I Geology F Geological Provinces 4 Hot Spots









- Slides: 19

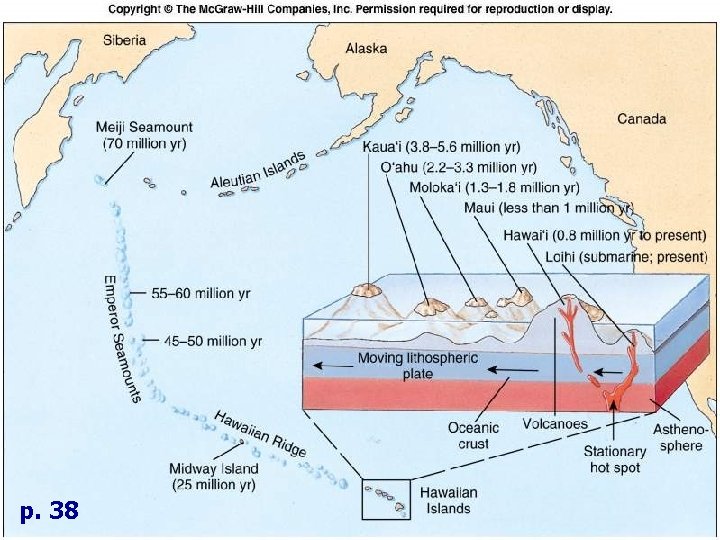
I. Geology F. Geological Provinces 4. Hot Spots • • • Stationary locations where magma rises from mantle • Heat crust, producing volcano Crust moves over mantle • Forms chain of volcanoes, seamounts, islands Hawaiian Islands • Hawaiian Ridge • Emperor Seamounts

p. 38

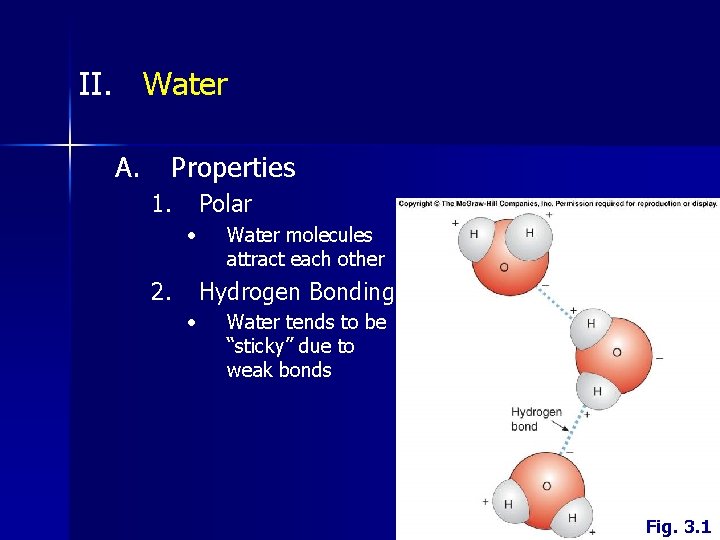
II. Water A. Properties 1. Polar • 2. Water molecules attract each other Hydrogen Bonding • Water tends to be “sticky” due to weak bonds Fig. 3. 1

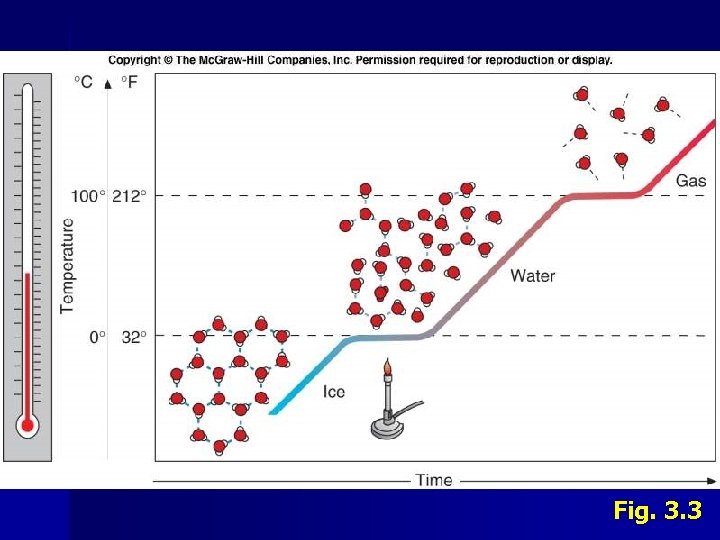
II. Water A. Properties 3. Surface Tension • • • 4. Stickiness at the surface due to cohesion Water surface can support weight of very small organisms Air-water interface supports numerous organisms • Microbes in surface films Viscosity • • 5. Stickiness to objects due to adhesion Objects moving through water drag some water with them Changes in State • • • Fresh water reaches maximum density at 4 o. C Sea water gets denser as it cools Ice is less dense than liquid water

