Hypoplastic left heart syndrome and the Rastelli procedure



- Slides: 24

Hypoplastic left heart syndrome and the Rastelli procedure Catherine Kim-Gavino, PGY 4 Loyola University Medical Center

Admixture Lesions • Mixing of R & L circulations across a large VSD, ASD, PDA • Admixed blood flows to pulmonary circulation • Cyanotic with increased PBF

HLHS (Aortic Atresia) • Complex combination of cardiac malformations (8%) • Most common cause cardiac death 1 st week of life • 4 th most common cardiac malformation within 1 st year of life § Behind VSD, d-TGA, TOF • M: F 2: 1 • Invariably lethal if left untreated § 25% early cardiac deaths in neonate

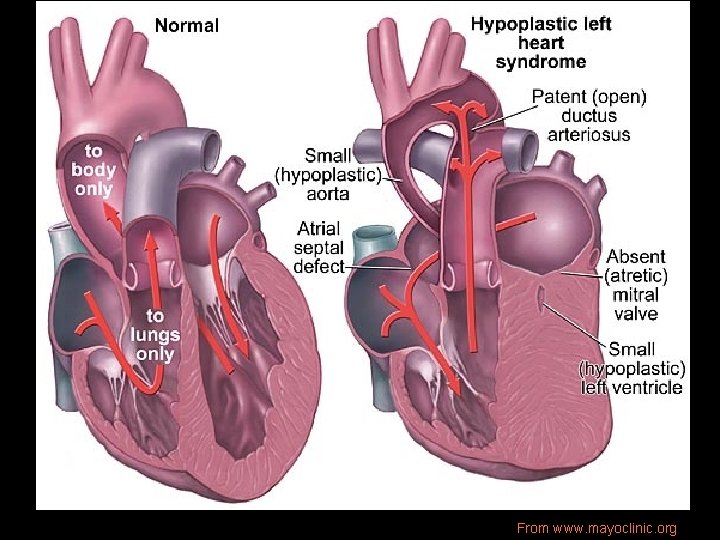
Pathophysiology • Hypoplastic/absent LV, small sinuses of Valsalva, small Ao annulus/ascending Ao, LA, § 25% have a small MV or atresia • L-sided outflow tract obstruction • Variable severity • Systemic perfusion of aorta entirely thru PDA@ pulmonary pressures • Occasional ASD or VSD

