Hydrogen Generation using Chemical Catalysts Martin Wills David









- Slides: 26

Hydrogen Generation using Chemical Catalysts. Martin Wills, David J. Morris, Tarn Johnson, Department of Chemistry, University of Warwick, Coventry, UK. Joe Wood, Bushra Al-Duri, Suzanne Al-Samaq School of Chemical Engineering, University of Birmingham, UK.

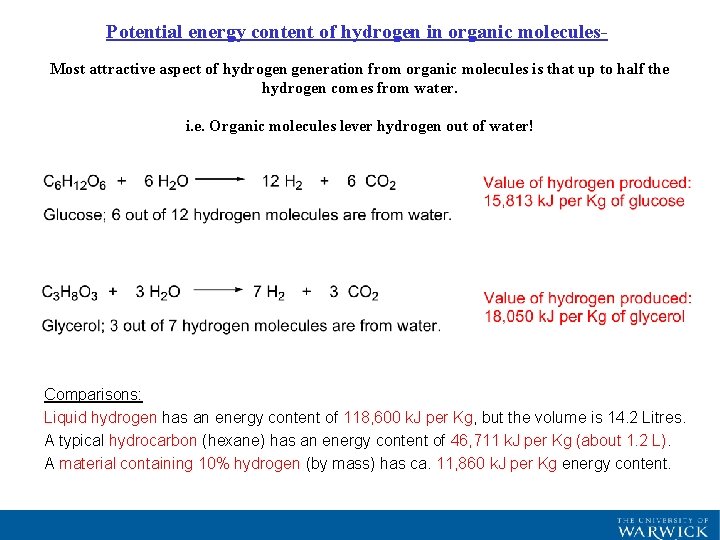
Potential energy content of hydrogen in organic molecules. Most attractive aspect of hydrogen generation from organic molecules is that up to half the hydrogen comes from water. i. e. Organic molecules lever hydrogen out of water! Comparisons: Liquid hydrogen has an energy content of 118, 600 k. J per Kg, but the volume is 14. 2 Litres. A typical hydrocarbon (hexane) has an energy content of 46, 711 k. J per Kg (about 1. 2 L). A material containing 10% hydrogen (by mass) has ca. 11, 860 k. J per Kg energy content.

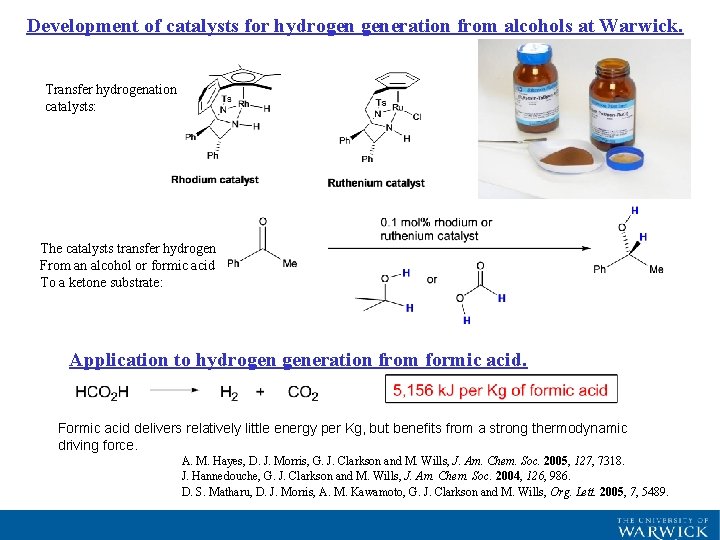
Development of catalysts for hydrogen generation from alcohols at Warwick. Transfer hydrogenation catalysts: The catalysts transfer hydrogen From an alcohol or formic acid To a ketone substrate: Application to hydrogen generation from formic acid. Formic acid delivers relatively little energy per Kg, but benefits from a strong thermodynamic driving force. A. M. Hayes, D. J. Morris, G. J. Clarkson and M. Wills, J. Am. Chem. Soc. 2005, 127, 7318. J. Hannedouche, G. J. Clarkson and M. Wills, J. Am. Chem. Soc. 2004, 126, 986. D. S. Matharu, D. J. Morris, A. M. Kawamoto, G. J. Clarkson and M. Wills, Org. Lett. 2005, 7, 5489.

