Hydrogen Bonding Hydrogen Bond an attraction between a

Hydrogen Bonding Hydrogen Bond = an attraction between a slightly positive hydrogen atom and a slightly negative atom, often oxygen or nitrogen

Hydrogen bonds What are they? • A special case of permanent dipole-dipole interactions They are stronger than van der Waals forces. Molecules with hydrogen bonds have higher boiling points than molecules that don’t.
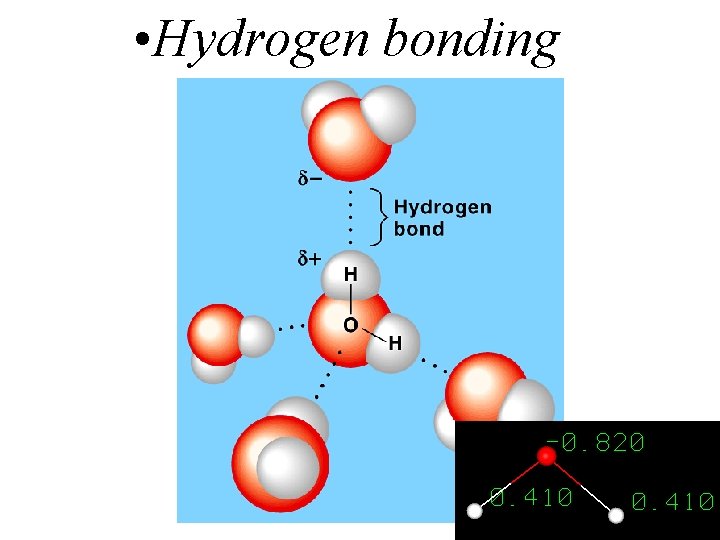
• Hydrogen bonding 3

Hydrogen bonds What do you need? • A hydrogen atom covalently bonded to an electronegative atom … N, O or F. • A lone pair of electrons on the electronegative atom. If only one of these conditions is met, you don’t get hydrogen bonding.
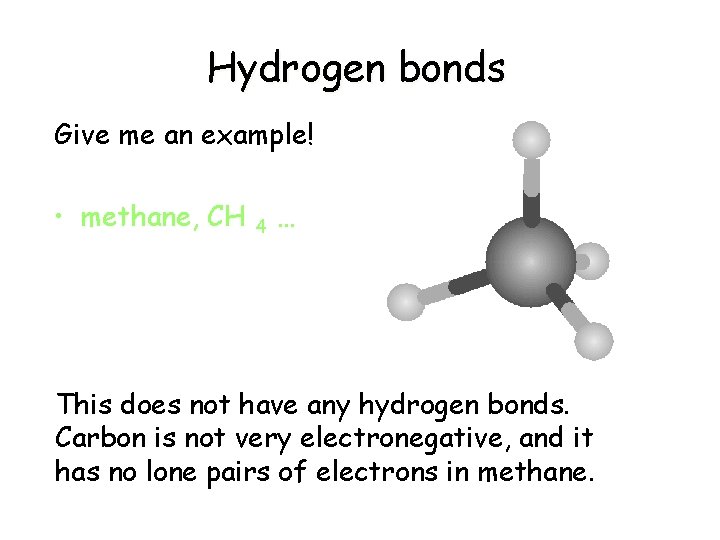
Hydrogen bonds Give me an example! • methane, CH 4 … This does not have any hydrogen bonds. Carbon is not very electronegative, and it has no lone pairs of electrons in methane.