Fig. 3. 3

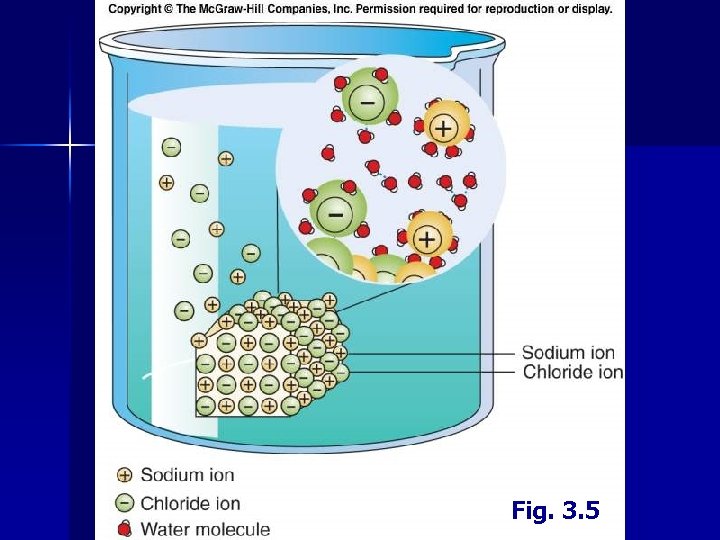
II. Water A. Properties 6. Heat Capacity • • 7. Water requires an unusually large amount of heat to raise its temperature • Energy needed to break hydrogen bonds High latent heat of melting/fusion High latent heat of evaporation (evaporative cooling) Ocean tends to have a stable temperature Solvent Properties • Electrical charges on water (polar molecule) • Help to pull apart molecules held together by electrical charge (salts) • Charged ions dissociate in water

Fig. 3. 5

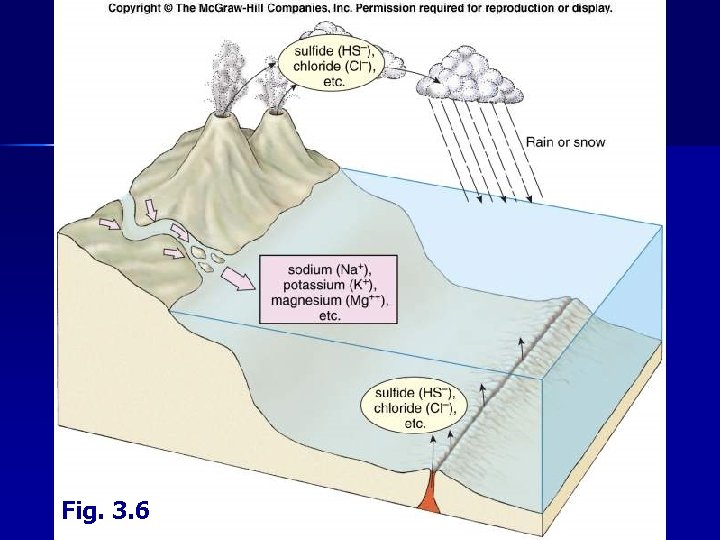
II. Water B. Dissolved Salts • Mostly derived from weathering of rocks on land, hydrothermal vents, volcanic ejecta and precipitation

Fig. 3. 6

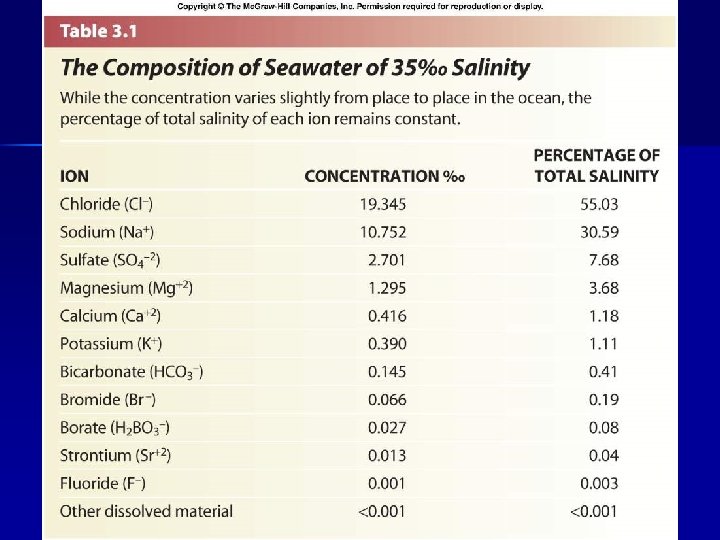
II. Water B. Dissolved Salts 1. • Constituents Salinity = salt content • • • Usually expressed in ‰ (g per kg) “Average” sea water ~34. 7 ‰ • 33 -37 ‰ in open ocean • higher in enclosed seas • lower in areas with extensive river runoff Fresh water < 0. 5 ‰ Brackish water – 0. 5 -17 ‰ Six ions make up 98% of total dissolved solids in sea water Most major constituents used by organisms


II. Water B. Dissolved Salts 1. • Constituents Rule of Constant Proportions • • • Salinity may change from place to place, but proportions of major ions remain constant Makes it easier for marine organisms to regulate their physiology Salinity affected by • • • Evaporation Precipitation Ice formation

