Use Pipette & Balance Volumetric & Mass measurements Glassware Pipettes Measurement Balances Pipetting & Reagent preparation Calculation Reagent Preparation
Volumetric & Mass Measurements facweb. furman. edu/~wworthen/bio 222/volumes. ppt
Mass Measurements Mass – a measure of the amount of matter an object has; constant regardless of position Weight – a measure of how strongly an object is pulled by the earth’s gravity; varies with the distance from the earth’s center As long as you are working at or near the earth’s surface, weight is an accurate indicator of mass. Basic unit = Gram (g).

Laboratory Balances: Measuring Mass Scientists usually refer to a “balance” not “scale” Most are now electronic, with digital readout and few moving parts. Maximum capacity of balance is usually shown on the instrument. DO NOT EXCEED. Be sure that surface is levelled (check bubble).

Common laboratory balances Top-loading balance: Weighs to 0. 01 g. Wind screen not needed. Analytical balance: Weighs to 0. 0001 g (i. e. 1/10 of a milligram or 0. 1 mg). Has a wind screen to protect the balance pan from breezes. Microbalance: Weighs to 0. 000001 g (1µg) or less. For specialized uses only. Intermediate sizes exist as well.

Balances Top-loading Analytical
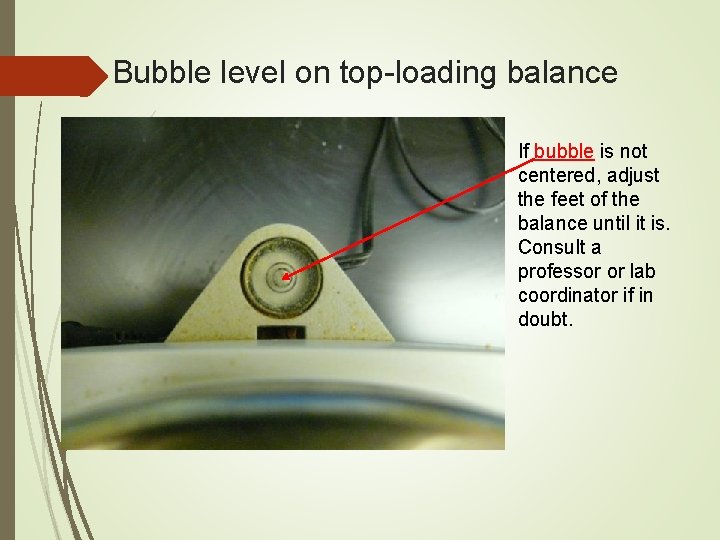
Bubble level on top-loading balance If bubble is not centered, adjust the feet of the balance until it is. Consult a professor or lab coordinator if in doubt.

Typical weighing procedure Use weighing paper or plastic “boat” Place on pan. Close windscreen. Press tare (or zero) to bring the display back to zero. Open windscreen. Carefully add substance. Wait for reading to stabilize. As you are near the desired weight, tap spatula to add a few grains at a time. Close windscreen before final measurement. When finished, clean balance.
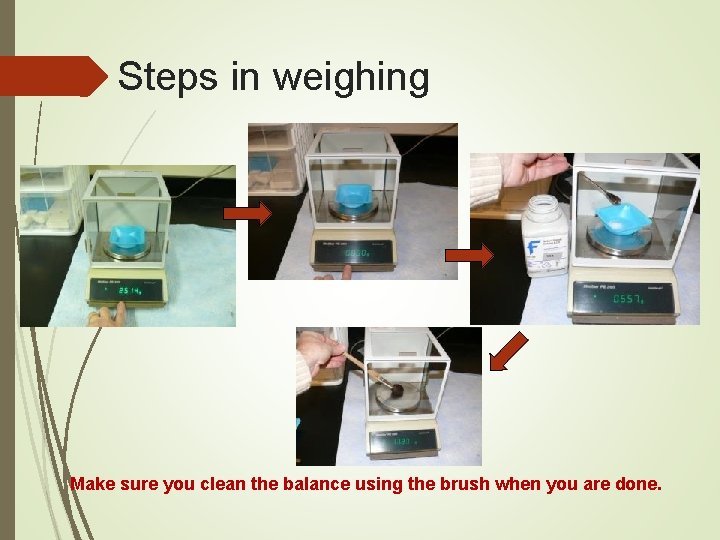
Steps in weighing Make sure you clean the balance using the brush when you are done.

