Hydrogen Ions and Acidity Hydrogen Ions from Water






- Slides: 12

Hydrogen Ions and Acidity

Hydrogen Ions from Water • Water molecules are highly polar and are in constant motion. • Occasionally these collisions result in reactions. • A water molecule that gains a hydrogen ion becomes a hydronium ion. (H 3 O+) • A water molecule that loses a hydrogen ion becomes a hydroxide ion. (OH-)

Self Ionization • The reaction in which water molecules produce ions is called the self ionization of water. • H 2 O(l) H+ + OH • In pure water the concentration of hydrogen ions is only 1 x 10 -7 M. • The concentration of OH- is also 1 x 10 -7 M

Neutral Solution • Any aqueous solution in which H+ and OH- are equal is a neutral solution.

Ion Product Constant for Water • The ionization for water is a reversible reaction, so Le Chatelier’s principle applies. • Adding either hydrogen ions or hydroxide ions to an aqueous solution is a stress to the system. • The equilibrium will shift toward to formation of water. • When [H+] increases [OH-] decreases and vice versa.

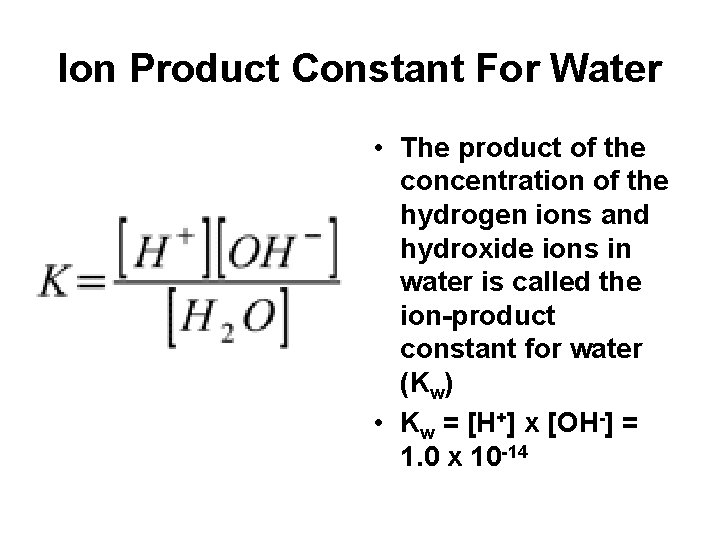
Ion Product Constant For Water • The product of the concentration of the hydrogen ions and hydroxide ions in water is called the ion-product constant for water (Kw) • Kw = [H+] x [OH-] = 1. 0 x 10 -14

Acidic Solutions • HCl H+ + Cl • In hydrochloric acid, the hydrogen-ion concentration is greater than the hydroxide-ion concentration. • A solution in which [H+] is greater that [OH-] is an acidic solution. • The H+ is greater than 1 x 10 -7 M

Basic Solution • Na. OH Na+ + OH • When sodium hydroxide dissolves in water there are more OH- ions. • A basic solution is one in which [H+] is less than [OH-]. • The [H+] is less than 1 x 10 -7 M • Also known as alkaline solutions.

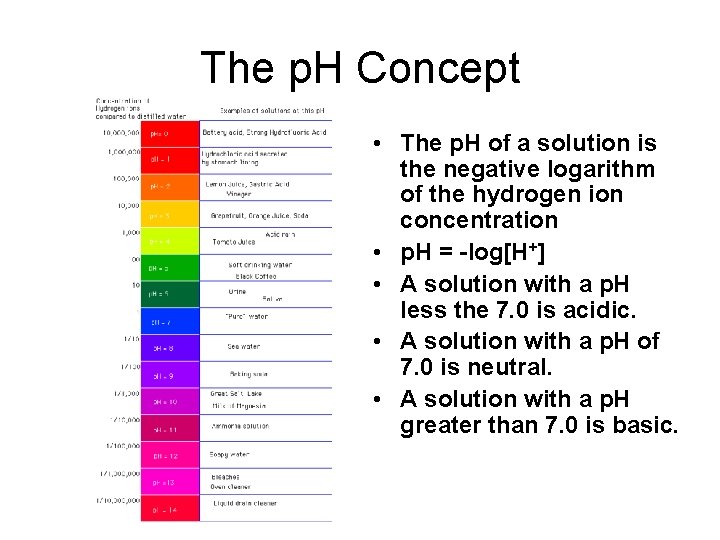
The p. H Concept • The p. H of a solution is the negative logarithm of the hydrogen ion concentration • p. H = -log[H+] • A solution with a p. H less the 7. 0 is acidic. • A solution with a p. H of 7. 0 is neutral. • A solution with a p. H greater than 7. 0 is basic.

Measuring p. H • Either acid-base indicators or p. H meters can be used to measure p. H.

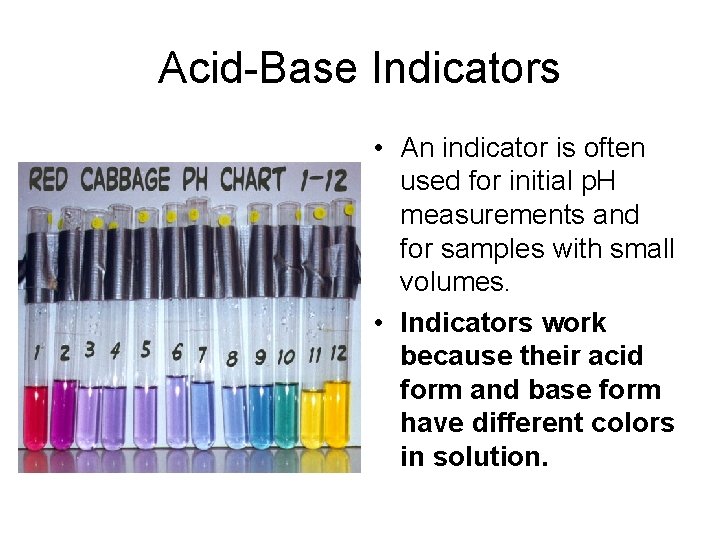
Acid-Base Indicators • An indicator is often used for initial p. H measurements and for samples with small volumes. • Indicators work because their acid form and base form have different colors in solution.

p. H Meters • A p. H meter is used to make rapid, continuous measurements of p. H.