From www. mayoclinic. org

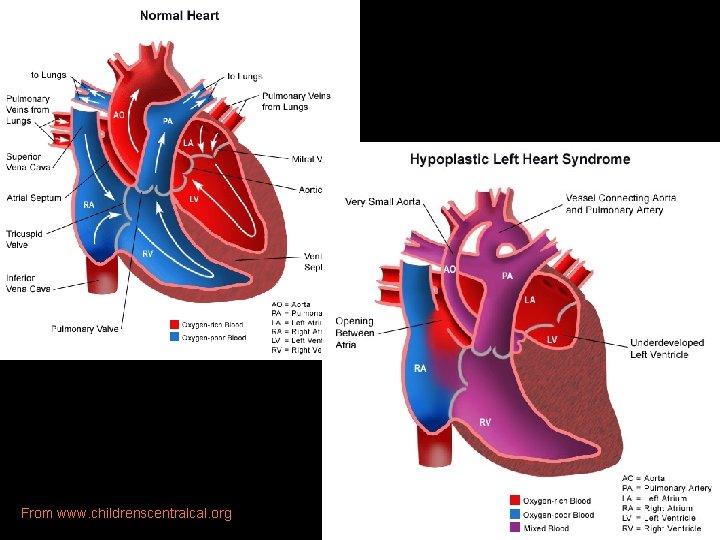
From www. childrenscentralcal. org

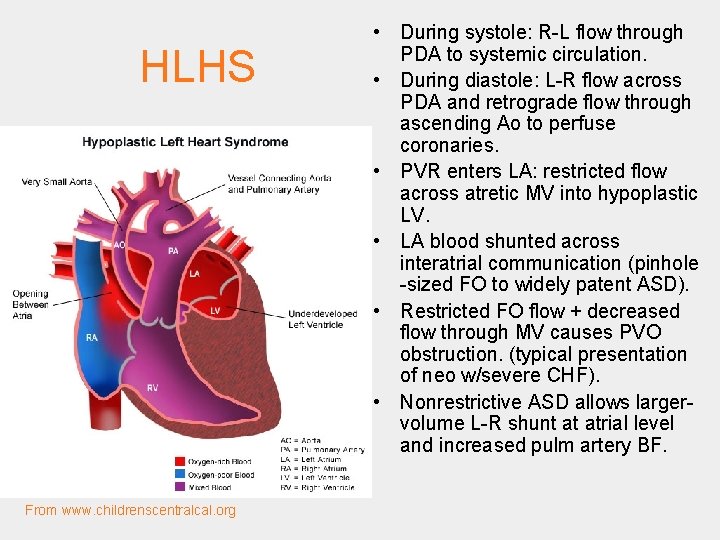
HLHS From www. childrenscentralcal. org • During systole: R-L flow through PDA to systemic circulation. • During diastole: L-R flow across PDA and retrograde flow through ascending Ao to perfuse coronaries. • PVR enters LA: restricted flow across atretic MV into hypoplastic LV. • LA blood shunted across interatrial communication (pinhole -sized FO to widely patent ASD). • Restricted FO flow + decreased flow through MV causes PVO obstruction. (typical presentation of neo w/severe CHF). • Nonrestrictive ASD allows largervolume L-R shunt at atrial level and increased pulm artery BF.

HLHS • Pulmonary, systemic, coronary circulation depends on RV • Rt-sided HF occurs rapidly, esp as ductus is closing (24 -48 hrs) § Dusky infant in failure in first 48 hrs of life = HLHS § Maintain PDA patency with IV PGE 1.

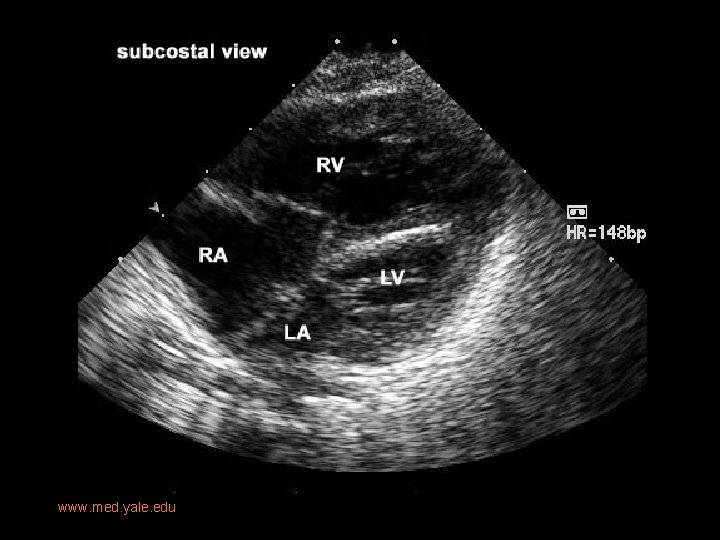
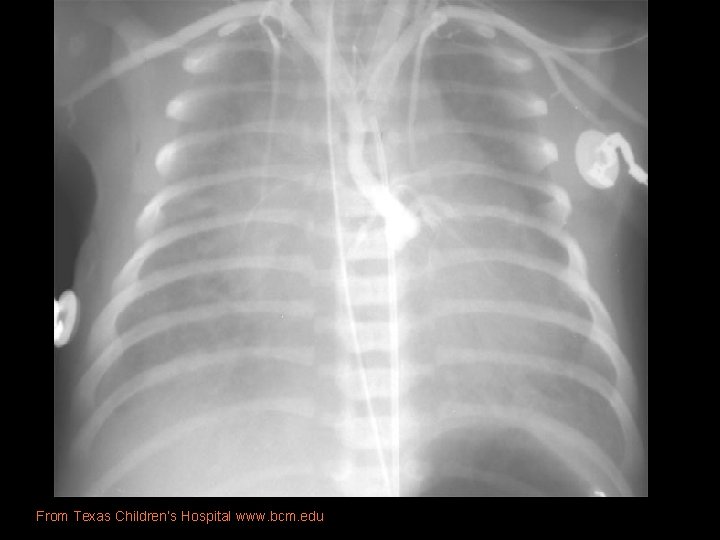
Imaging • Prenatal US: diastolic flow reversal in extremely narrow ascending Ao is diagnostic for HLHS. • Real-time echo: § sm, thick walled LV, sm MV w/restricted motion § Dilated RH/PA, lg PDA § Sm ascending Ao • CXR: § Moderate-marked CM § Globular cardiac silhouette suggesting combined chamber enlargement § PV congestion with interstitial/pleural fluid

