Hydrogen ion homeostasis and blood gases Overview Hydrogen
Hydrogen ion homeostasis and blood gases

Overview Hydrogen homeostasis Sources of hydrogen in the body Acid base control Assessment of acid base status Disorders of hydrogen ion homeostasis 1. Acidosis 2. Alkalosis

Hydrogen homeostasis Hydrogen ion homeostasis is essential for life. Examples: ü Mitochondrial functioning ü Charge and shape of proteins ü Ionization of Ca++ and Mg++
![Normal [H+] Concentrations 35 – 45 nmol/L in the ECF 40 – 40, 000 Normal [H+] Concentrations 35 – 45 nmol/L in the ECF 40 – 40, 000](http://slidetodoc.com/presentation_image_h2/5e5e8b1fe2f4ea05997d6b7ed69eef9b/image-4.jpg)
Normal [H+] Concentrations 35 – 45 nmol/L in the ECF 40 – 40, 000 nmol/L in urine 1, 000, 000 nmol/L in gastric acid
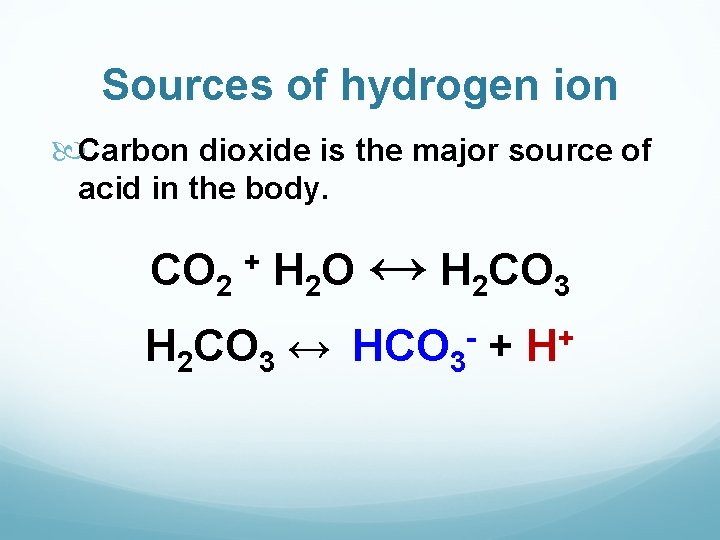
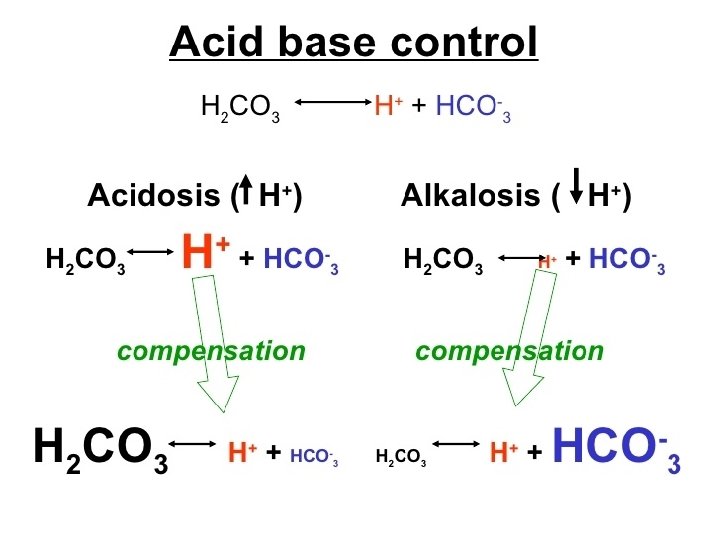
Sources of hydrogen ion Carbon dioxide is the major source of acid in the body. CO 2 H 2 O ↔ H 2 CO 3 + H 2 CO 3 ↔ HCO 3 + - + H

