Hydrolysis of salts The concept of hydrolysis Mechanism





- Slides: 12

Hydrolysis of salts. The concept of hydrolysis. Mechanism of hydrolysis of cations, anions and compatible hydrolysis. Hydrolysis of salts as an equilibrium process: the degree and constant of hydrolysis and the factors that determine their value. Equilibrium shift of protolytic reactions. The role of protolytic reactions in drug metabolism, in the analysis of drugs, technology of their manufacture and storage. Lecture 8

Hydrolysis of salts. Hydrolysis of salt is the interaction of salt ions with water ions, which leads to the formation of a weak electrolyte and changes in the p. H of the medium. Conditions under which salt is subject to hydrolysis: 1. Salt is soluble in water; 2. The salt is formed either by a cation of a weak base, or an anion of a weak acid, or both at the same time. Water-soluble salts, which include cations of weak bases, or anions of weak acids, or both, are hydrolyzed. These ions bind to water ions H + or OH- with the formation of a weak electrolyte, resulting in a violation of the balance of electrolytic dissociation of water H 2 O ⇄ H + + OHH + or OH- ions accumulate in the solution, giving it an acidic or alkaline reaction. Salts formed with a strong base and a strong acid (Na. Cl, Na. NO 3, K 2 SO 4, Ba. Cl 2, Li. NO 3) were not hydrolyzed. In this case, neither the cation nor the anion of the salt will NOT bind water ions into poorly dissociated products, so the equilibrium of water dissociation is not disturbed. The reaction medium in solutions of such salts is neutral, p. H ~ 7

Types of hydrolysis • There are three types of hydrolysis: • 1. Hydrolysis by anion • 2. Hydrolysis of the cation • 3. Hydrolysis by anion and cation.

Hydrolysis by anion • Hydrolysis by anion occurs in solutions of salts consisting of anions of weak acids and cations of strong bases (CH 3 COOK, KNО 2, Na 2 CO 3, Na 3 PO 4). In this case, the weak acid anion binds to H + water ions to form a weak electrolyte. • As an example, consider the hydrolysis of potassium nitrite KNO 2. This salt is formed by a strong KOH base and a weak HNO 2 acid. When dissolved in water, KNO 2 completely dissociates into K + and NO 2 - ions. K + cations cannot bind OH-water ions, as KOH is a strong electrolyte. NO 2 - anions bind H + ions of water, as a result of which molecules of weak acid HNO 2 and hydroxide ions OH- appear in the solution.

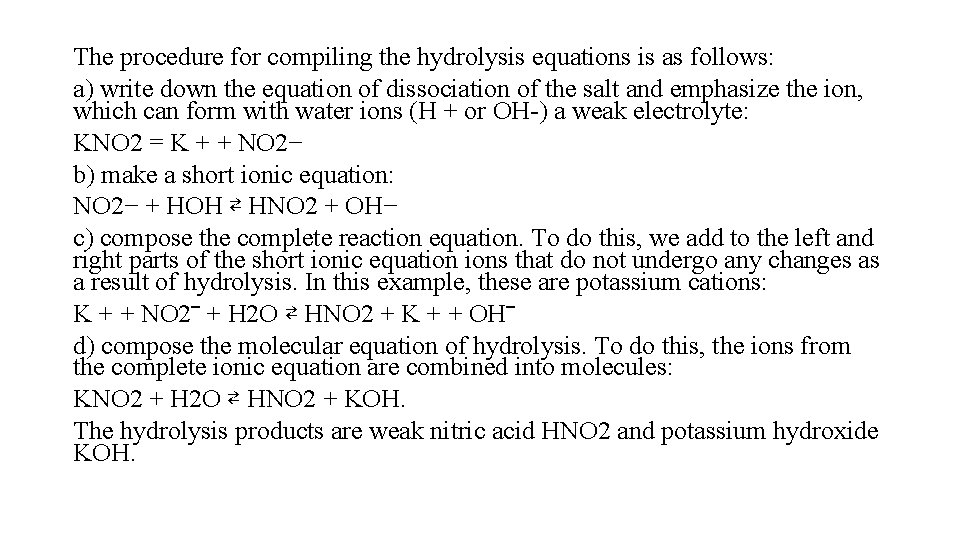
The procedure for compiling the hydrolysis equations is as follows: a) write down the equation of dissociation of the salt and emphasize the ion, which can form with water ions (H + or OH-) a weak electrolyte: KNO 2 = K + + NO 2− b) make a short ionic equation: NO 2− + НОН ⇄ HNO 2 + OH− c) compose the complete reaction equation. To do this, we add to the left and right parts of the short ionic equation ions that do not undergo any changes as a result of hydrolysis. In this example, these are potassium cations: K + + NO 2‾ + H 2 O ⇄ HNO 2 + K + + OH‾ d) compose the molecular equation of hydrolysis. To do this, the ions from the complete ionic equation are combined into molecules: KNO 2 + H 2 O ⇄ HNO 2 + KOH. The hydrolysis products are weak nitric acid HNO 2 and potassium hydroxide KOH.