www. med. yale. edu

From Texas Children’s Hospital www. bcm. edu

Prognosis • CHF and death --> MI secondary to restrictive coronary flow or poor AV mixing. • Universally fatal with 80% patient dying within the first week. • Heart transplantation or staged Norwood procedure alternative forms of therapy.

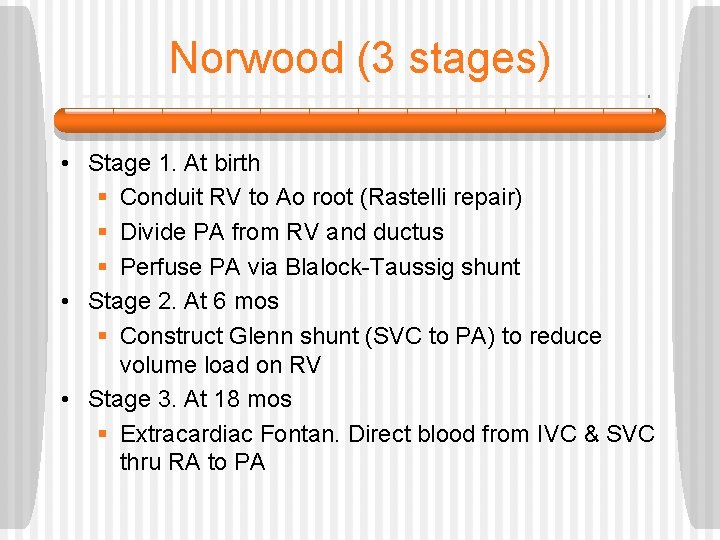
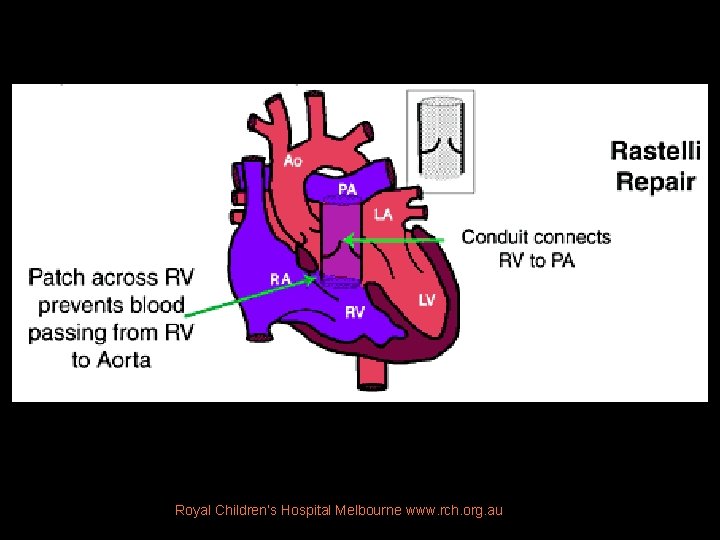
Norwood (3 stages) • Stage 1. At birth § Conduit RV to Ao root (Rastelli repair) § Divide PA from RV and ductus § Perfuse PA via Blalock-Taussig shunt • Stage 2. At 6 mos § Construct Glenn shunt (SVC to PA) to reduce volume load on RV • Stage 3. At 18 mos § Extracardiac Fontan. Direct blood from IVC & SVC thru RA to PA

But wait…. • Not all patients undergo this 3 stage palliative procedure. • If LV still functional, both ventricles can participate in cardiac circulation