Other sources of Hydrogen Glycolysis lactic acid Lipolysis Free fatty acid Ureagenesis Urea synthesis Ketogenesis Ketoacids Renal excretions of buffered acids H+
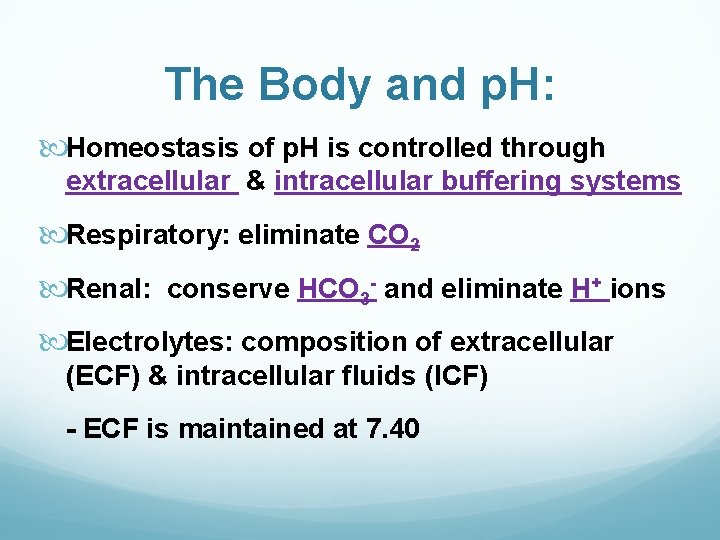
The Body and p. H: Homeostasis of p. H is controlled through extracellular & intracellular buffering systems Respiratory: eliminate CO 2 Renal: conserve HCO 3 - and eliminate H+ ions Electrolytes: composition of extracellular (ECF) & intracellular fluids (ICF) - ECF is maintained at 7. 40
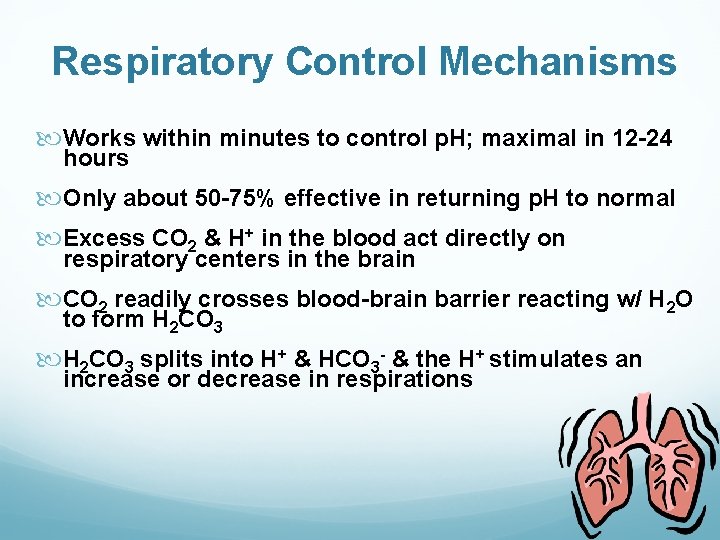
Respiratory Control Mechanisms Works within minutes to control p. H; maximal in 12 -24 hours Only about 50 -75% effective in returning p. H to normal Excess CO 2 & H+ in the blood act directly on respiratory centers in the brain CO 2 readily crosses blood-brain barrier reacting w/ H 2 O to form H 2 CO 3 splits into H+ & HCO 3 - & the H+ stimulates an increase or decrease in respirations