Volumetric measurements Basic units of volumetric measurement is liter (abbreviation is letter L – upper or lower case) 1 m. L = 1 cc 1 m. L of water weighs 1 g 1 liter of water weighs 1 kg Consider the purposes of common glassware:

Beakers: Mixing and dispensing Wide mouth, good for stirring but hard to seal for storage Lip, good for pouring m. L graduations, if present, are VERY approximate; neither accurate nor precise

Conical (Erlenmeyer) Flasks: Mixing and storing Shape is good for swirling to mix solutions. Narrow mouth is easy to seal for storage (using stopper or Parafilm). Not as easy to pour things into or out of it, compared to a beaker. Like a beaker, graduations are neither accurate nor precise.

Graduated cylinders: Measuring Cylindrical shape gives good precision and accuracy. Graduations can measure any volume within the size range of the cylinder.

Volumetric flasks: measuring and making solutions Narrow neck gives excellent precision. Individually calibrated for excellent accuracy. Shape good for swirling to dissolve. Can only be used to measure one volume Common sizes: 10, 25, 50, 100, 250, 500, 1000 m. L Expensive – do not use for storage.

Smaller volumes: Pipettes Glass/plastic serological pipettes 1 m. L, 5 m. L, 10 m. L, 20 m. L Pipetters with disposable tips Most are adjustable up to a specified max. volume 10 µL, 20 µL, 100 µL, 200 µL, 1000 µL, 5000 µL

Most labware can be glass or plastic Plastic inexpensive and disposable not very fragile can’t be heated on hotplate or burner may be reactive or contaminate sample often the graduations are not accurate Glass More expensive less reactive or likely to contaminate may adsorb ions and other molecules wettable: a film of water is left clinging to glass

Wettability issues (esp. glass) If you put exactly 100 m. L of water into a glass container, when you pour it out it will dispense less than 100 m. L. The rest is clinging to the glass. Accurate instruments (graduated cylinders, volumetric flasks, serological pipets) may be marked TC or TD. TC = “to contain” TD = “to deliver”

TC vs. TD When an instrument marked TC is filled to a particular graduation, it contains that volume. When emptied, it will deliver less than that volume, due to wettability/clinging water. To compensate for this, TD instruments actually contain slightly more than the marked volume, but they deliver the specified amount. This assumes the liquid is water. Can’t use TD for other liquids, because greater or lesser amount might cling (e. g. maple syrup, alcohol).
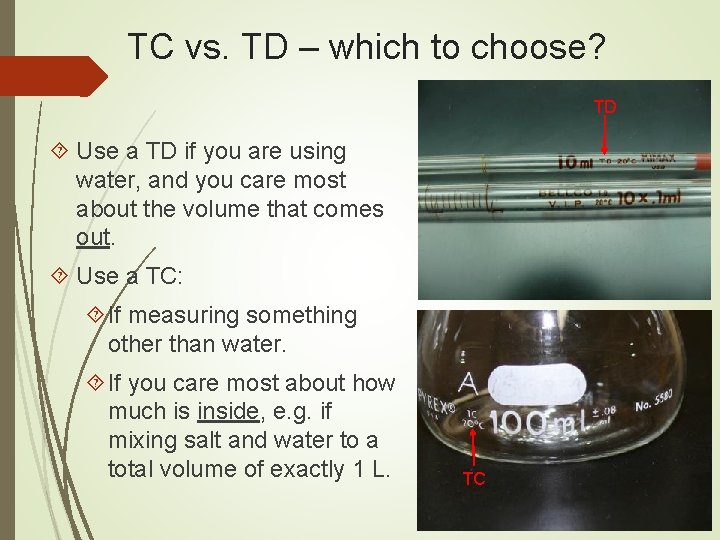
TC vs. TD – which to choose? TD Use a TD if you are using water, and you care most about the volume that comes out. Use a TC: If measuring something other than water. If you care most about how much is inside, e. g. if mixing salt and water to a total volume of exactly 1 L. TC

