I Chemistry A Elements and Atoms 1 Elements












- Slides: 27

I. Chemistry

A. Elements and Atoms 1. Elements- Substance which cannot be broken down into a simpler substance A) 96% of all life is Carbon, Hydrogen, oxygen and nitrogen B) 3. 2% Calcium, Phosphorous, Potassium and Sulfur

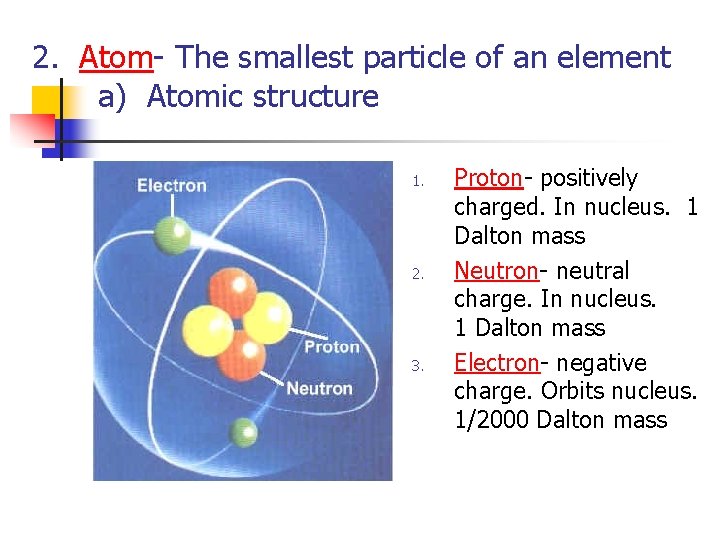
2. Atom- The smallest particle of an element a) Atomic structure 1. 2. 3. Proton- positively charged. In nucleus. 1 Dalton mass Neutron- neutral charge. In nucleus. 1 Dalton mass Electron- negative charge. Orbits nucleus. 1/2000 Dalton mass

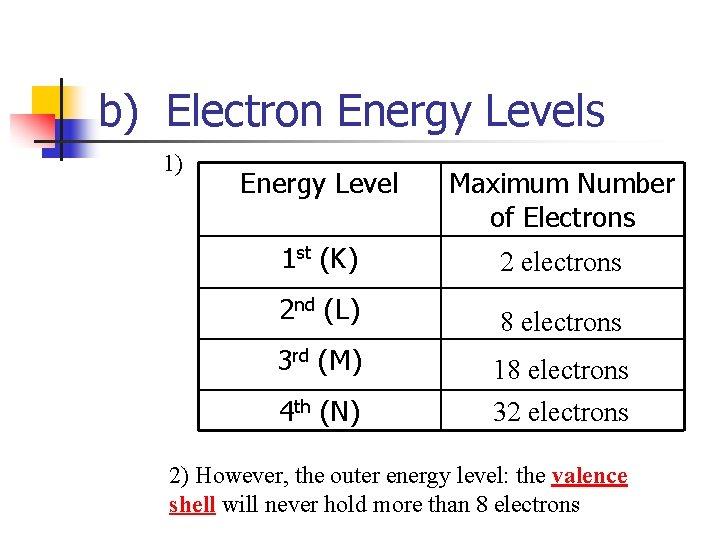
b) Electron Energy Levels 1) Energy Level Maximum Number of Electrons 1 st (K) 2 electrons 2 nd (L) 8 electrons 3 rd (M) 4 th (N) 18 electrons 32 electrons 2) However, the outer energy level: the valence shell will never hold more than 8 electrons