Renal Control Mechanisms: Don’t work as fast as the respiratory system; function for days to restore p. H to, or close to, normal Regulate p. H through excreting acidic or alkaline urine; excreting excess H+ & regenerating or reabsorbing HCO 3 - Excreting acidic urine decreases acid in the EC fluid & excreting alkaline urine removes base
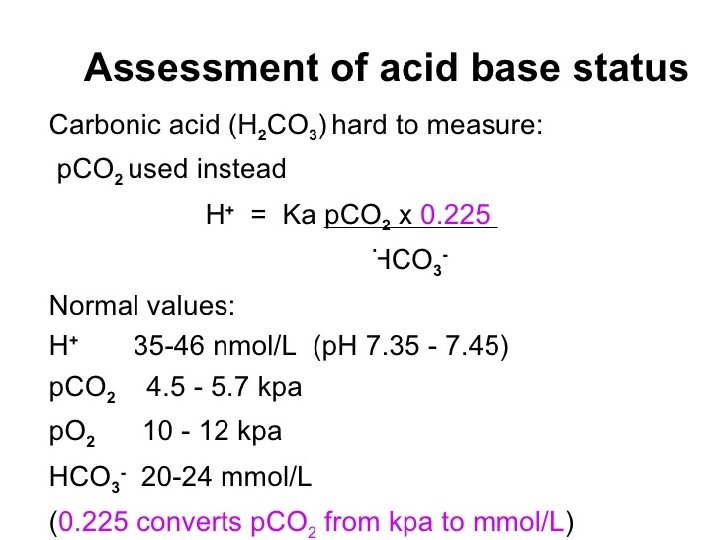
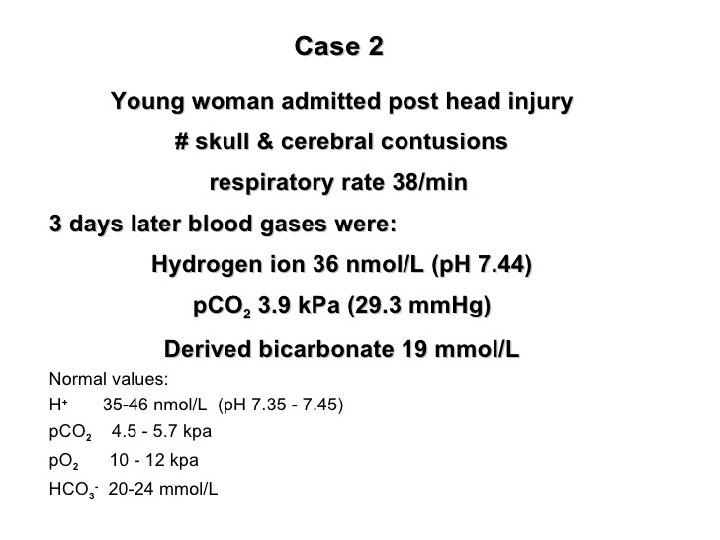
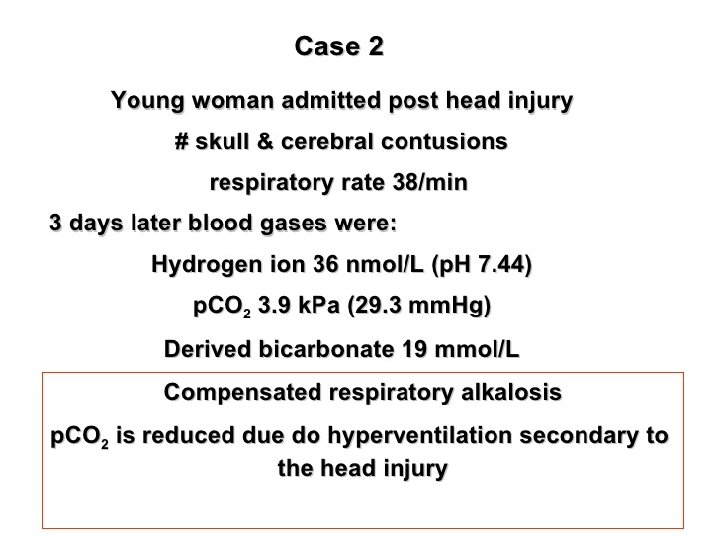
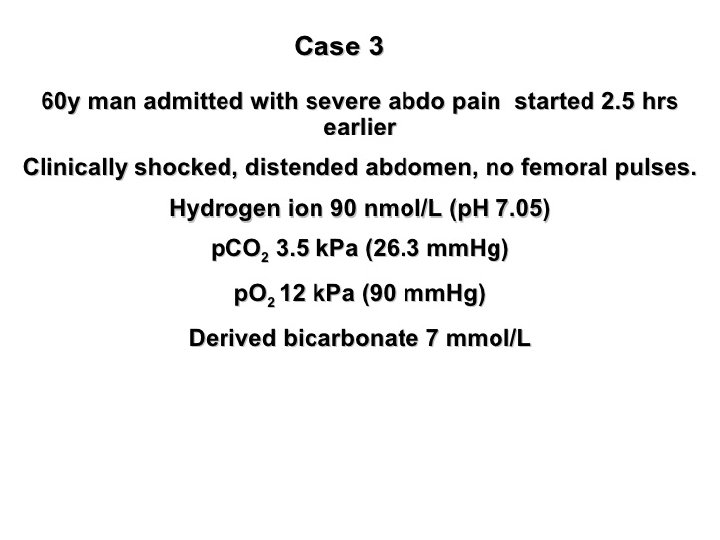
Assessment of acid base status Direct measurements: ü H+ ü PH ü PCO 2 ü PO 2 Derived measures: ü Bicarbonate (HCO 3)
Use heparinized blood and measure within 10 minutes
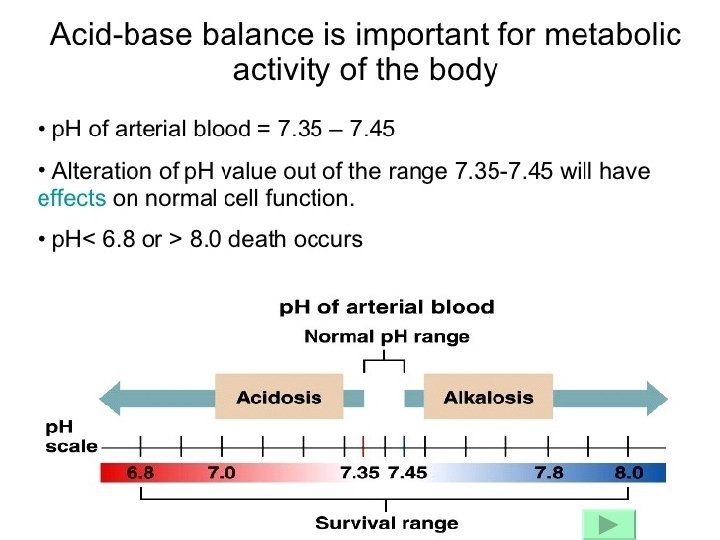

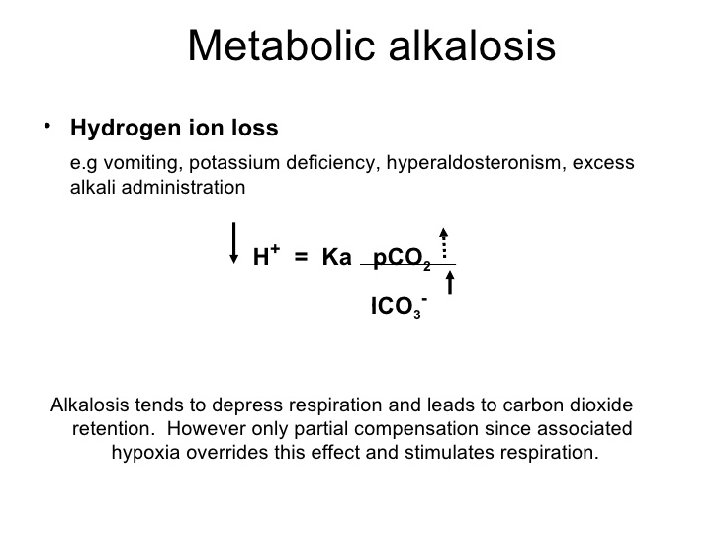
Metabolic Disturbances: Alkalosis: elevated HCO 3 - (>26 m. Eq/L) Causes include: Cl- depletion (vomiting, prolonged nasogastric suctioning), Cushing’s syndrome, K+ deficiency, massive blood transfusions, ingestion of antacids, etc. Acidosis: decreased HCO 3 - (<22 m. Eq/L) Causes include: DKA, shock, sepsis, renal failure, diarrhea, salicylates (aspirin), etc.