Pipetting & Reagent Preparation
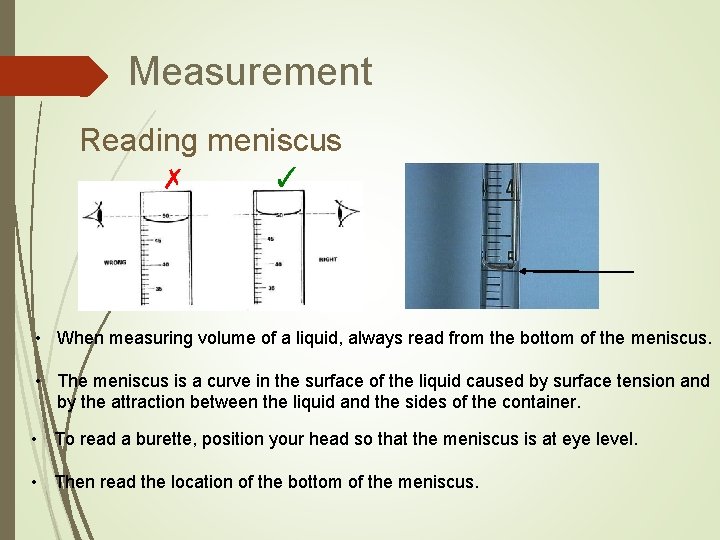
Measurement Reading meniscus ✗ ✓ • When measuring volume of a liquid, always read from the bottom of the meniscus. • The meniscus is a curve in the surface of the liquid caused by surface tension and by the attraction between the liquid and the sides of the container. • To read a burette, position your head so that the meniscus is at eye level. • Then read the location of the bottom of the meniscus.
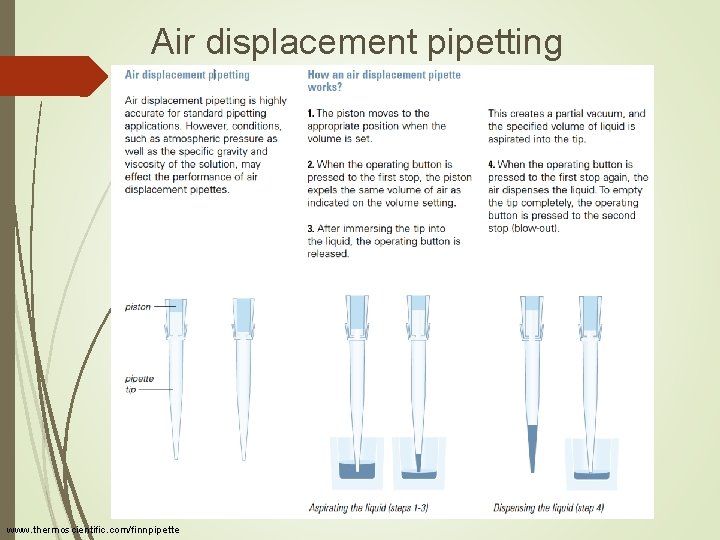
Air displacement pipetting www. thermoscientific. com/finnpipette
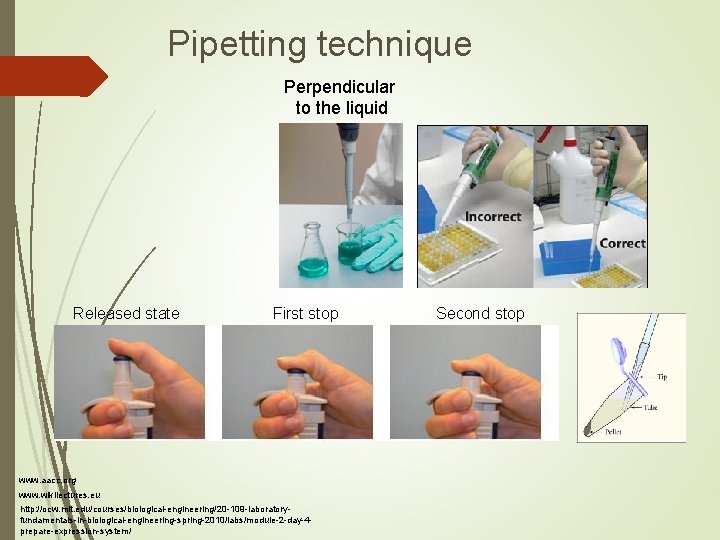
Pipetting technique Perpendicular to the liquid Released state First stop www. aacc. org www. wikilectures. eu http: //ocw. mit. edu/courses/biological-engineering/20 -109 -laboratoryfundamentals-in-biological-engineering-spring-2010/labs/module-2 -day-4 prepare-expression-system/ Second stop
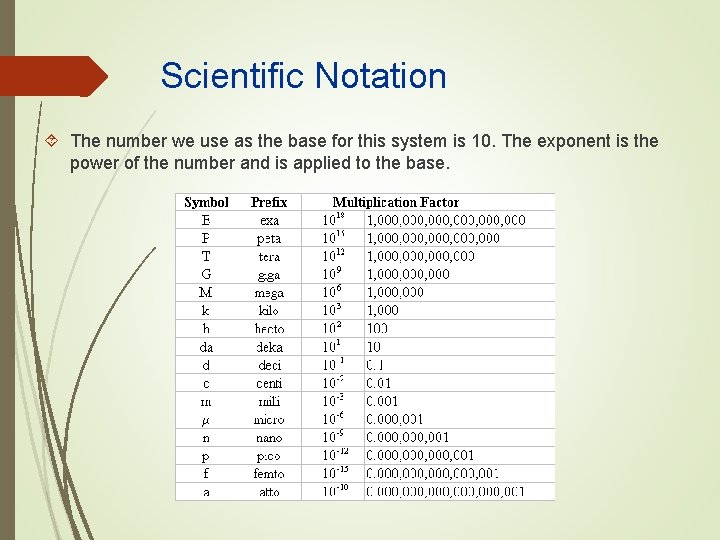
Scientific Notation The number we use as the base for this system is 10. The exponent is the power of the number and is applied to the base.