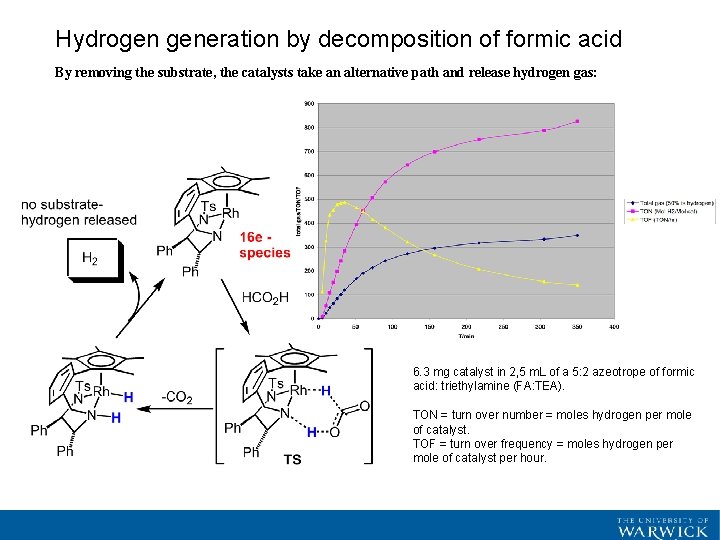
Hydrogen generation by decomposition of formic acid By removing the substrate, the catalysts take an alternative path and release hydrogen gas: 6. 3 mg catalyst in 2, 5 m. L of a 5: 2 azeotrope of formic acid: triethylamine (FA: TEA). TON = turn over number = moles hydrogen per mole of catalyst. TOF = turn over frequency = moles hydrogen per mole of catalyst per hour.

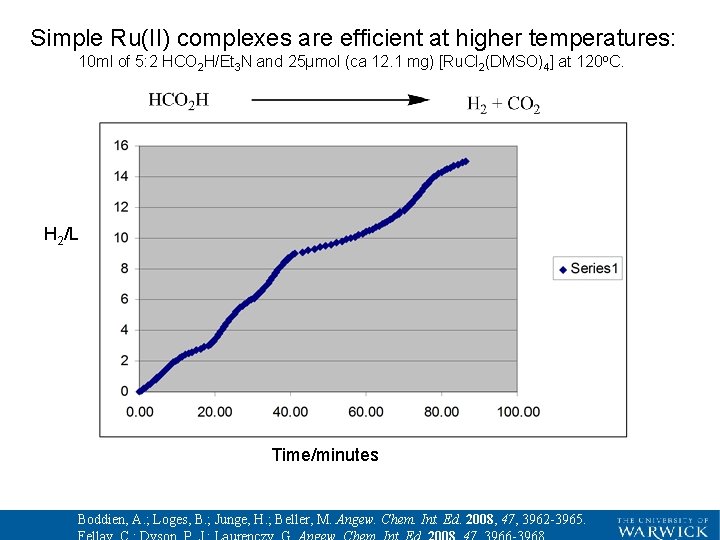
Simple Ru(II) complexes are efficient at higher temperatures: 10 ml of 5: 2 HCO 2 H/Et 3 N and 25µmol (ca 12. 1 mg) [Ru. Cl 2(DMSO)4] at 120 o. C. H 2/L Time/minutes Boddien, A. ; Loges, B. ; Junge, H. ; Beller, M. Angew. Chem. Int. Ed. 2008, 47, 3962 -3965.

The formic acid sample is heated in the round-bottom flask fitted with a thermometer to monitor internal temperature. Two gas syringes are attached, allowing seamless monitoring of gas flow. In the picture above, the gas is being diverted into a small PEM fuel cell, which is running an electric fan. Reproduced with permission of Aman Dhir, Department of Chemical Engineering, Birmingham University.

