I have the worst cold EVER l Reading









- Slides: 16

I have the worst cold EVER!!! l Reading: l Chapter 5, sections 5, 6, 7 Mastering Chemistry l Nomenclature lists 1, 2, 3, 4 l Quiz Friday l Tutoring l 1

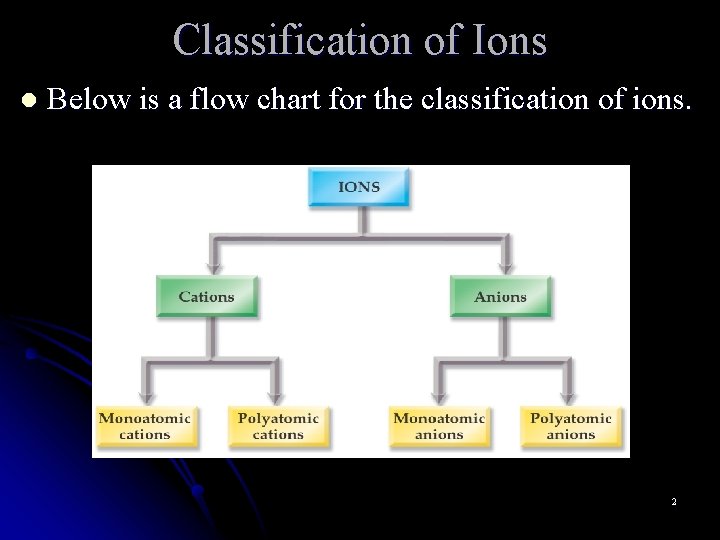
Classification of Ions l Below is a flow chart for the classification of ions. 2

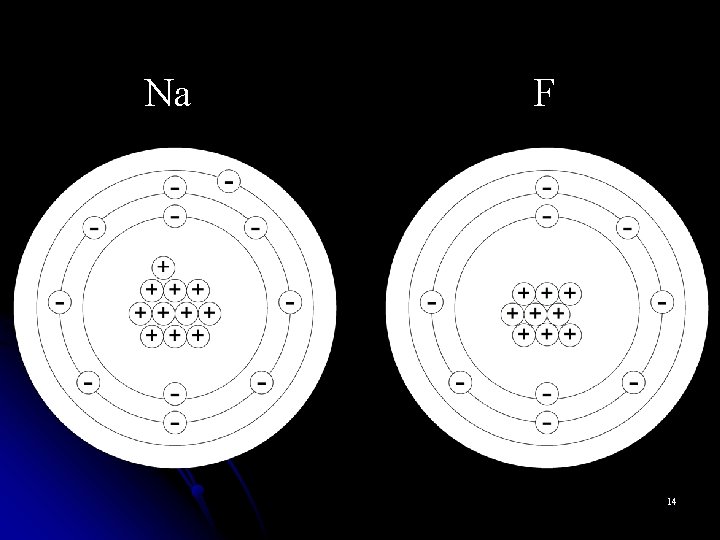
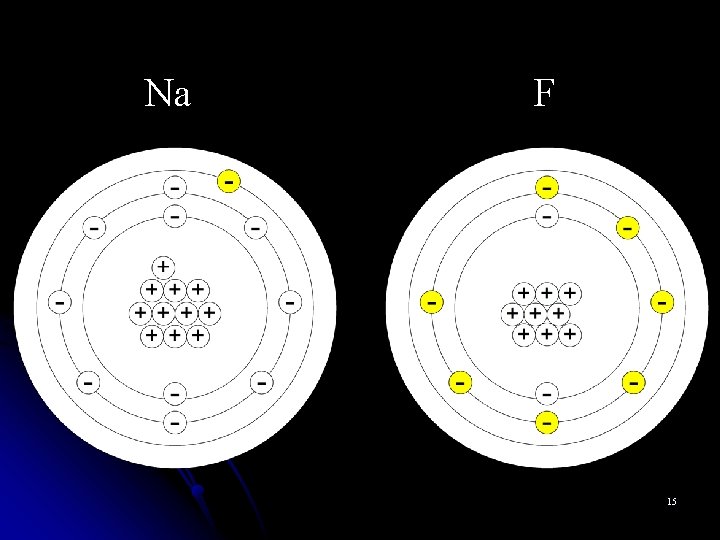
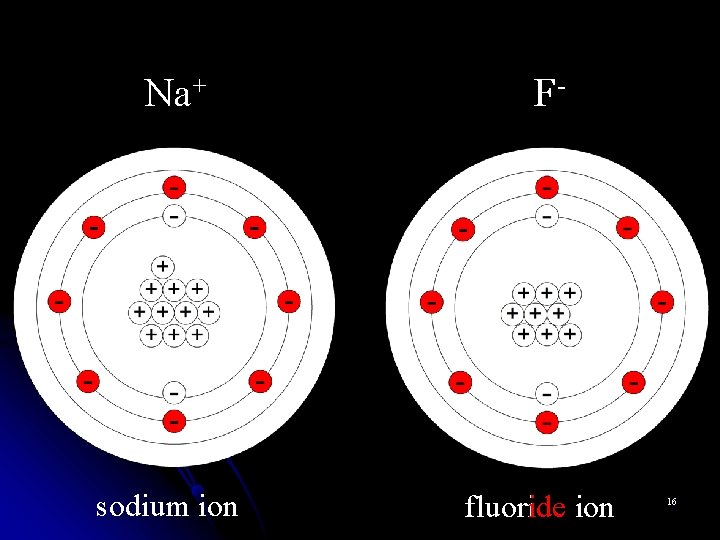
Monoatomic Cations l Metal atoms can lose valence electrons and become positively charged cations. l Cations are named for the parent atom followed by the word “ion. ” l Na+ is named “sodium ion” l Al 3+ is named “aluminum ion” 3