For example Write the following large number using Scientific Notation : 146, 000, 000. Step 1: place the decimal after the first digit and drop all the zeros. 1. 46 000, 000 - Step 2: count the number of places from the decimal to the end of the number. - There are 11 places after the decimal point; therefore the exponent is 11. 1. 46 X 1011

Solution Preparation C 1 V 1 = C 2 V 2 (Concentration of existing solution) x (Volume of existing solution) = (Desired concentration of new solution) x (Desired volume of new solution)
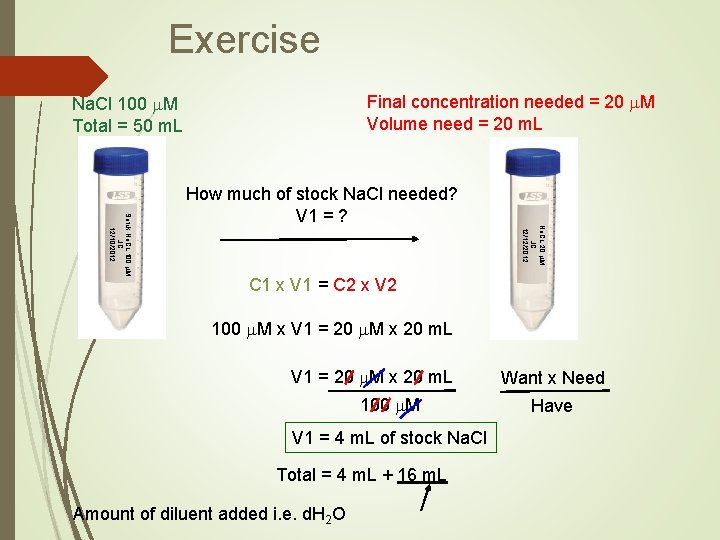
Exercise Final concentration needed = 20 m. M Volume need = 20 m. L Na. Cl 100 m. M Total = 50 m. L Na. CL 20 m. M JC 12/12/2012 Sotck Na. CL 100 m. M JC 12/10/2012 How much of stock Na. Cl needed? V 1 = ? C 1 x V 1 = C 2 x V 2 100 m. M x V 1 = 20 m. M x 20 m. L 100 m. M V 1 = 4 m. L of stock Na. Cl Total = 4 m. L + 16 m. L Amount of diluent added i. e. d. H 2 O Want x Need Have