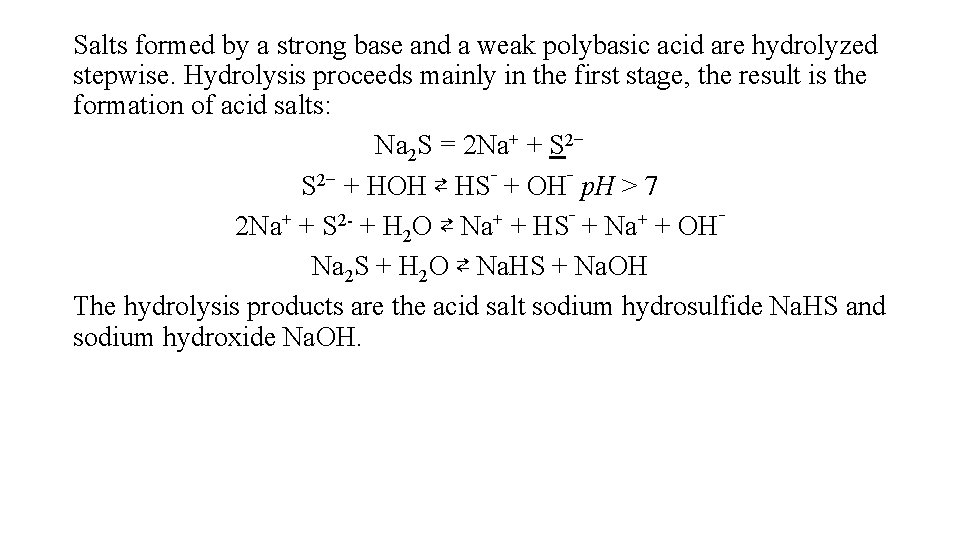
Salts formed by a strong base and a weak polybasic acid are hydrolyzed stepwise. Hydrolysis proceeds mainly in the first stage, the result is the formation of acid salts: Na 2 S = 2 Na+ + S 2− + НOН ⇄ HS‾ + OH‾ p. H > 7 2 Na+ + S 2 - + H 2 O ⇄ Na+ + HS‾ + Na+ + OH‾ Na 2 S + H 2 O ⇄ Na. HS + Na. OH The hydrolysis products are the acid salt sodium hydrosulfide Na. HS and sodium hydroxide Na. OH.