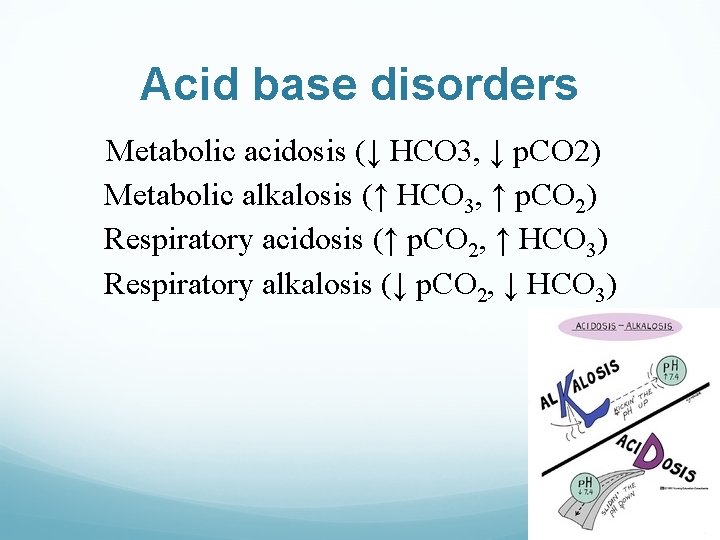
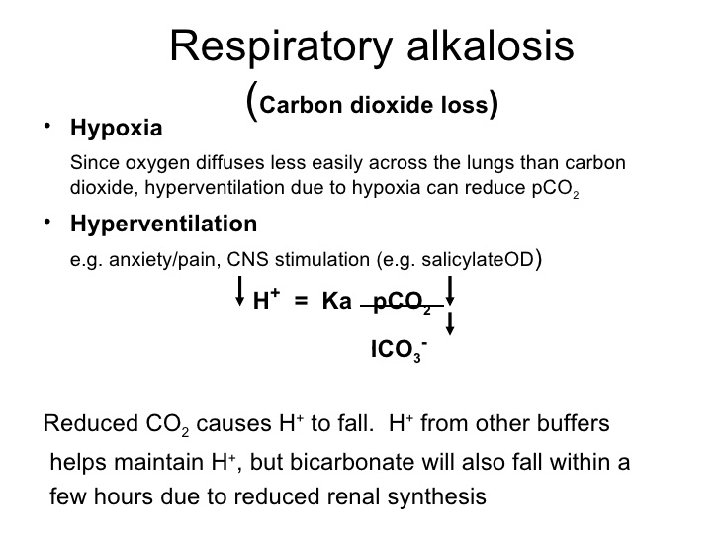
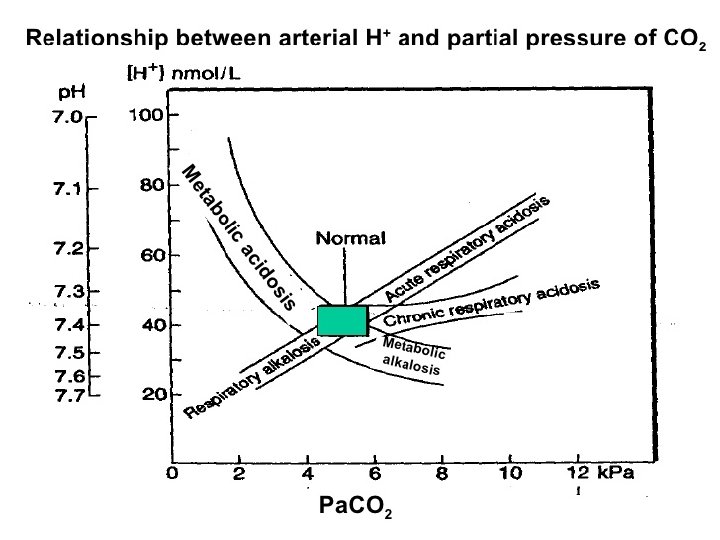
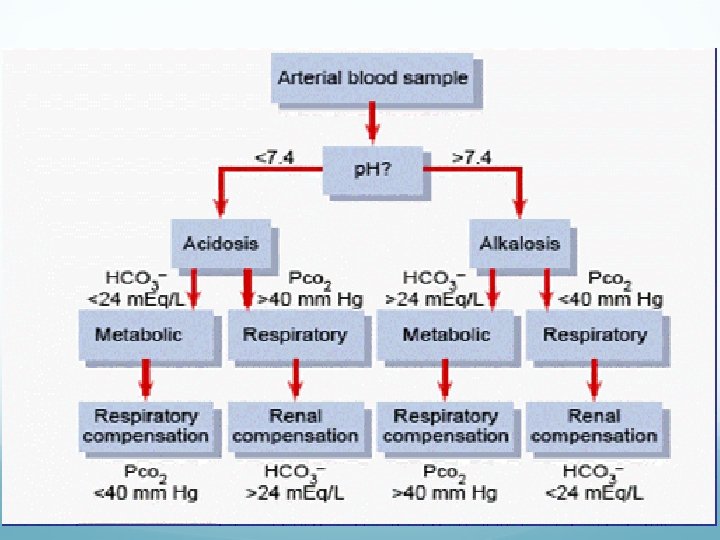
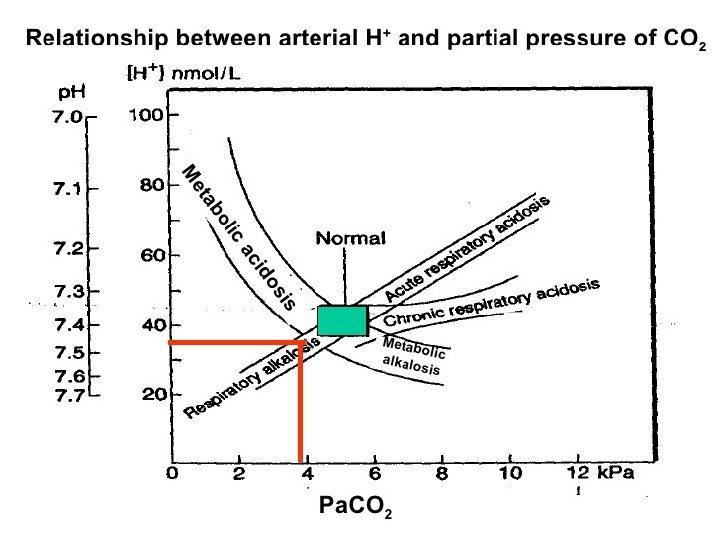
Acid base disorders Metabolic acidosis (↓ HCO 3, ↓ p. CO 2) Metabolic alkalosis (↑ HCO 3, ↑ p. CO 2) Respiratory acidosis (↑ p. CO 2, ↑ HCO 3) Respiratory alkalosis (↓ p. CO 2, ↓ HCO 3)
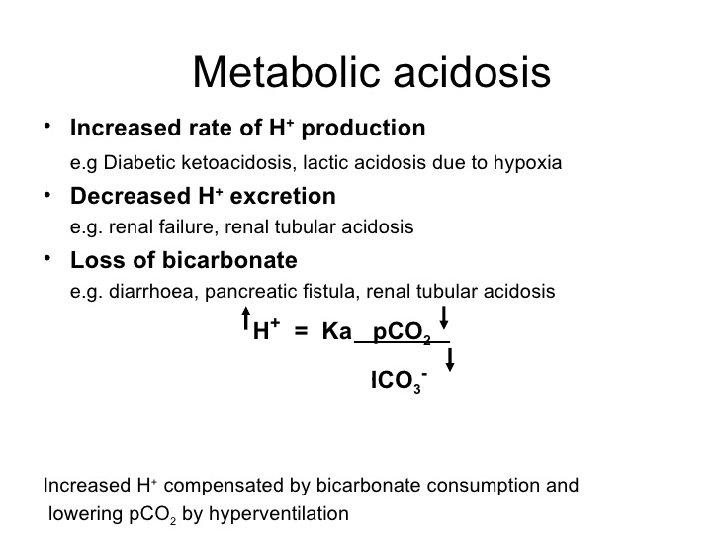
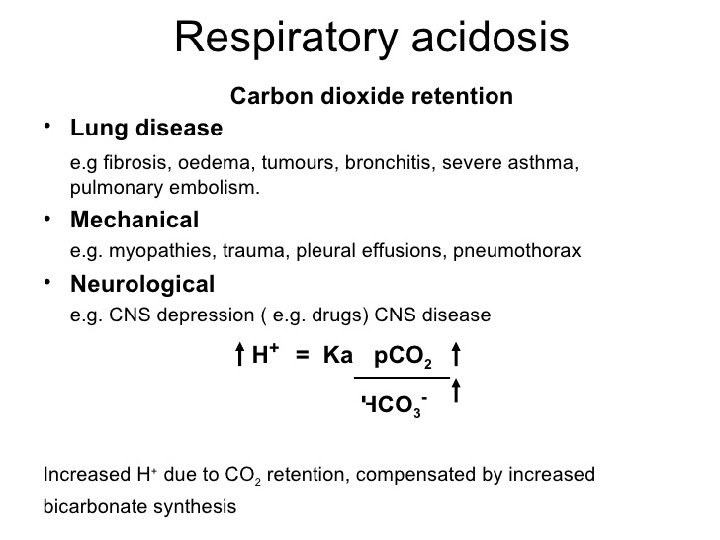
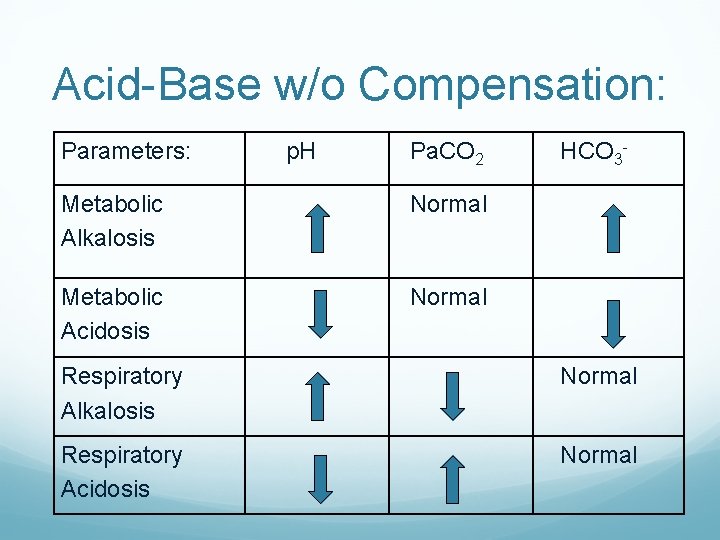