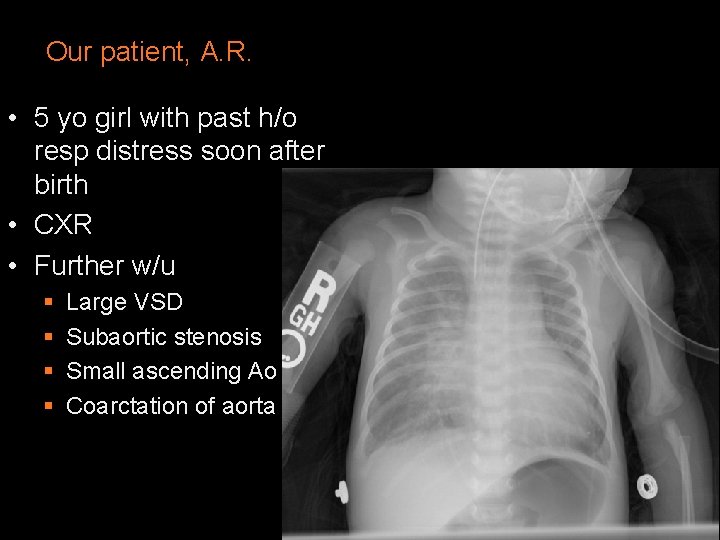
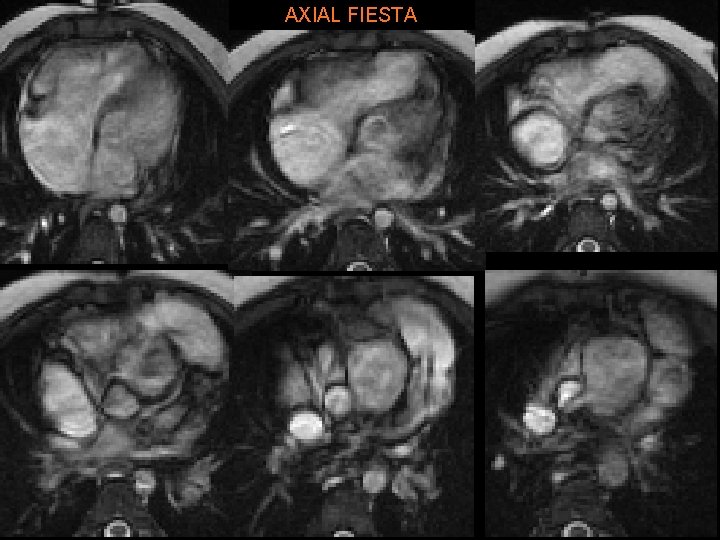
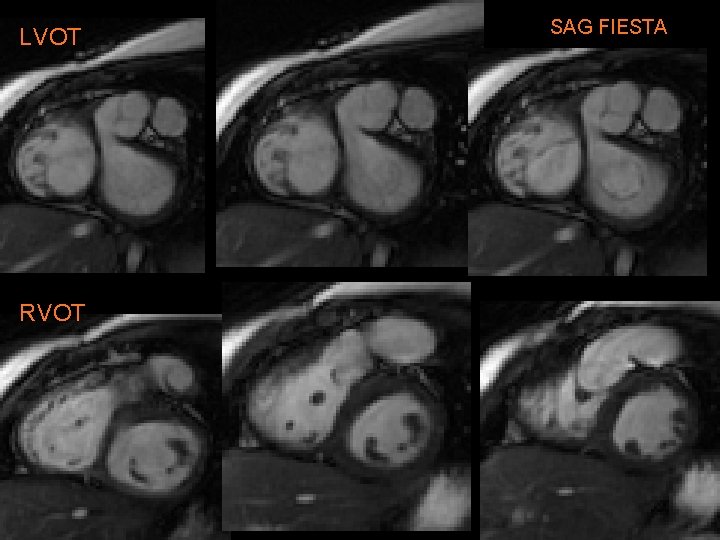
Our patient, A. R. • 5 yo girl with past h/o resp distress soon after birth • CXR • Further w/u § § Large VSD Subaortic stenosis Small ascending Ao Coarctation of aorta