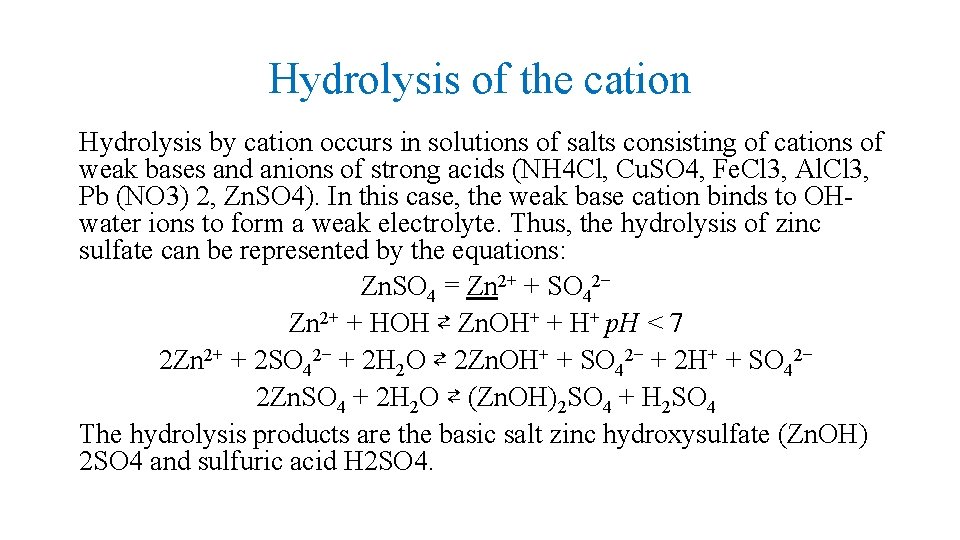
Hydrolysis of the cation Hydrolysis by cation occurs in solutions of salts consisting of cations of weak bases and anions of strong acids (NH 4 Cl, Cu. SO 4, Fe. Cl 3, Al. Cl 3, Pb (NO 3) 2, Zn. SO 4). In this case, the weak base cation binds to OHwater ions to form a weak electrolyte. Thus, the hydrolysis of zinc sulfate can be represented by the equations: Zn. SO 4 = Zn 2+ + SO 42− Zn 2+ + HOН ⇄ Zn. OH+ + H+ р. Н < 7 2 Zn 2+ + 2 SO 42− + 2 H 2 O ⇄ 2 Zn. OH+ + SO 42− + 2 H+ + SO 42− 2 Zn. SO 4 + 2 H 2 O ⇄ (Zn. OH)2 SO 4 + H 2 SO 4 The hydrolysis products are the basic salt zinc hydroxysulfate (Zn. OH) 2 SO 4 and sulfuric acid H 2 SO 4.

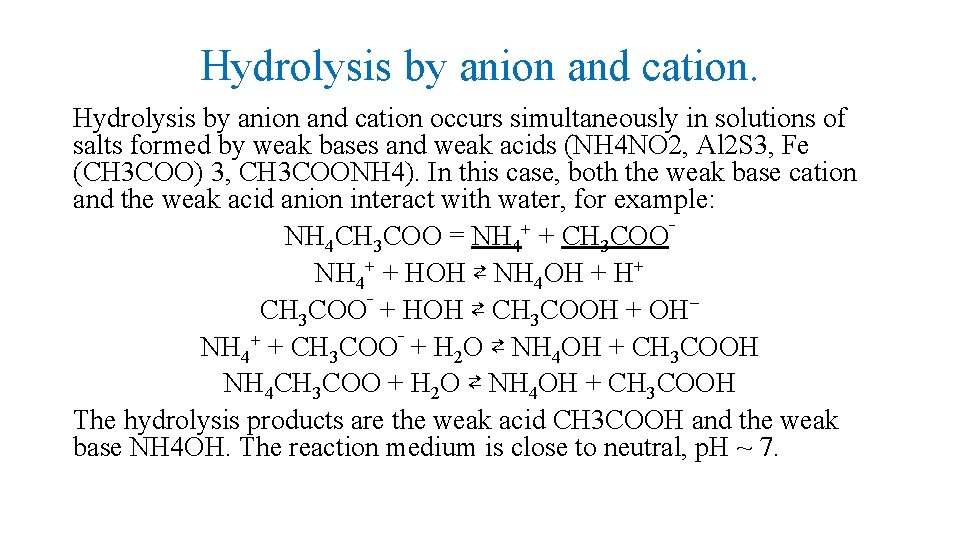
Hydrolysis by anion and cation occurs simultaneously in solutions of salts formed by weak bases and weak acids (NH 4 NO 2, Al 2 S 3, Fe (CH 3 COO) 3, CH 3 COONH 4). In this case, both the weak base cation and the weak acid anion interact with water, for example: NH 4 CH 3 COO = NH 4+ + CH 3 COO‾ NH 4+ + HOН ⇄ NH 4 OH + H+ CH 3 COO‾ + HOН ⇄ CH 3 COOH + ОН− NH 4+ + CH 3 COO‾ + H 2 O ⇄ NH 4 OH + CH 3 COOH NH 4 CH 3 COO + H 2 O ⇄ NH 4 OH + CH 3 COOH The hydrolysis products are the weak acid CH 3 COOH and the weak base NH 4 OH. The reaction medium is close to neutral, p. H ~ 7.