Acid-Base w/o Compensation: Parameters: p. H Pa. CO 2 Metabolic Alkalosis Normal Metabolic Acidosis Normal HCO 3 - Respiratory Alkalosis Normal Respiratory Acidosis Normal
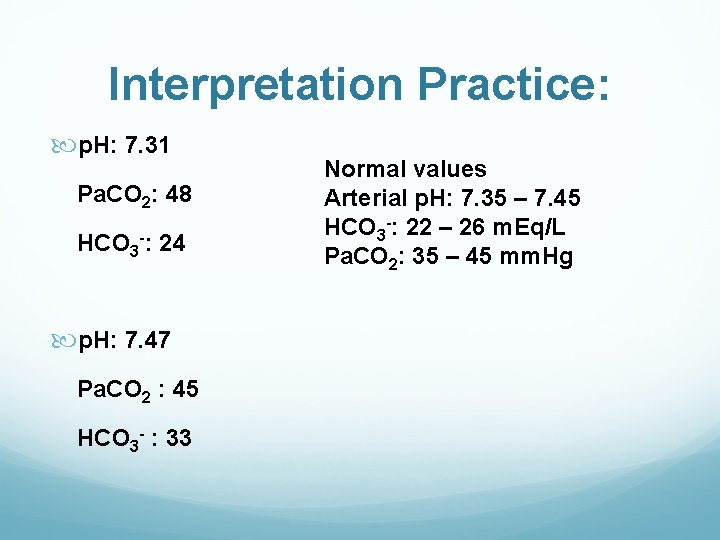
Interpretation Practice: p. H: 7. 31 Pa. CO 2: 48 HCO 3 -: 24 p. H: 7. 47 Pa. CO 2 : 45 HCO 3 - : 33 Normal values Arterial p. H: 7. 35 – 7. 45 HCO 3 -: 22 – 26 m. Eq/L Pa. CO 2: 35 – 45 mm. Hg
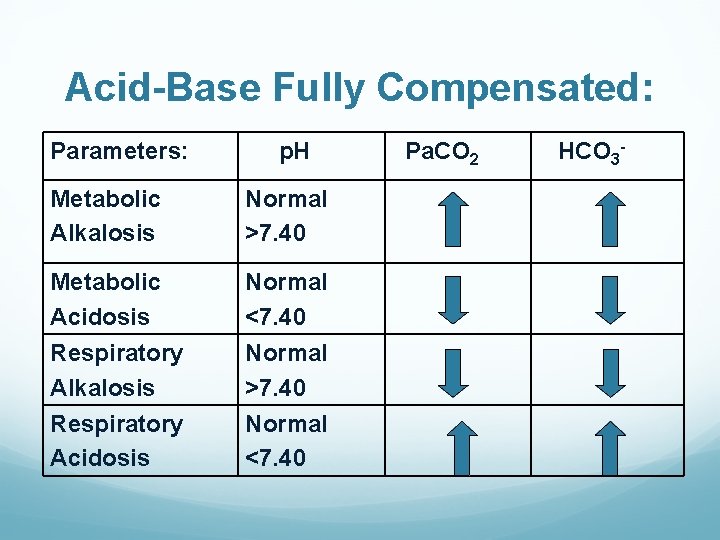
Acid-Base Fully Compensated: Parameters: p. H Metabolic Alkalosis Normal >7. 40 Metabolic Acidosis Normal <7. 40 Respiratory Alkalosis Normal >7. 40 Respiratory Acidosis Normal <7. 40 Pa. CO 2 HCO 3 -