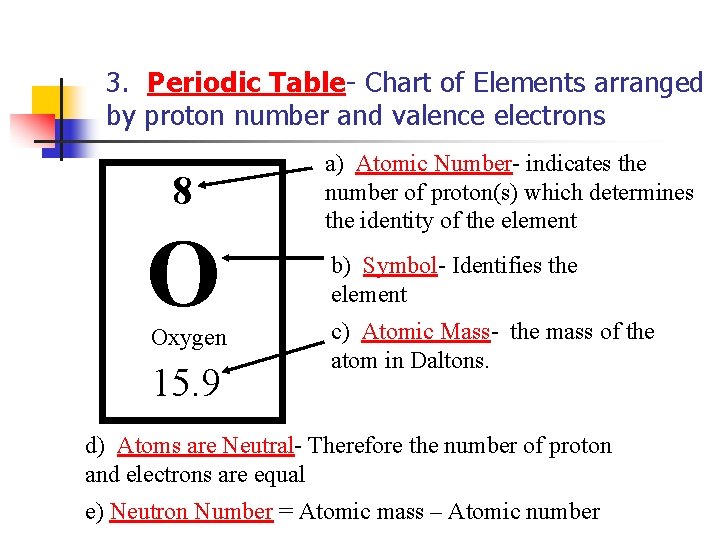
3. Periodic Table- Chart of Elements arranged by proton number and valence electrons 8 O Oxygen 15. 9 a) Atomic Number- indicates the number of proton(s) which determines the identity of the element b) Symbol- Identifies the element c) Atomic Mass- the mass of the atom in Daltons. d) Atoms are Neutral- Therefore the number of proton and electrons are equal e) Neutron Number = Atomic mass – Atomic number

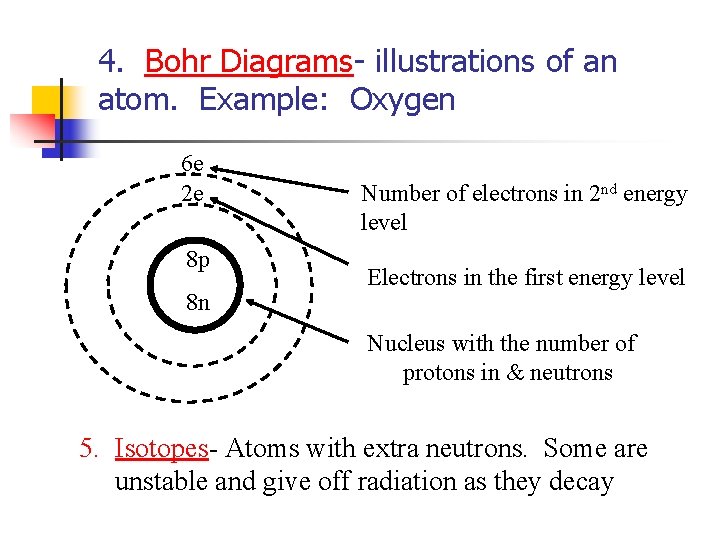
4. Bohr Diagrams- illustrations of an atom. Example: Oxygen 6 e 2 e 8 p 8 n Number of electrons in 2 nd energy level Electrons in the first energy level Nucleus with the number of protons in & neutrons 5. Isotopes- Atoms with extra neutrons. Some are unstable and give off radiation as they decay

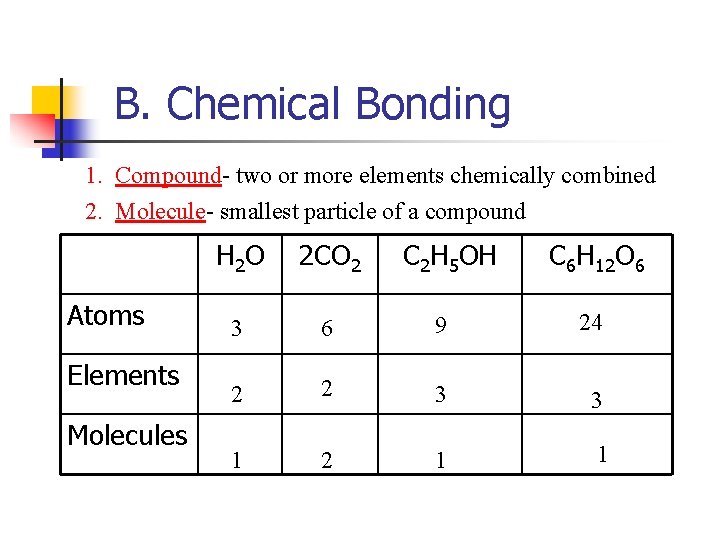
B. Chemical Bonding 1. Compound- two or more elements chemically combined 2. Molecule- smallest particle of a compound Atoms Elements Molecules H 2 O 2 CO 2 C 2 H 5 OH C 6 H 12 O 6 3 6 9 24 2 2 3 3 1 2 1 1

What Determines Whether Atoms Bond? 3. 4. Atoms with their outer energy level filled are stable. He, Ne, Ar, Kr, Xe, Rn (Noble Gasses). Keep in mind that helium’s first energy level is its outer energy level and therefore requires only two electrons in its valence shell for stability Unstable atoms will share or transfer electrons to become stable