Percentage Calculation • C 1 V 1 = C 2 V 2 also applied • Dilution of 95% Ethanol to 70% Ethanol, Volume need = 500 m. L • Now you try…

Dilution • Stock 10 x. TBE (Tris-Base EDTA) Make 1 x. TBE, volume = 1 L • C 1 V 1 = C 2 V 2 works too Dilution factor • 1: 1000 in 1000 μL = 1 μL of stock chemical + 999 μL of diluent (i. e. d. H 2 O)

Labeling reagents you prepared!!! • Name of the solution, Concentration, Date, Initials • Make it readable for you and others! If you opened the new stock bottle, WRITE DOWN THE DATE!!

Glassware sterilization www. ehs. unc. edu www. ecu. edu/cs-dhs/. . . /upload/Autoclave-20 Training-20 Final. ppt

Principles of Autoclave Operation • Steam penetrates objects in the autoclave • Condensation creates negative pressure and draws in additional steam • Moist heat kills microorganisms via coagulation of proteins • Two types of autoclaves • Gravity Displacement • Vacuum/Gravity Assisted
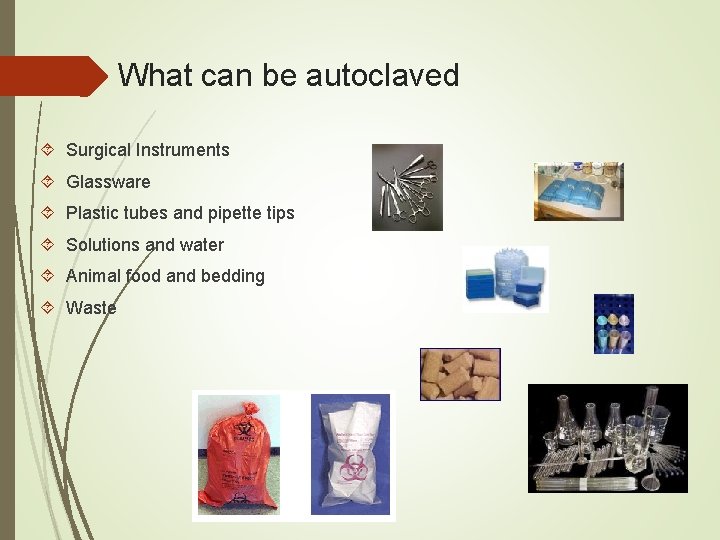
What can be autoclaved Surgical Instruments Glassware Plastic tubes and pipette tips Solutions and water Animal food and bedding Waste

Pre-Autoclaving Procedures Indicator Tape Write down the date you are autoclaving on the tape as well Indicator tape should change color after autoclaving (i. e. the word ‘Autoclaved’ appears on the tape after treatment. ) Anything that has cap on, need to be loosen!!! Before autoclave.

Autoclave Sterilization Procedures. Liquid Materials The autoclave must have a ‘LIQUID’ setting that can be used for liquid materials. The settings for liquids run for a longer period at a lower temperature to minimize liquid evaporation and spills. Liquids should be placed in borosilicate (Kimax or Pyrex) or polypropylene containers for autoclaving and these containers should be filled to no more than 75% capacity.
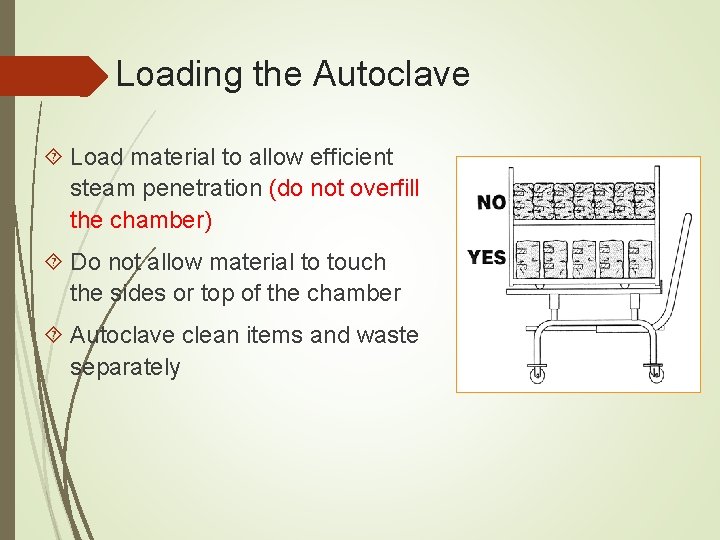
Loading the Autoclave Load material to allow efficient steam penetration (do not overfill the chamber) Do not allow material to touch the sides or top of the chamber Autoclave clean items and waste separately