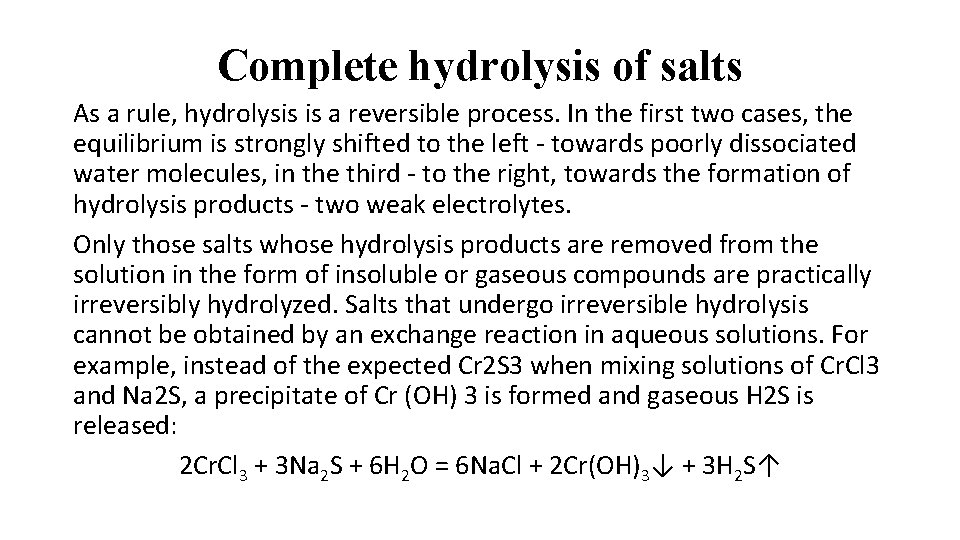
Complete hydrolysis of salts As a rule, hydrolysis is a reversible process. In the first two cases, the equilibrium is strongly shifted to the left - towards poorly dissociated water molecules, in the third - to the right, towards the formation of hydrolysis products - two weak electrolytes. Only those salts whose hydrolysis products are removed from the solution in the form of insoluble or gaseous compounds are practically irreversibly hydrolyzed. Salts that undergo irreversible hydrolysis cannot be obtained by an exchange reaction in aqueous solutions. For example, instead of the expected Cr 2 S 3 when mixing solutions of Cr. Cl 3 and Na 2 S, a precipitate of Cr (OH) 3 is formed and gaseous H 2 S is released: 2 Cr. Cl 3 + 3 Na 2 S + 6 H 2 O = 6 Na. Cl + 2 Cr(OH)3↓ + 3 H 2 S↑

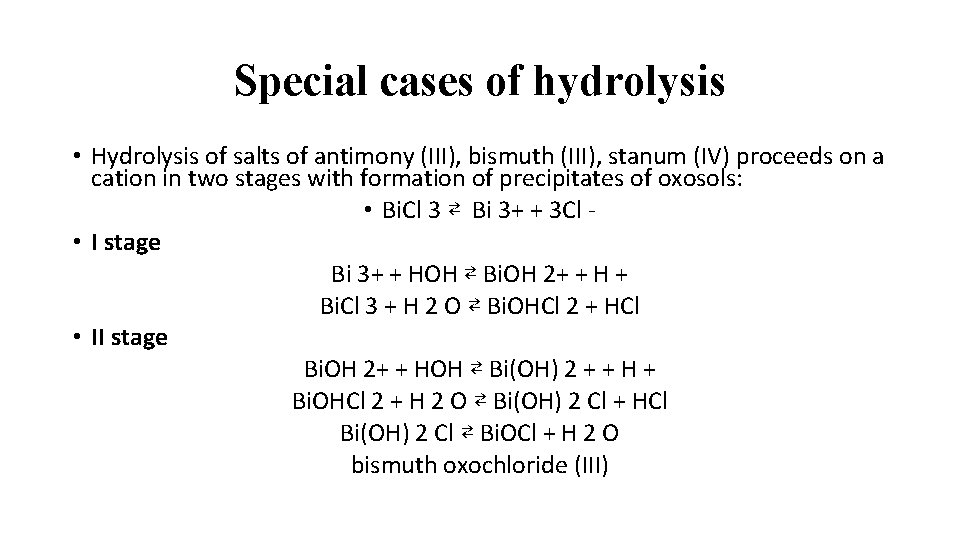
Special cases of hydrolysis • Hydrolysis of salts of antimony (III), bismuth (III), stanum (IV) proceeds on a cation in two stages with formation of precipitates of oxosols: • Bi. Cl 3 ⇄ Bi 3+ + 3 Cl • I stage Bi 3+ + HOH ⇄ Bi. OH 2+ + H + Bi. Cl 3 + H 2 O ⇄ Bi. OHCl 2 + HCl • II stage Bi. OH 2+ + HOH ⇄ Bi(OH) 2 + + H + Bi. OHCl 2 + H 2 O ⇄ Bi(OH) 2 Cl + HCl Bi(OH) 2 Cl ⇄ Bi. OCl + H 2 O bismuth oxochloride (III)