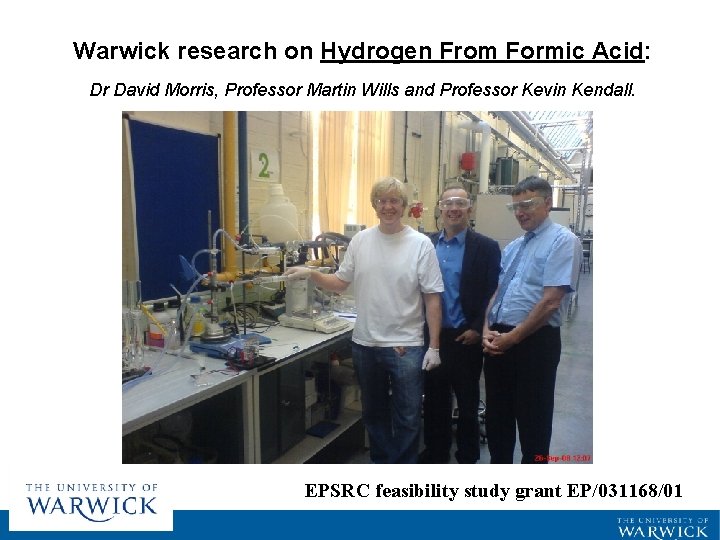
Warwick research on Hydrogen From Formic Acid: Dr David Morris, Professor Martin Wills and Professor Kevin Kendall. EPSRC feasibility study grant EP/031168/01

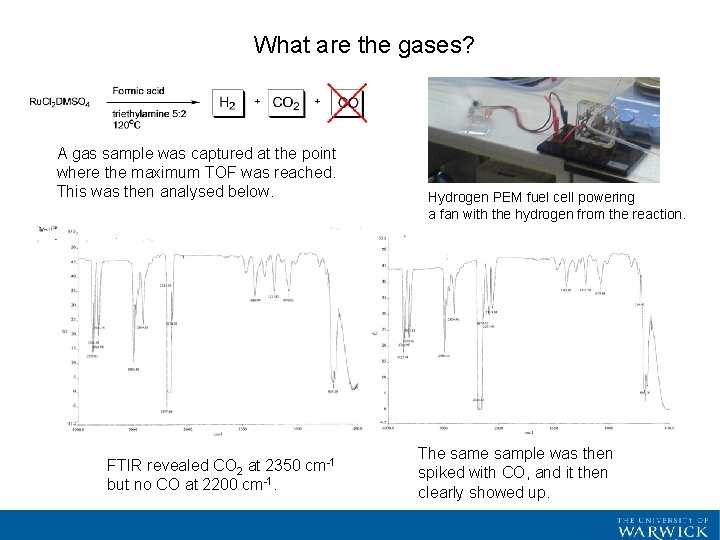
What are the gases? A gas sample was captured at the point where the maximum TOF was reached. This was then analysed below. FTIR revealed CO 2 at 2350 cm-1 but no CO at 2200 cm-1. Hydrogen PEM fuel cell powering a fan with the hydrogen from the reaction. The sample was then spiked with CO, and it then clearly showed up.

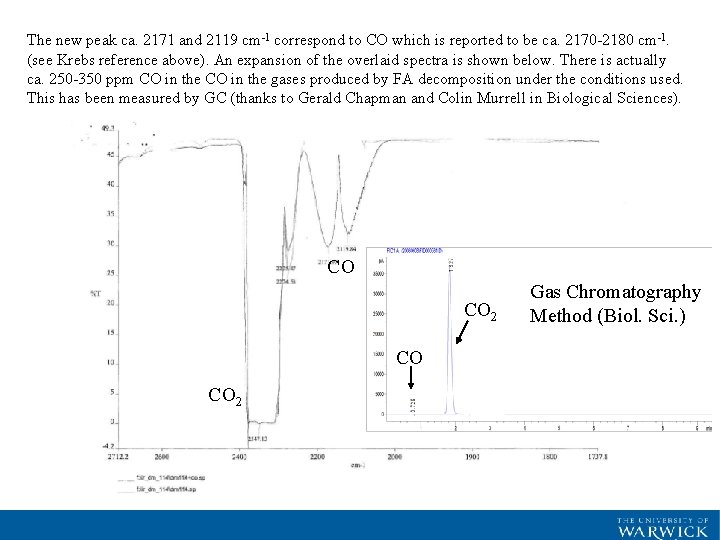
The new peak ca. 2171 and 2119 cm-1 correspond to CO which is reported to be ca. 2170 -2180 cm-1. (see Krebs reference above). An expansion of the overlaid spectra is shown below. There is actually ca. 250 -350 ppm CO in the gases produced by FA decomposition under the conditions used. This has been measured by GC (thanks to Gerald Chapman and Colin Murrell in Biological Sciences). CO CO 2 Gas Chromatography Method (Biol. Sci. )