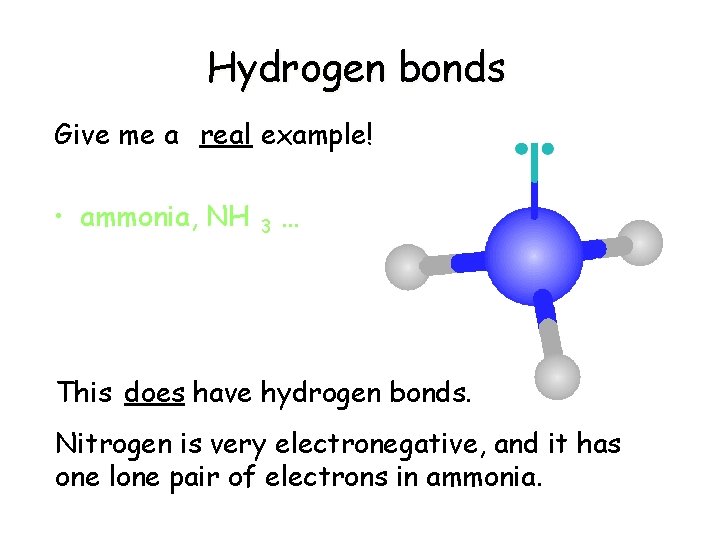
Hydrogen bonds Give me a real example! • ammonia, NH 3 … This does have hydrogen bonds. Nitrogen is very electronegative, and it has one lone pair of electrons in ammonia.
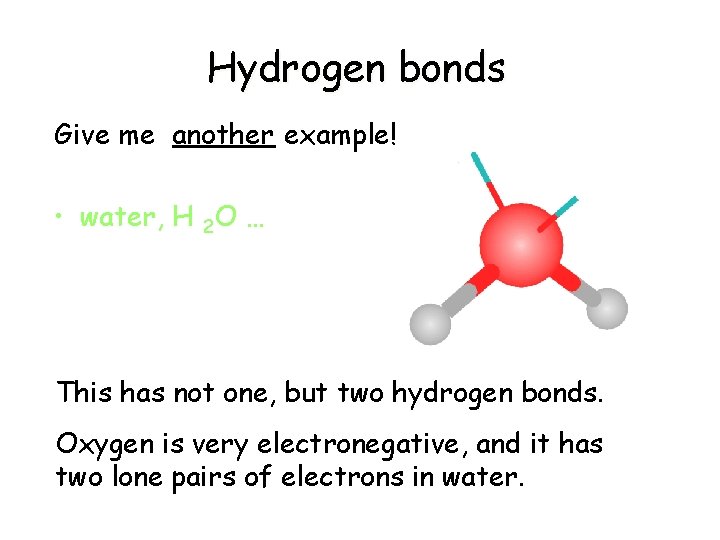
Hydrogen bonds Give me another example! • water, H 2 O … This has not one, but two hydrogen bonds. Oxygen is very electronegative, and it has two lone pairs of electrons in water.
Water Chemistry The polarity of water causes it to be cohesive and adhesive. cohesion: water molecules stick to other water molecules by hydrogen bonding adhesion: water molecules stick to other polar molecules by hydrogen bonding 8
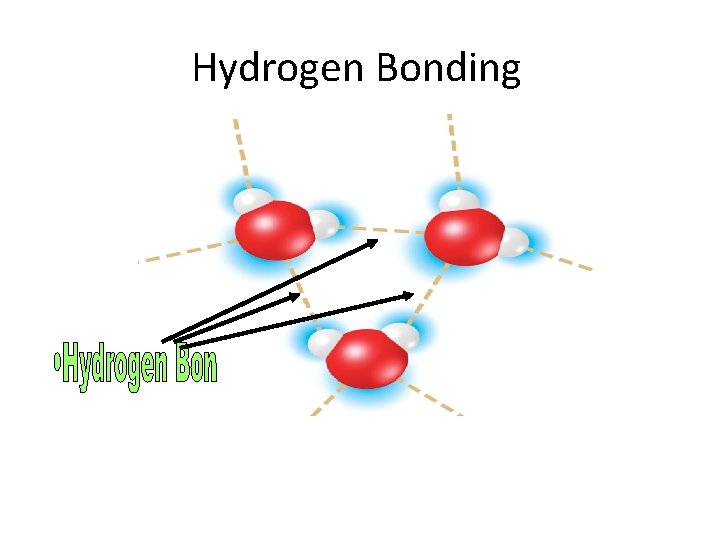
Hydrogen Bonding