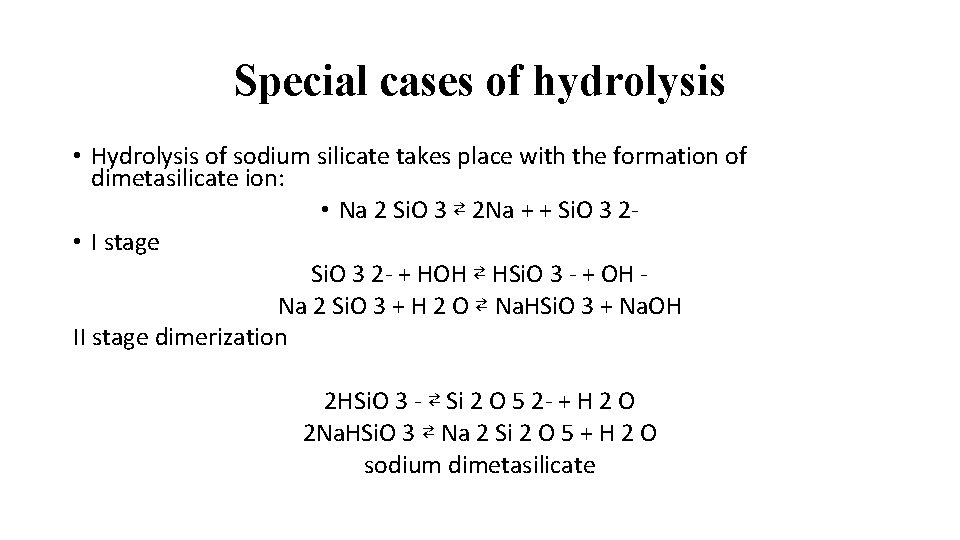
Special cases of hydrolysis • Hydrolysis of sodium silicate takes place with the formation of dimetasilicate ion: • Na 2 Si. O 3 ⇄ 2 Na + + Si. O 3 2 • I stage Si. O 3 2 - + HOH ⇄ HSi. O 3 - + OH Na 2 Si. O 3 + H 2 O ⇄ Na. HSi. O 3 + Na. OH II stage dimerization 2 HSi. O 3 - ⇄ Si 2 O 5 2 - + H 2 O 2 Na. HSi. O 3 ⇄ Na 2 Si 2 O 5 + H 2 O sodium dimetasilicate

Equilibrium of hydrolysis • The balance of hydrolysis is influenced by temperature and concentration. The equilibrium shift of hydrolysis occurs according to the principle of Le Chatelier. Hydrolysis is a reaction inverse to neutralization, and neutralization is an exothermic process, so hydrolysis is endothermic. Therefore, increasing the temperature enhances the hydrolysis, ie shifts the equilibrium to the right. At a constant temperature, the hydrolysis equilibrium can be shifted to the right and thus increase the hydrolysis by diluting the solution with water and removing the hydrolysis products. Hydrolysis slows down (equilibrium shifts to the left) if you increase the concentration of hydrolysis products.