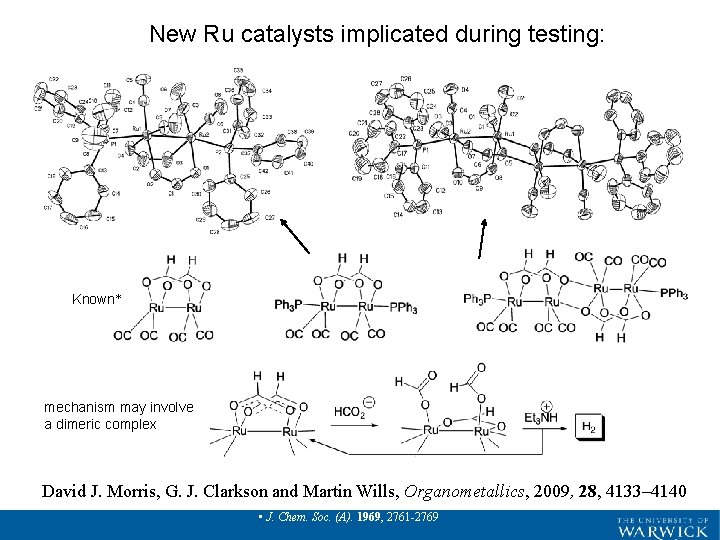
New Ru catalysts implicated during testing: Known* mechanism may involve a dimeric complex David J. Morris, G. J. Clarkson and Martin Wills, Organometallics, 2009, 28, 4133– 4140 • Crooks, G. R. ; Johnson, B. F. G. ; Lewis, J. ; Williams, I. G. ; Gamien, G. • J. Chem. Soc. (A). 1969, 2761 -2769

Medium scale hydrogen generation from formic acid: Larger scale set up which allows continuous formation of hydrogen from formic acid, with Artur Majewski. D. J. Morris and M. Wills, Organometallics, 2009, 28, 4133 -4140.

Medium scale hydrogen generation from formic acid – Example of continuous production: 462 L H 2 Larger scale set up which allows continuous formation of hydrogen from formic acid, with Artur Majewski. D. J. Morris and M. Wills, Organometallics, 2009, 28, 4133 -4140.

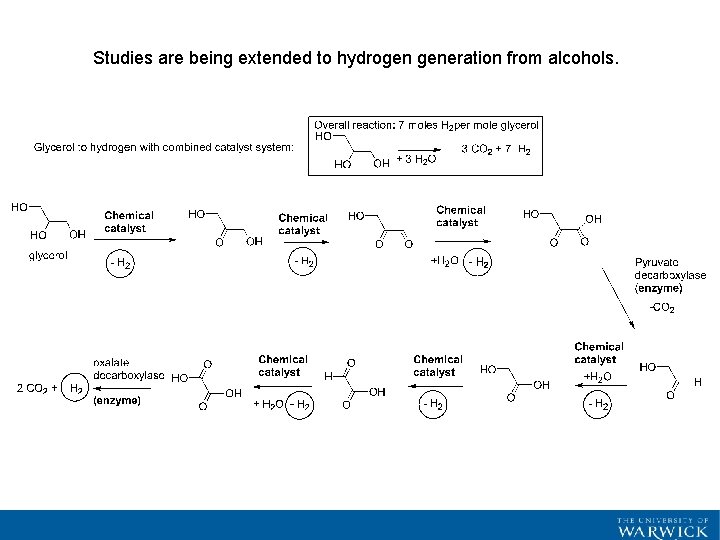
Studies are being extended to hydrogen generation from alcohols.

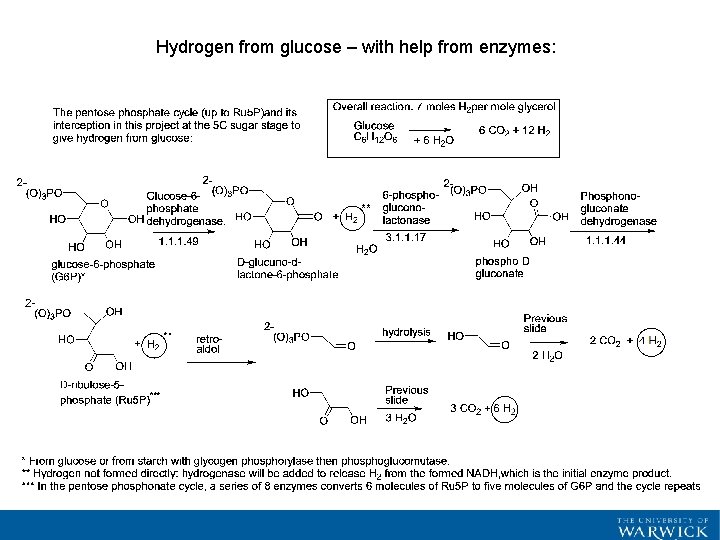
Hydrogen from glucose – with help from enzymes:

Conclusions Hydrogen is a clean fuel, at point of use. ‘Clean’ hydrogen generation represents a significant challenge. Acknowledgements: Funding: EPSRC (EP/031168/01) Science: Dr David Morris HDelivery


The Birmingham/Warwick Science Cities Hydrogen Energy Project. A Collaborative Research Project that is part of the larger Energy Futures projects between: The University of Warwick (Physics, Chemistry, Engineering, Biological Sciences, WHRI) and The University of Birmingham (Project leader Prof. Kevin Kendall, Chemical Engineering, Chemistry, Economics, Biological Sciences, Materials) Part of Warwick strategy in energy research centred on WISER Project started as an £ 8. 3 M project with AWM funding at £ 6. 2 M – in January 2007 and has already attracted another £ 1. 5 M of funding via Supply Chain Research Applied To Clean Hydrogen (SCRATCH) EPSRC project (Kendall PI)

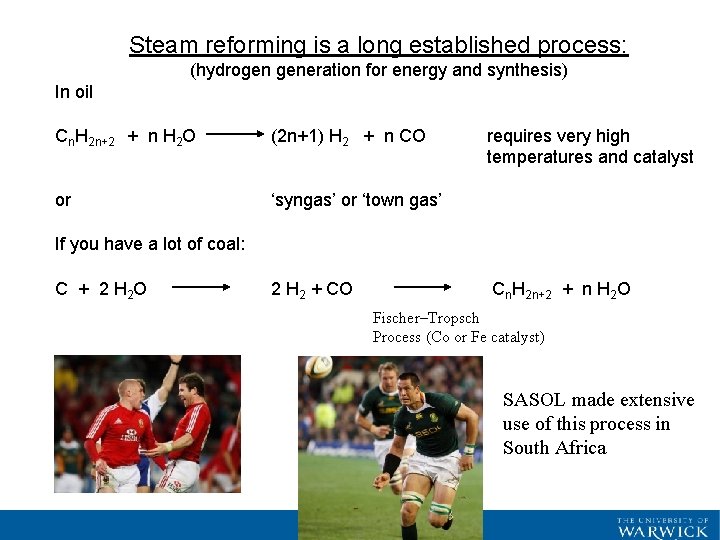
Steam reforming is a long established process: (hydrogen generation for energy and synthesis) In oil Cn. H 2 n+2 + n H 2 O (2 n+1) H 2 + n CO or ‘syngas’ or ‘town gas’ requires very high temperatures and catalyst If you have a lot of coal: C + 2 H 2 O 2 H 2 + CO Cn. H 2 n+2 + n H 2 O Fischer–Tropsch Process (Co or Fe catalyst) SASOL made extensive use of this process in South Africa

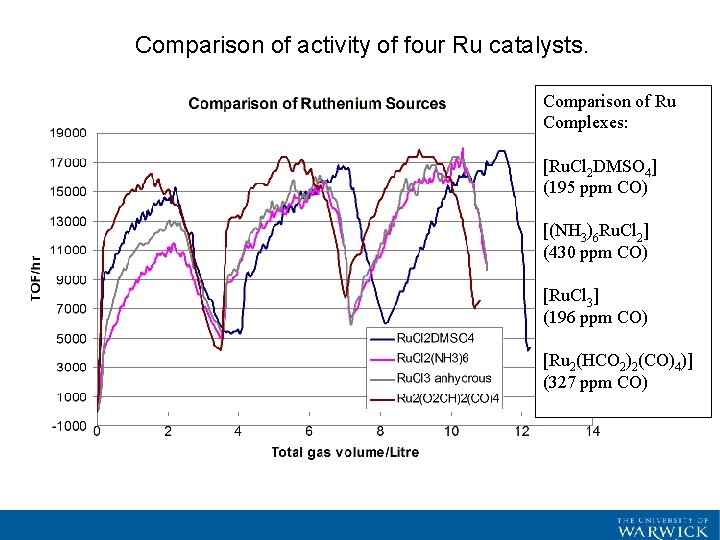
Comparison of activity of four Ru catalysts. Comparison of Ru Complexes: [Ru. Cl 2 DMSO 4] (195 ppm CO) [(NH 3)6 Ru. Cl 2] (430 ppm CO) [Ru. Cl 3] (196 ppm CO) [Ru 2(HCO 2)2(CO)4)] (327 ppm CO)