Metals That Form Multiple Ions l If a metal can form more than one cation, it is named for the parent, followed by the charge in Roman numerals in parentheses, followed by the word “ion. ” l Fe 2+ is the iron(II) ion l Fe 3+ is the iron(III) ion 4

Monoatomic Anions l Nonmetals can gain valence electrons and become negatively charged anions. l Monoatomic anions are named by dropping the end of the element name and adding the suffix –ide. l Br- is the bromide ion l O 2 - is the oxide ion l N 3 - is the nitride ion 5

Did you get it? Element Valence e- Ion charge Ion name Noble gas iodine 7 -1 iodide xenon aluminum 3 +3 aluminum ion neon phosphorus 5 -3 phosphide argon barium 2 +2 barium ion xenon 6

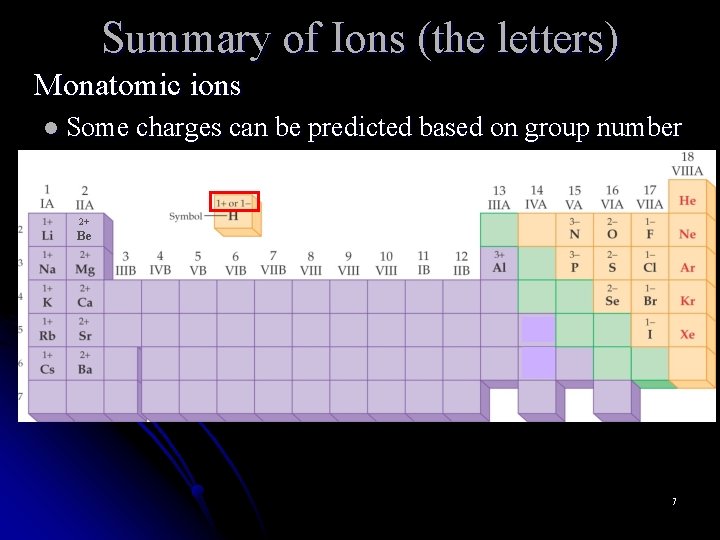
Summary of Ions (the letters) Monatomic ions l Some charges can be predicted based on group number 2+ Be 7

Summary of Ions (the letters) Monatomic ions l Some charges can be predicted based on group number l Cation name is the same as element name with element: zinc (Zn) element: cesium (Cs) ion added ion: zinc ion (Zn 2+) ion: cesium ion (Cs+) l Anion name changes ending of element name to ide element: nitrogen (N) element: iodine (I) ion: nitride (N 3 -) ion: iodide (I-) 8