Properties Related to Hydrogen Bonding Life depends on hydrogen bonds in water • High Specific Heat = water resists changes in temp. - helps regulate cells
Properties Related to Hydrogen Bonding (cont) • Cohesion = attractive forces between particles of the same kind - example: surface tension of water • Adhesion = the attractive forces between unlike substances - example: meniscus curve in a graduated cylinder

Surface Tension
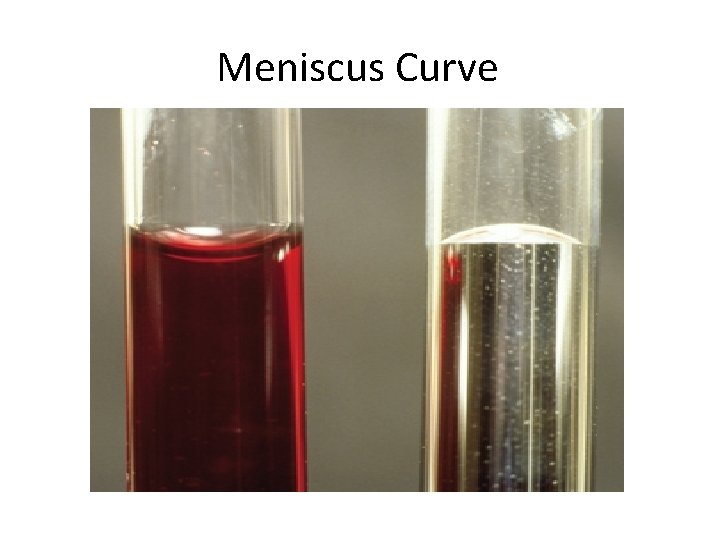
Meniscus Curve

Properties Related to Hydrogen Bonding (cont) • Capillarity = the ability of water to move up through narrow tubes against gravity - due to cohesion and adhesion
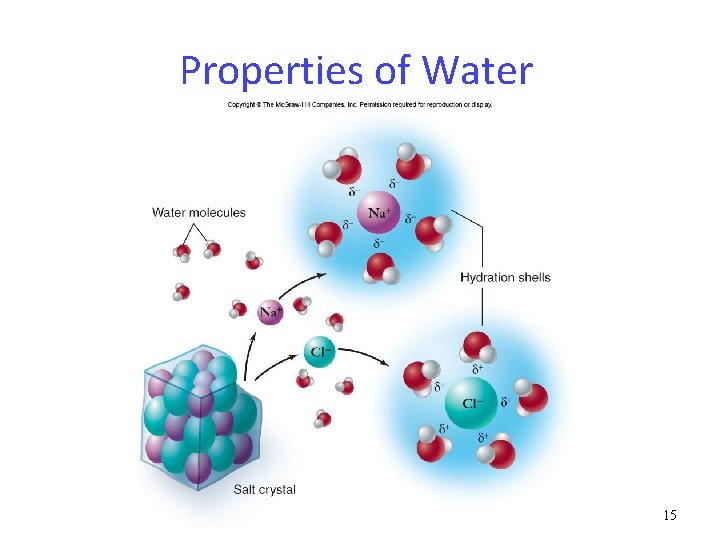
Properties of Water 15
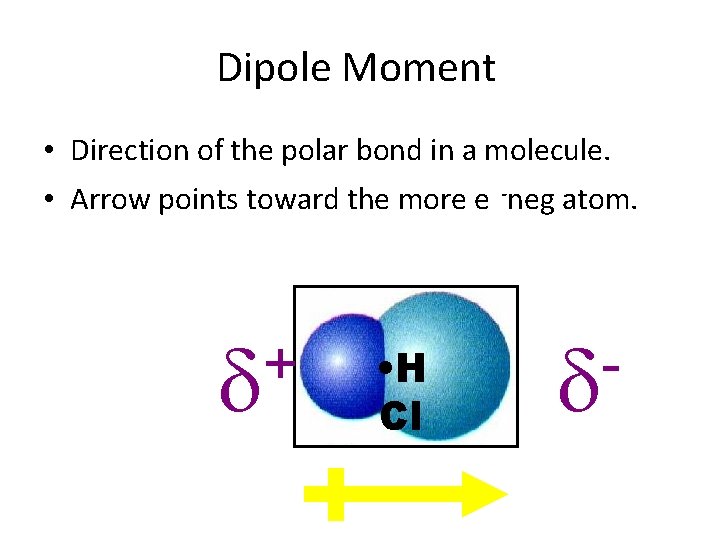