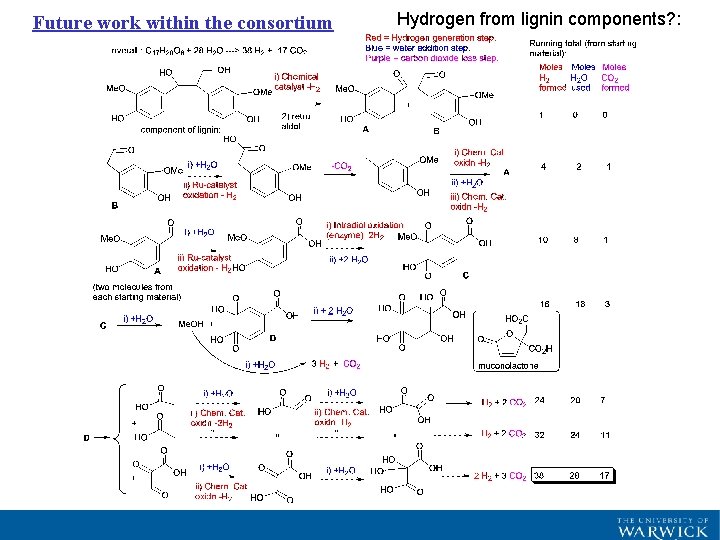
Future work within the consortium Hydrogen from lignin components? :

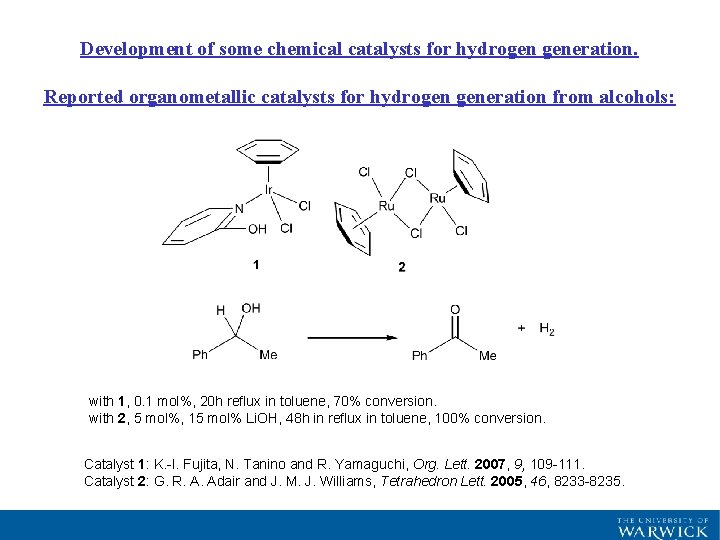
Development of some chemical catalysts for hydrogen generation. Reported organometallic catalysts for hydrogen generation from alcohols: with 1, 0. 1 mol%, 20 h reflux in toluene, 70% conversion. with 2, 5 mol%, 15 mol% Li. OH, 48 h in reflux in toluene, 100% conversion. Catalyst 1: K. -I. Fujita, N. Tanino and R. Yamaguchi, Org. Lett. 2007, 9, 109 -111. Catalyst 2: G. R. A. Adair and J. M. J. Williams, Tetrahedron Lett. 2005, 46, 8233 -8235.