Operating the Autoclave Be sure the autoclave is functioning properly before use Record information in User Log Check strainer and remove any debris Close door properly and securely Choose the correct conditions for your material Ø Make sure door to autoclave room remains closed as this prevents the loss of negative air pressure, therefore preventing the release of odors.
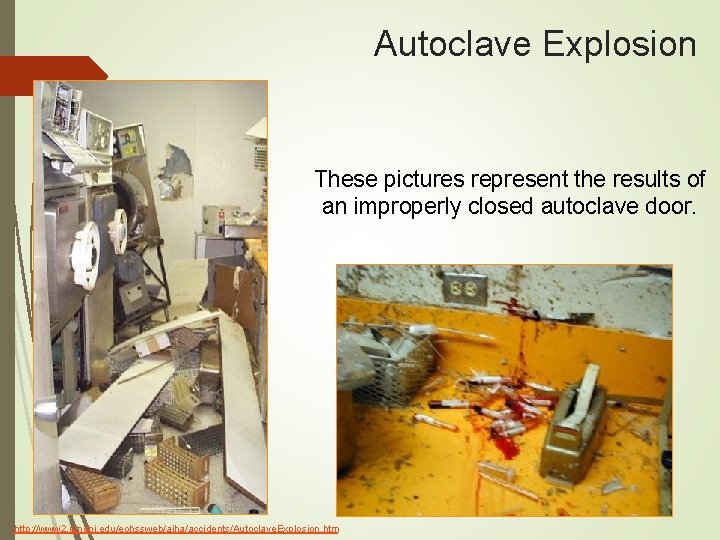
Autoclave Explosion These pictures represent the results of an improperly closed autoclave door. http: //www 2. umdnj. edu/eohssweb/aiha/accidents/Autoclave. Explosion. htm

Results of Improper Autoclave Use Make sure you put everything you autoclave is in the TRAY!!!
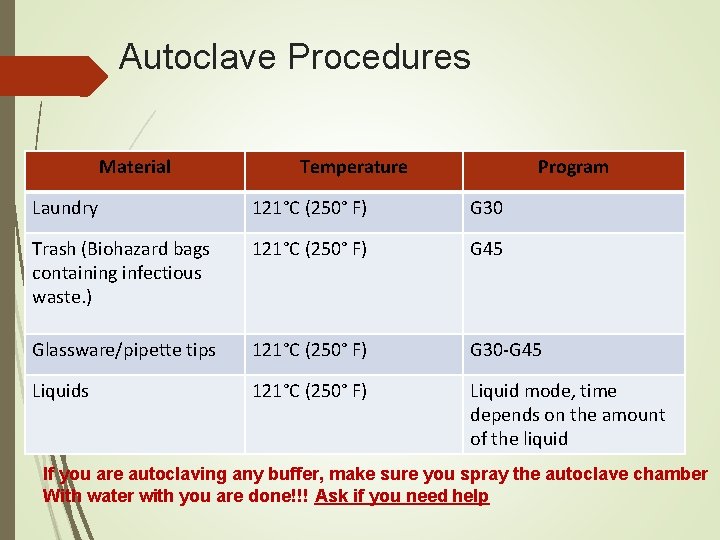
Autoclave Procedures Material Temperature Program Laundry 121°C (250° F) G 30 Trash (Biohazard bags containing infectious waste. ) 121°C (250° F) G 45 Glassware/pipette tips 121°C (250° F) G 30 -G 45 Liquids 121°C (250° F) Liquid mode, time depends on the amount of the liquid If you are autoclaving any buffer, make sure you spray the autoclave chamber With water with you are done!!! Ask if you need help

42 If you have questions, always ask someone. We are here to help.
- Slides: 42