Hydrophobic Rocks By Manuel Soto Andrew Derrick Purpose

Hydrophobic Rocks By Manuel Soto & Andrew Derrick

Purpose There are tons of sites around the world affected by the acid rain, and even lowering the amount of SO 3 produced won’t completely get rid of acid rain being made, it will just be slower. It may be possible to use hydrophobic spray to prevent corrosion for long periods of time. Buildings like the Taj Mahal, and the Pantheon are being corroded by acid rain, and causing the buildings to become brittle and soft.

Background Research ● Hydrophobicity is how much an object repels water. You can tell how high the hydrophobicity is by the angle of a water drop on the surface of an object. ● SO 3 & SO 2 are air pollutants produced by factories, and small amounts react with evaporated water molecules in the air, making sulphuric acid. This reaction is H 2 SO 4 + Ca. CO 3 → Ca. SO 4 + H 2 CO 3.

Background Research ● Plenty of buildings across the world are made from marble or other rocks that react with sulphuric acid. ● The two biggest rocks affected by acid rain are marble(reacts to make gypsum, a soft & brittle material), and limestone(reacts to make water and carbon dioxide). ● Water molecules are polar, meaning they have a side that’s more positive or negative than the other.

How Hydrophobic Spray Works Hydrophobicity is caused by the complex architecture of the surface. The smaller this pattern is, the more hydrophobic it is. Since water molecules are polar, they are attracted to other polar substances. Hydrophobic spray is non-polar, so water does not stick to it. Architecture of hydrophobic coating

How does hydrophobic coating affect the rate of reaction between sulphuric acid and limestone? Hypothesis: We hypothesize that the treated rocks will be more resistant towards the reaction of sulphuric acid between limestone, because it repels liquids and lowers the amount of contact.

Experimental Design Independent variable: Treated or untreated rocks Dependant variable: Weight loss in rocks Constants: Concentration of acid, amount of acid, size of beaker, rock type, & temperature. Materials: - 10 chunks of limestone - Sulphuric acid - Hydrophobic spray(Never-Wet) - scale - 10 beakers - tongs - wax paper - rubber bands

Procedure 1) Make a 1. 7 m. L/L of water solution of H 2 SO 4. 2) Gather 10 chunks of limestone & 10 beakers. 3) Treat 5 chunks of limestone with hydrophobic spray. 4) Separate the 1 L solution of H 2 SO 4 into 10 beakers evenly. 5) Label each beaker, 5 as untreated, 5 as treated(number them).

Procedure 6) Measure weight & record data of all 10 rocks before putting in solution of H 2 SO 4. 7) Put each rock in designated beaker of H 2 SO 4 solution. 8) Use rubber bands & wax paper to cover each beaker to prevent evaporation of H 2 SO 4 solution. 9) Measure the weight of each rock every day for 1 week & record data.
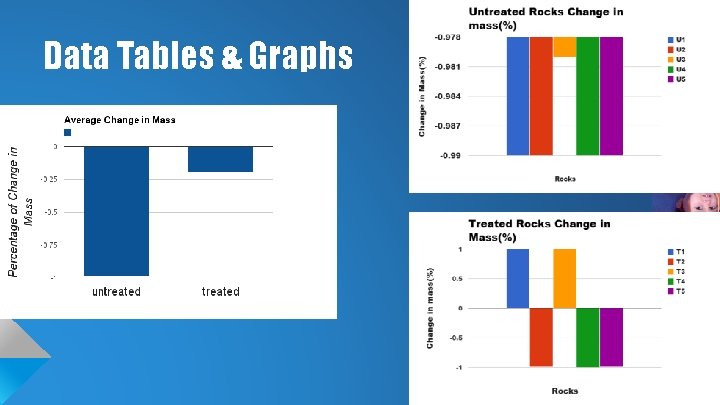
Data Tables & Graphs

Conclusion We found that the treated rocks lost less weight than the untreated rocks, and this may be a useful method to preserving limestone structures. Our hypothesis was correct, and the rocks sprayed with Never-Wet lost less weight than the rocks that weren’t treated. Although we couldn’t get trials for days 4, 5 & 6, we believe our data is sufficient enough to say that our methods worked.

Future Work We wish to take more trials over a long period of time, with more types of hydrophobic coating. getting more trials in with different types of spray may show more promising results.

Sources Elmhurst. edu (2003). Limestone Neutralization. Acid Rain Effects: acid rain effects. Retrieved from http: //elmhurst. edu/~chm/vchembook/196 soil. html Watson, J. (1997, July 21). The effects of acid rain on buildings. How Does Acid Precipitation Affect Marble and Limestone Buildings? Retrieved from http: //pubs. usgs. gov/gip/acidrain/5. html Diep, F. (2013, June 19). Super-hydrophobic Spray. Super-Hydrophobic Spray Makes All Your Stuff Liquid-Proof. Retrieved from http: //www. popsci. com/science/article/2013 -06/super-hydrophobic-spraymakes-all-your-stuff-liquid-proof Mink, J. (2009, January 9). 5 places around the world affected by acid rain. Top 5 Endangered Heritage Sites - Acid Rain. Retrieved from http: //archive. cyark. org/top-5 -endangered-heritage-sitesacid-rain-blog
- Slides: 13