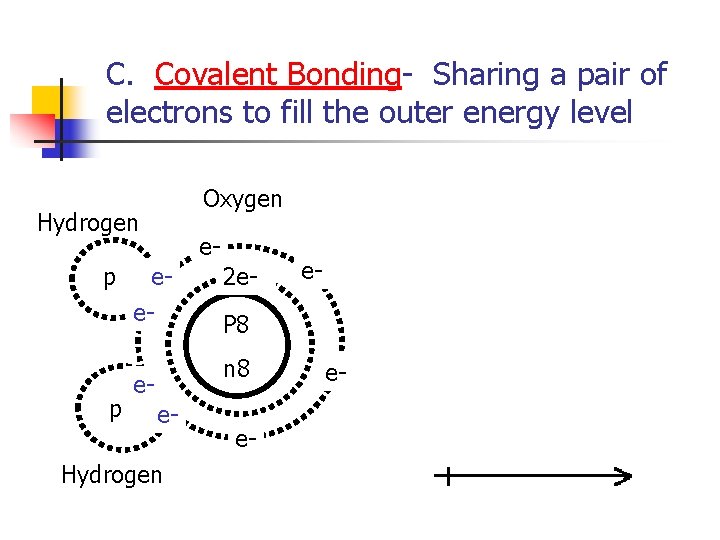
C. Covalent Bonding- Sharing a pair of electrons to fill the outer energy level Oxygen Hydrogen p p e- ee- 2 e. P 8 n 8 ee- Hydrogen e- e- e-

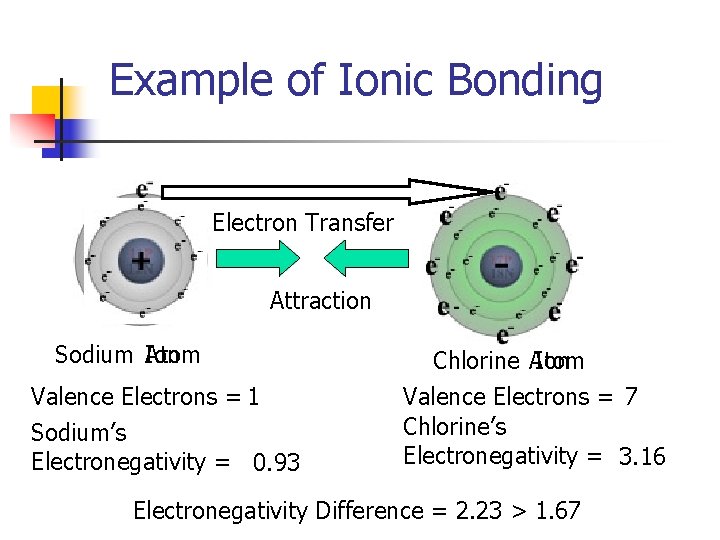
Example of Ionic Bonding Electron Transfer Attraction Sodium Ion Atom Valence Electrons = 1 Sodium’s Electronegativity = 0. 93 Chlorine Atom Ion Valence Electrons = 7 Chlorine’s Electronegativity = 3. 16 Electronegativity Difference = 2. 23 > 1. 67

D. Ionic Bonding- The transfer of electrons from one element to another to fill the outer energy level 1. 2. Electronegativity- a measure of the attraction of electrons to an atom. When atoms with high electrogenativities are placed with atoms with low electronegativities (the difference is greater than 1. 67), Ionic bonding occurs a) b) The transfer of the electron from one atom to another causes the formation of particles called ions Ions with opposite charges attract