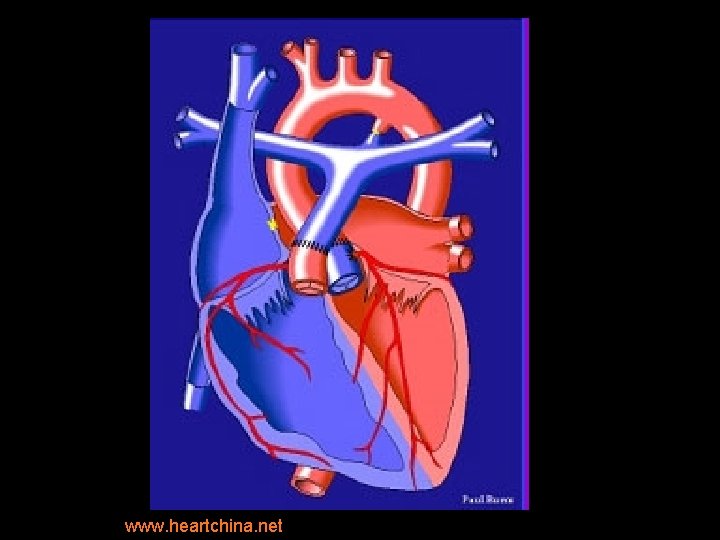
Rastelli surgery • Intracardiac conduit/baffle: § establishes continuity b/w LV and Ao § performed usu in DORV, forms of transposition (particularly w/VSD and subpulmonic stenosis). • Extracardiac conduit: § b/w RV and PAs during repair of TOF w/pulmonary atresia, TA, DORV. § Either a homograft AV w/segment of ascend Ao or a Dacron conduit with porcine AV is used.

Royal Children’s Hospital Melbourne www. rch. org. au

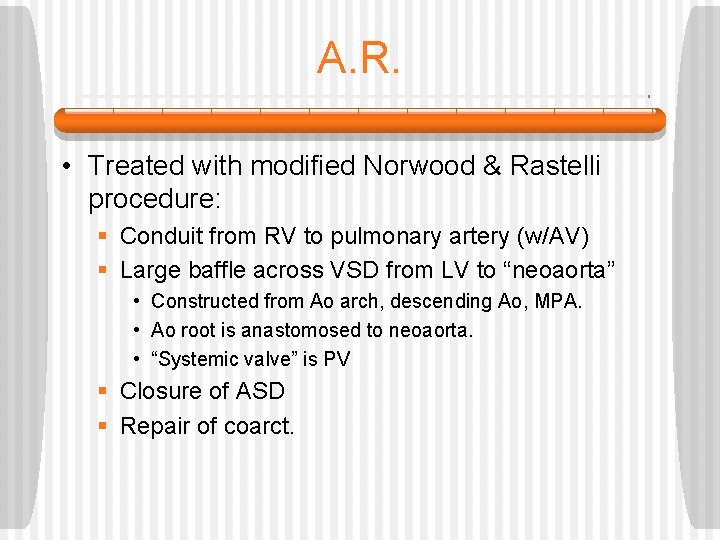
A. R. • Treated with modified Norwood & Rastelli procedure: § Conduit from RV to pulmonary artery (w/AV) § Large baffle across VSD from LV to “neoaorta” • Constructed from Ao arch, descending Ao, MPA. • Ao root is anastomosed to neoaorta. • “Systemic valve” is PV § Closure of ASD § Repair of coarct.

www. heartchina. net

AXIAL FIESTA

LVOT RVOT SAG FIESTA

Complications • Obstruction b/w LV & Ao (VSD level) or other anastomotic sites • Incorrect placement of intracardiac baffle. § If VSD must be enlarged, may get complete HB, necessitating pacemaker. • Calcification of conduit/prosthetic valve ( conduit obstruction). • Obstruction/narrowing within conduit (neointimal fibrotic peel), thrombosis, periconduit abscess. • Pseudoaneurysms at anastomotic sites.

The End This is how I’m feeling, post-call

References • Kersting-Sommerhoffi BA, Seelos KC, Hardy C, Kondo C, Higgins SS, Higgins CB. The Evaluation of Surgical Procedures for Cyanotic Congenital Heart Disease by Using MR Imaging. AJR 1990; 155: 259 -266, • Bardo DME, Frankel DG, Applegate KE, Murphy DJ, Saneto RP. Hypoplastic Left Heart Syndrome. Radio. Graphics 2001; 21: 705717 • Lonergan GL. Congenital Heart Disease. “Radiologic Pathology, ” from the Armed Forces Institute of Pathology. Vol 3. 2006 -2007. • Kuhn JP, Slovis TL, Haller JO. Caffey’s Pediatric Diagnostic Imaging. 10 th ed. Mosby, Philadelphia. 2004