Dipole Moment • Direction of the polar bond in a molecule. • Arrow points toward the more e -neg atom. + • H Cl
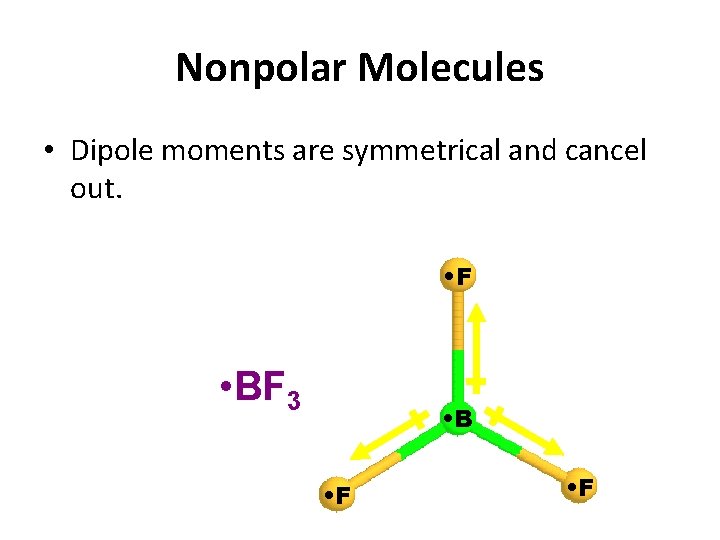
Nonpolar Molecules • Dipole moments are symmetrical and cancel out. • F • BF 3 • B • F
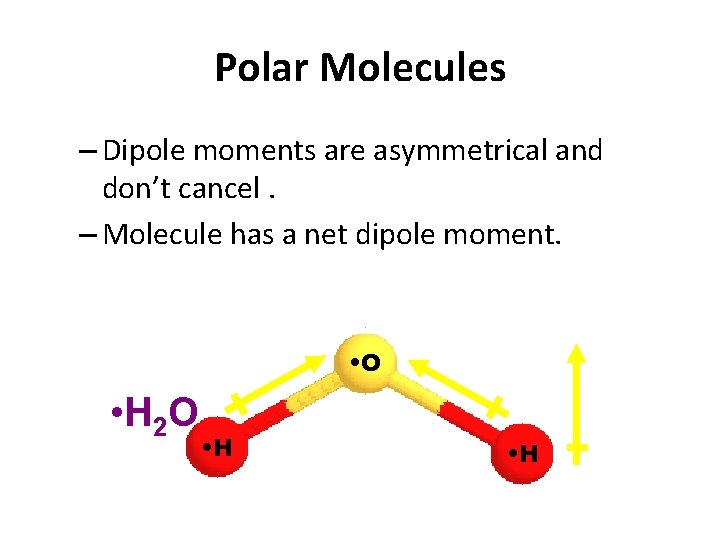
Polar Molecules – Dipole moments are asymmetrical and don’t cancel. – Molecule has a net dipole moment. • O • H 2 O • H • net • dipole • moment
Determining Molecular Polarity • Depends on: – dipole moments – molecular shape
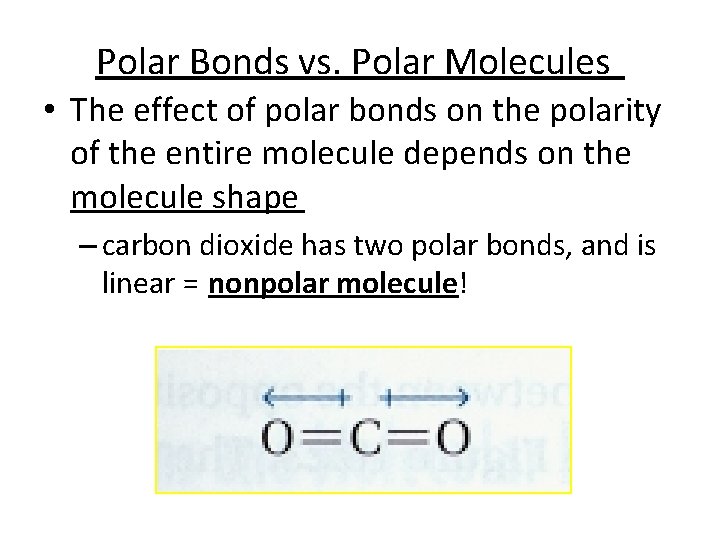
Polar Bonds vs. Polar Molecules • The effect of polar bonds on the polarity of the entire molecule depends on the molecule shape – carbon dioxide has two polar bonds, and is linear = nonpolar molecule!