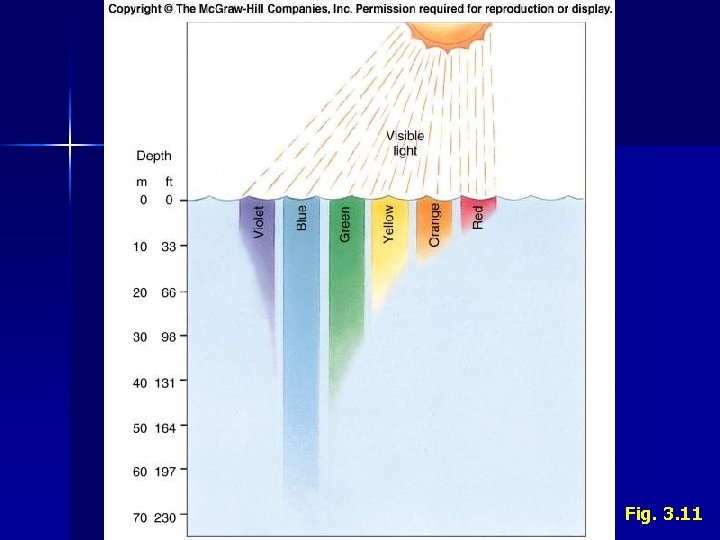
II. Water C. 1. 2. 3. Properties • • • Density Determined by salinity and temperature • Temperature more influential than salinity Increases with depth where water column is stable Dissolved Gases Most important • O 2 – Used for respiration, produced by photosynthesis • CO 2 – Used for photosynthesis, produced by respiration • N 2 – Used as a nutrient in metabolism Exchanged between ocean and atmosphere (gas exchange) More soluble at lower temperatures Concentrations impacted by biological activity Transparency Penetration of light Affects • Photosynthesis • Vision • Behavior Spectral attenuation

Secchi Disk Fig. 3. 12

Fig. 3. 11

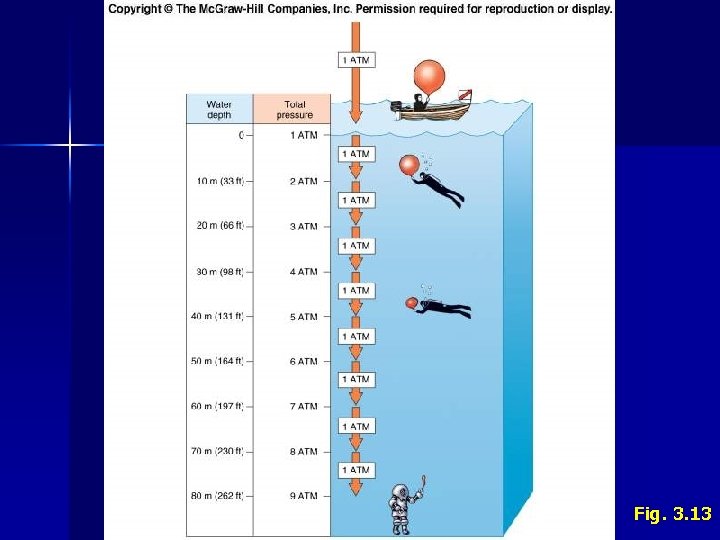
Fig. 3. 14 II. Water C. Properties 1. 2. 3. 4. Density Dissolved Gases Transparency Pressure • • • Changes predictably with depth • 10 m = 1 atmosphere (14. 7 psi) Affects gas-filled structures Constrains vertical movements of many animals

Fig. 3. 13

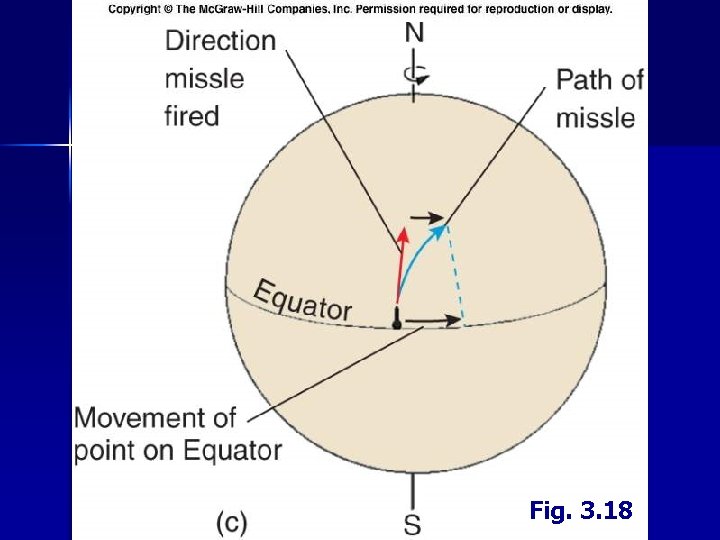
III. Circulation A. Coriolis Effect • • Affects motion of winds, currents and tides Caused by rotation of earth N. Hemisphere – Deflects motion to right S. Hemisphere – Deflects motion to left

Fig. 3. 18