Summary of Ions (the letters) Polyatomic ion Poly: more than one or many atomic: atoms ion: a positively or negatively charged particle Almost always anions (two exceptions) ammonium -- NH 4+ mercury (I) -- Hg 22+ 9

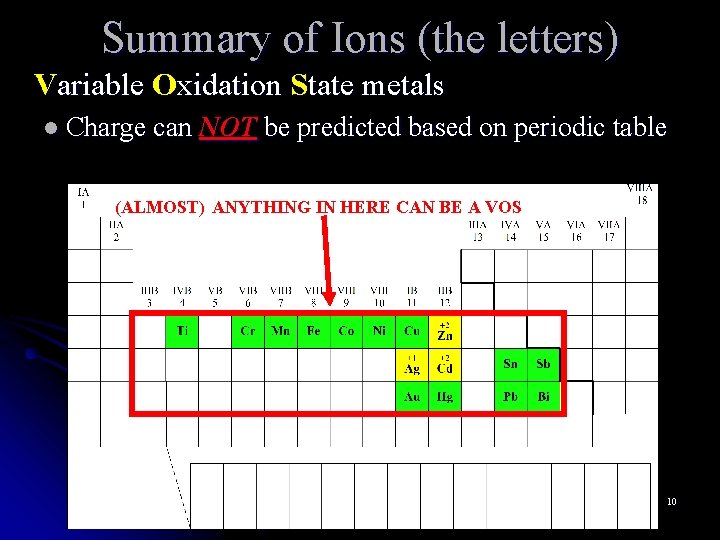
Summary of Ions (the letters) Variable Oxidation State metals l Charge can NOT be predicted based on periodic table (ALMOST) ANYTHING IN HERE CAN BE A VOS 10

Summary of Ions (the letters) Variable Oxidation State metals l Charge can NOT be predicted based on periodic table l Can assume multiple ion charges non VOS metal – potassium ion = K+ lead ion = Pb 2+ or Pb 4+ l The charge of VOS metals MUST be indicated in the name with the use of roman numerals Pb 2+ = lead (II ( ) ion Pb 4+ = lead (IV ( ) ion 11

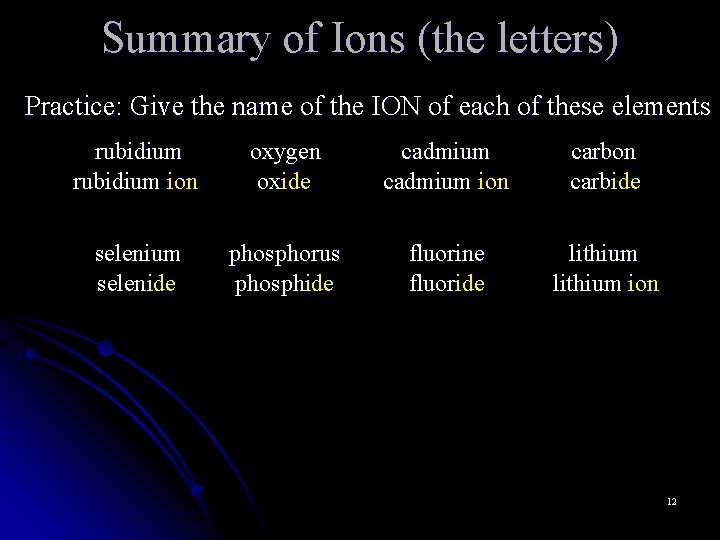
Summary of Ions (the letters) Practice: Give the name of the ION of each of these elements rubidium ion oxygen oxide cadmium ion carbide selenium selenide phosphorus phosphide fluorine fluoride lithium ion 12

Summary of Ions (the letters) Practice: Give the name OR symbol for these ions: tin (II) ion Sn 2+ Mg 2+ magnesium ion S 2 sulfide Fe 3+ iron (III) ion bromide Mn 4+ Brmanganese (IV) ion copper (I) ion Cu+ Ag+ silver ion 13

Na F 14

Na F 15

Na+ F- sodium ion fluoride ion 16