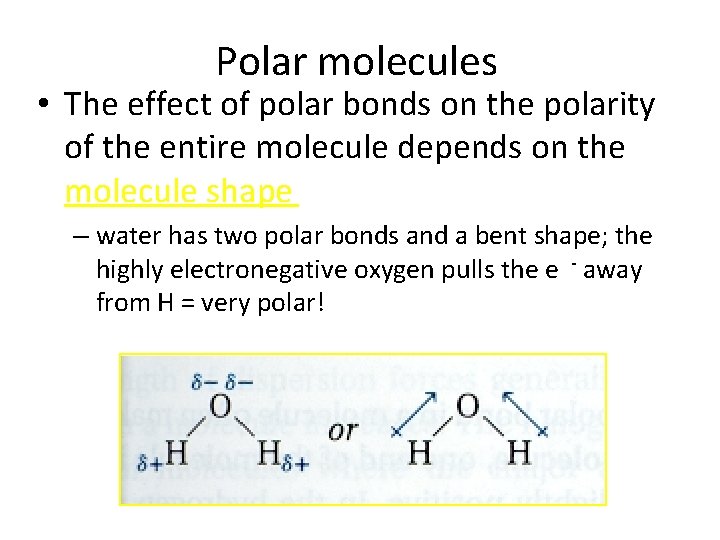
Polar molecules • The effect of polar bonds on the polarity of the entire molecule depends on the molecule shape – water has two polar bonds and a bent shape; the highly electronegative oxygen pulls the e - away from H = very polar!
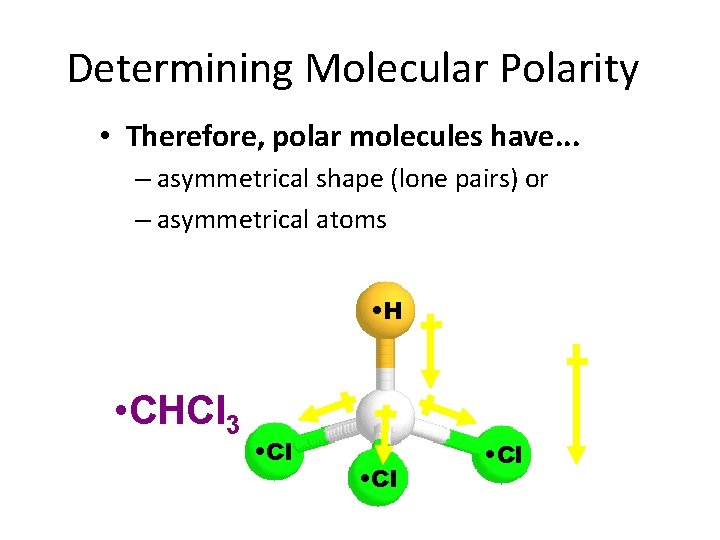
Determining Molecular Polarity • Therefore, polar molecules have. . . – asymmetrical shape (lone pairs) or – asymmetrical atoms • H • CHCl 3 • Cl • net • dipole • moment
- Slides: 22