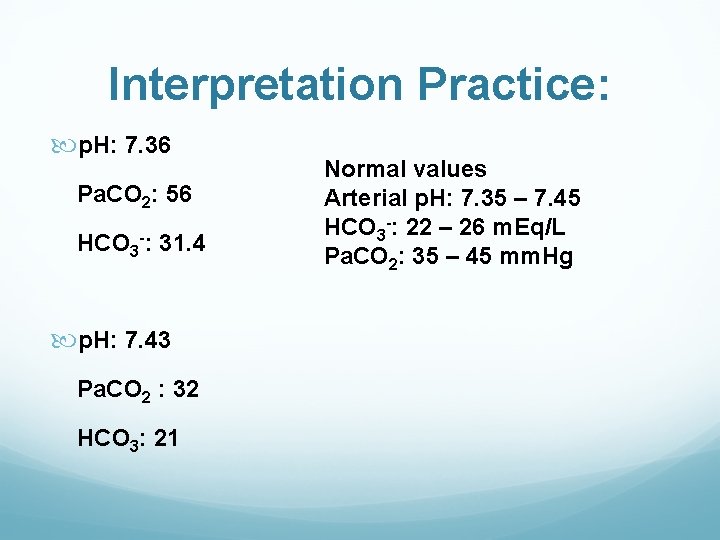
Interpretation Practice: p. H: 7. 36 Pa. CO 2: 56 HCO 3 -: 31. 4 p. H: 7. 43 Pa. CO 2 : 32 HCO 3: 21 Normal values Arterial p. H: 7. 35 – 7. 45 HCO 3 -: 22 – 26 m. Eq/L Pa. CO 2: 35 – 45 mm. Hg
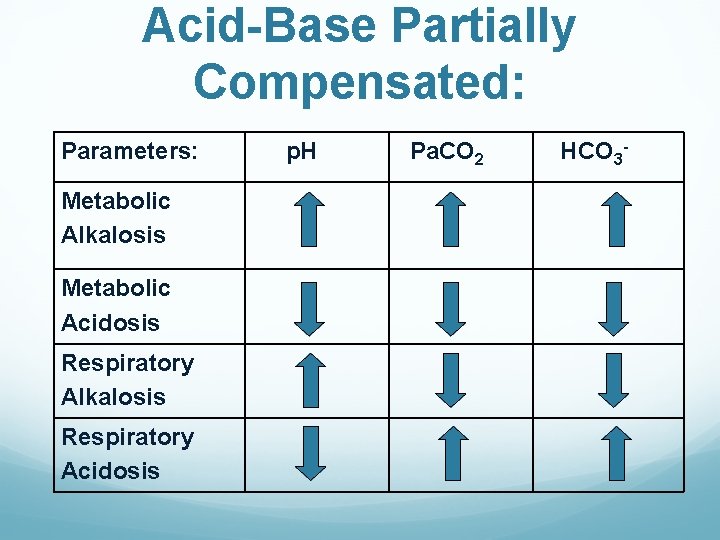
Acid-Base Partially Compensated: Parameters: Metabolic Alkalosis Metabolic Acidosis Respiratory Alkalosis Respiratory Acidosis p. H Pa. CO 2 HCO 3 -
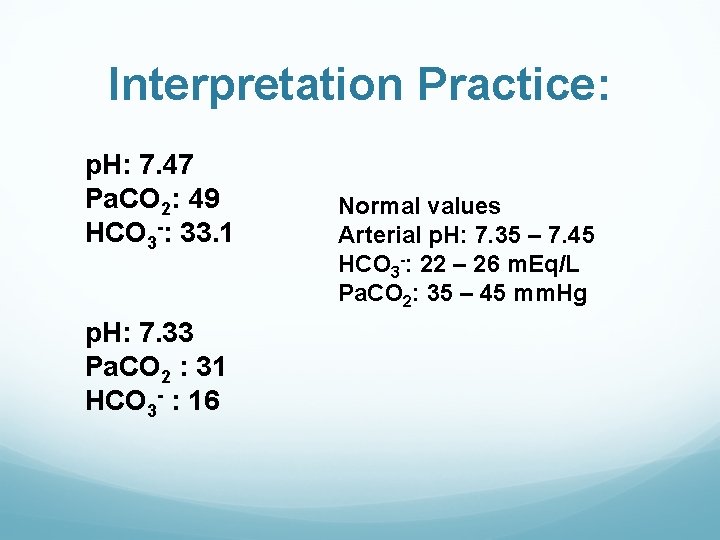