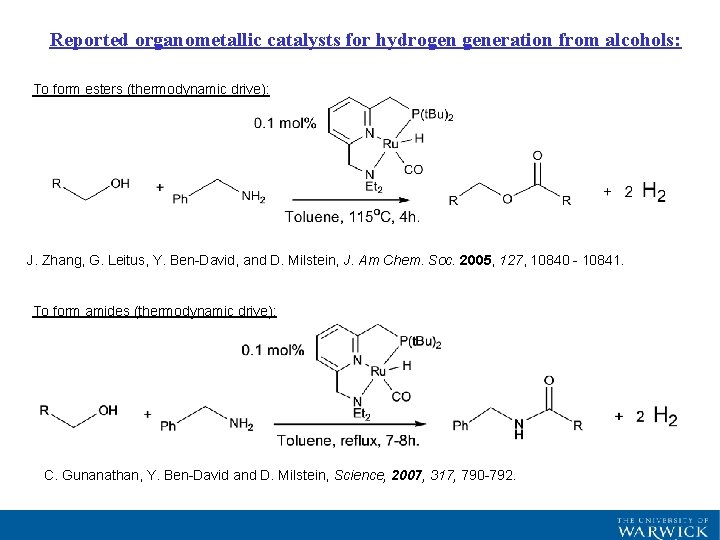
Reported organometallic catalysts for hydrogen generation from alcohols: To form esters (thermodynamic drive): J. Zhang, G. Leitus, Y. Ben-David, and D. Milstein, J. Am Chem. Soc. 2005, 127, 10840 - 10841. To form amides (thermodynamic drive): C. Gunanathan, Y. Ben-David and D. Milstein, Science, 2007, 317, 790 -792.

EPSRC funded ‘Delivery of Sustainable Hydrogen’ SUPERGEN project (14 partners, £ 5 M, 48 months, start date 1 st October 2008). Partners: John Irvine (St. Andrews), Management coordinator and PI. Ian Metcalfe (Newcastle), Finance coordinator. Chris Whitehead (Manchester) Bartek Glowacki (Cambridge) David Infield (Strathclyde) David Book (Birmingham) Martin Wills (Warwick) Kang Li (Imperial) Marcello Contestabile (Imperial) Shanwen Tao (Heriot-Watt) Neil Mc. Keown (Cardiff) Edman Tsang (Oxford) Malcolm Eames (Brunel) Valerie Dupont (Leeds)

Equipment now available at Warwick and Birmingham Solid state X-ray diffractometers, mass spectrometers, Solid state NMR. FTIR, / RAMAN, Glove boxes, Confocal Microscopy. High pressure reaction cells, pressure vessels, Fuel cells, large scale bioreactors, biohydrogen pilot plant. Extensive analytical equipment, Anaerobic growth reactors. Scanning electrochemical microscope and associated equipment. Full list available on request. Useful contact: Robert Hudson Science City Project Manager Hydrogen Energy (Based at Birmingham).

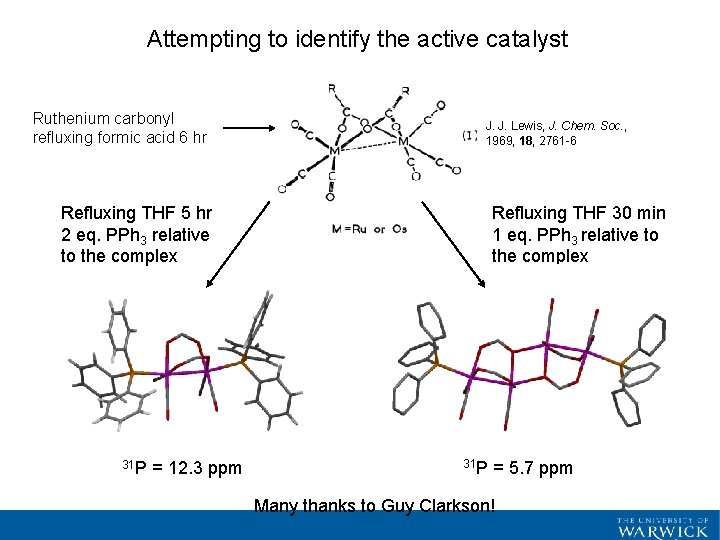
Attempting to identify the active catalyst Ruthenium carbonyl refluxing formic acid 6 hr J. J. Lewis, J. Chem. Soc. , 1969, 18, 2761 -6 Refluxing THF 5 hr 2 eq. PPh 3 relative to the complex 31 P = 12. 3 ppm Refluxing THF 30 min 1 eq. PPh 3 relative to the complex 31 P = 5. 7 ppm Many thanks to Guy Clarkson!

Hydrogen research: Examples of facilities Biological catalysis (Biological Sciences, Warwick HRI) Formation of hydrogen gas from Biomass in a Biomass reactor: Biomass at Warwick HRI is used as fuel for enzymatic decomposition in the biomass reactor (purchased with science cities funds) H 2 Recent icast: http: //www 2. warwick. ac. uk/newsandevents/icast/archive/s 2 week 2/ Other valuable products can be formed in this reactor.