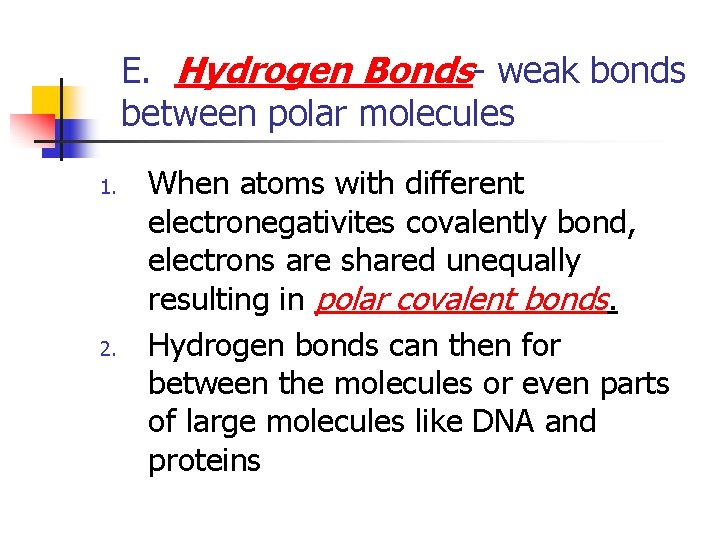
E. Hydrogen Bonds- weak bonds between polar molecules 1. 2. When atoms with different electronegativites covalently bond, electrons are shared unequally resulting in polar covalent bonds. Hydrogen bonds can then for between the molecules or even parts of large molecules like DNA and proteins
“Many have lived a life without love, not one without water. ”

F. Water’s Importance to Life 1. 2. All living things contain a large amount of water. Ex. Humans are 60 -65% water Living things are essentially water solutions A) B) C) Solution- a solute dissolved in a solvent Concentration- the amount of solute in the solvent Solutions are mixtures. The solute and the solvent do not chemically combine

3. The Properties of Water

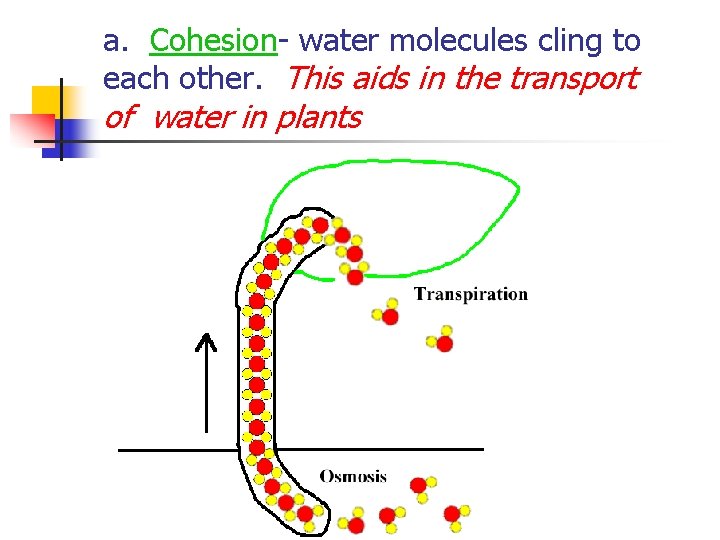
a. Cohesion- water molecules cling to each other. This aids in the transport of water in plants

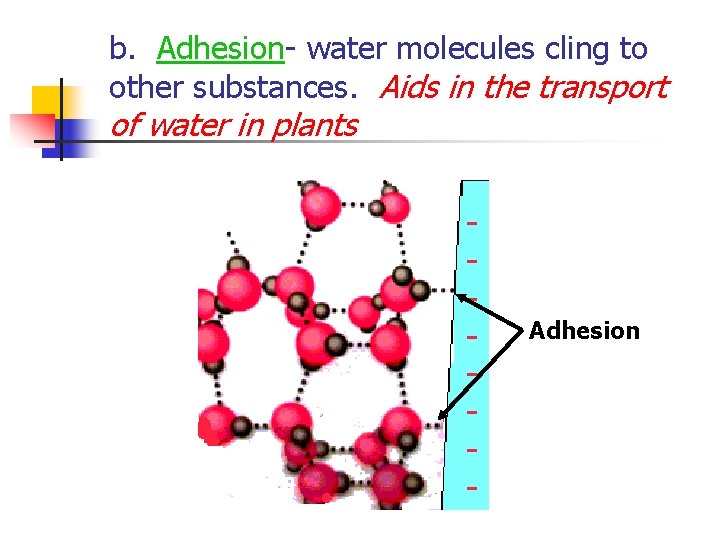
b. Adhesion- water molecules cling to other substances. Aids in the transport of water in plants Adhesion

c. High Heat Capacity- water resists changes in temperature. Moderates global temperatures

d. Water Expands when it Freezes- ice is less dense than liquid water. Insulates water in winter keeping it in liquid form

e. Water is an excellent solvent most substances dissolve in water. Allows for nutrient transportation in organisms

f. Water is clear- this allows light to penetrate to aquatic plants

G. Acids, Bases and p. H 1. 2. 3. Acids- release H+ ions (hydrogen ions) in solution Base- release OH- ions (Hydroxide ion) in solution p. H Scale- measure of the H+ ions in a solution