Interpretation Practice: p. H: 7. 47 Pa. CO 2: 49 HCO 3 -: 33. 1 p. H: 7. 33 Pa. CO 2 : 31 HCO 3 - : 16 Normal values Arterial p. H: 7. 35 – 7. 45 HCO 3 -: 22 – 26 m. Eq/L Pa. CO 2: 35 – 45 mm. Hg
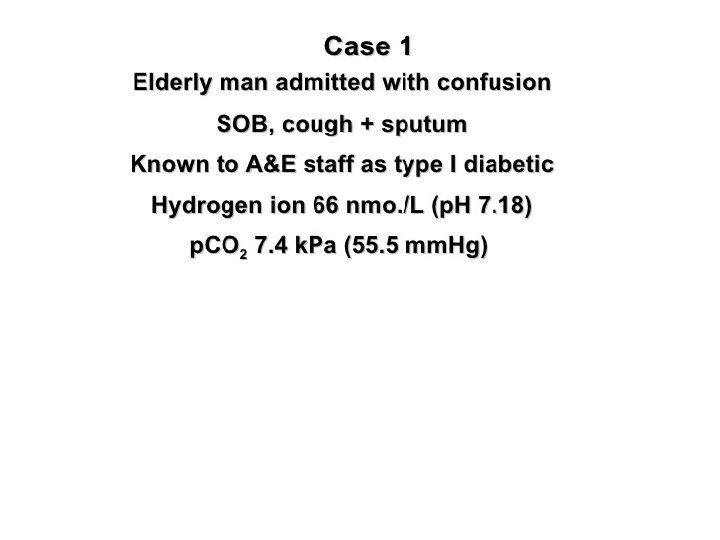
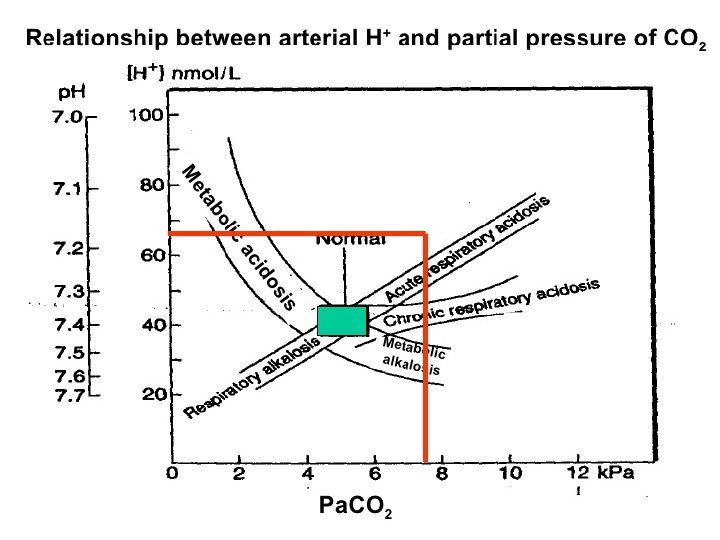
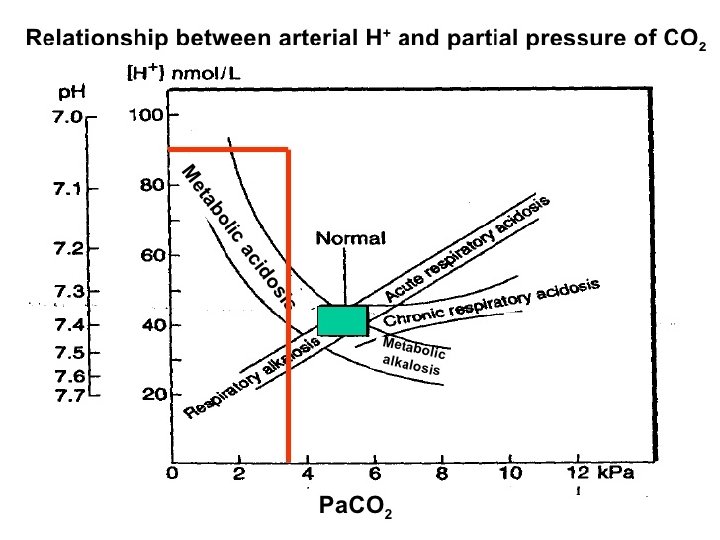
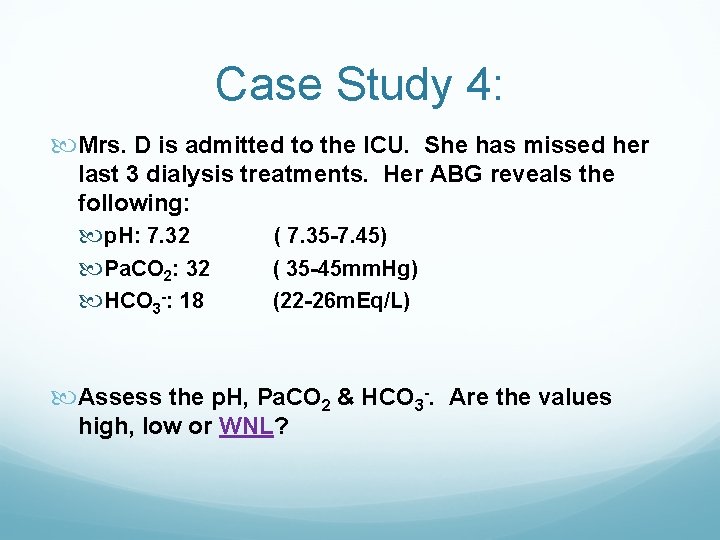
Case Study 4: Mrs. D is admitted to the ICU. She has missed her last 3 dialysis treatments. Her ABG reveals the following: p. H: 7. 32 ( 7. 35 -7. 45) Pa. CO 2: 32 ( 35 -45 mm. Hg) HCO 3 -: 18 (22 -26 m. Eq/L) Assess the p. H, Pa. CO 2 & HCO 3 -. Are the values high, low or WNL?

References http: //www. slideshare. net/parcellus/ss-ulecture 1 presentation http: //www. slideserve. com/Anita/acid-base-balanceinteractive-tutorial Marshall, W. and Bangert, S. (2008). Clinical chemistry (6 th ed. ). Edinburgh, London: Mosby Elsevier. ISBN 0723434557 (chapter 3) Gaw, A. et al. (2004). Clinical Biochemistry (3 rd ed. ) Beckett, G. et al. (2008). Clinical Biochemistry (8 th ed. ) Bishop. , et al. (2000). Clinical Chemistry (4 th ed. )
- Slides: 37