Acids, Bases and p. H 1. 2. 3. 4. 5. Dissociation of water molecules (H 2 O H+ + OH-) is a rare occurrence. Only one molecule of water is disassociated per 554 million water molecules. Thus, at 25 o. C, the number of hydrogen ions (H+) and hydroxide ions (OH-) is equal at 10 -7 M. In any solution, the sum of the OH- ions and H+ ions is 10 -14 The concentrations of hydrogen ions and hydroxide ions are exactly inverse! Increase one and you will decrease the other. The p. H scale is based on the power of 10

You Try a Problem If an acid was added to water to increase the hydrogen ion concentration to 10 -5, what would be the hydroxide ion concentration? What would be the p. H? If base was added to a solution until the hydroxide ion concentration was 10 -3, what would be the hydrogen ion concentration? What would be the p. H? If the p. H of a solution is 6, what is the hydrogen ion concentration? What is the hydroxide ion concentration?

H. Chemical Reactions- breaking of existing bonds and the formation of new ones 1. 2. Activation Energy- energy needed to start a reaction. (heat, stirring, electric. ) Atoms cannot be created or destroyed in a chemical reaction, they are only rearranged into different molecules

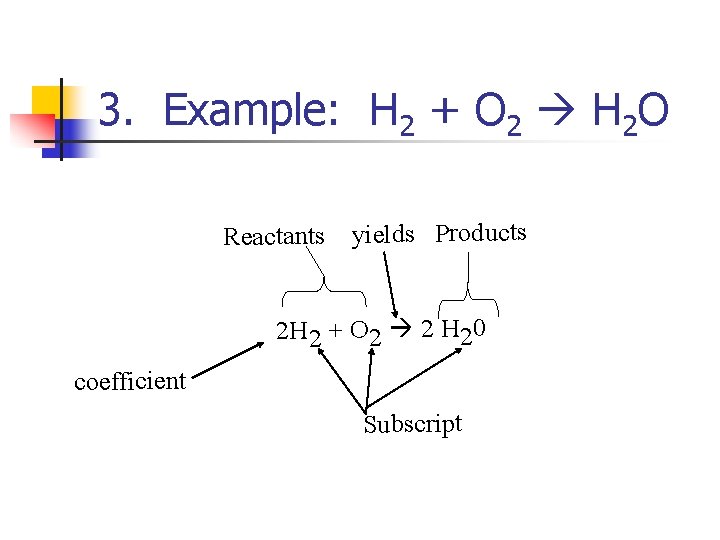
3. Example: H 2 + O 2 H 2 O Reactants yields Products 2 H 2 + O 2 2 H 20 coefficient Subscript

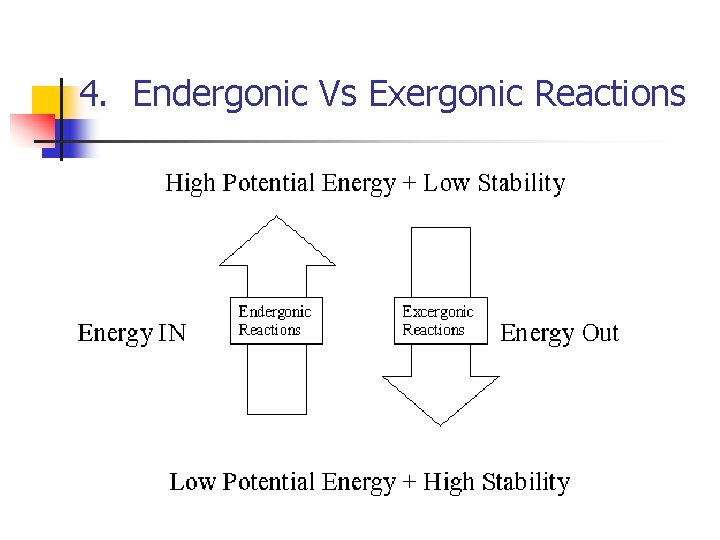
4. Endergonic Vs Exergonic Reactions