Hydrogen and Basic Elements The Periodic Table of

Hydrogen and Basic Elements
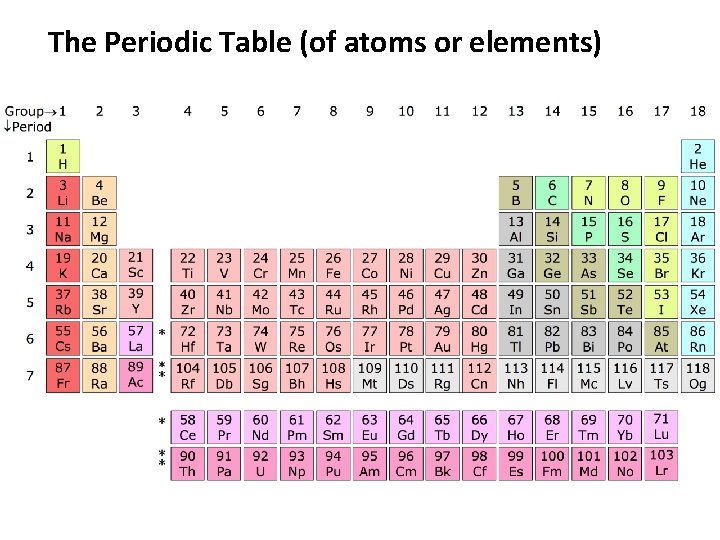
The Periodic Table (of atoms or elements)
Basics • An element/atom is chemically the most basic unit of matter – If you subdivide an element further, it is no longer that element. You get subatomic particles. – Electron(s) revolving around the nucleus – Nucleus has proton and neutrons – Electron is – (e-), Proton is + (p+), Neutrons is neutral (n) • For a neutral (non-ionized) atom, Np+ = Ne • Chemical properties are the intrinsic state by electronic configurations (not directly related to nucleus, p+ or n) • Spectral lines (energy emission or absorptions by atoms) are determined by electron transitions • Chemistry, molecular chemistry/physics and atomic physics are all properties of electron configurations.
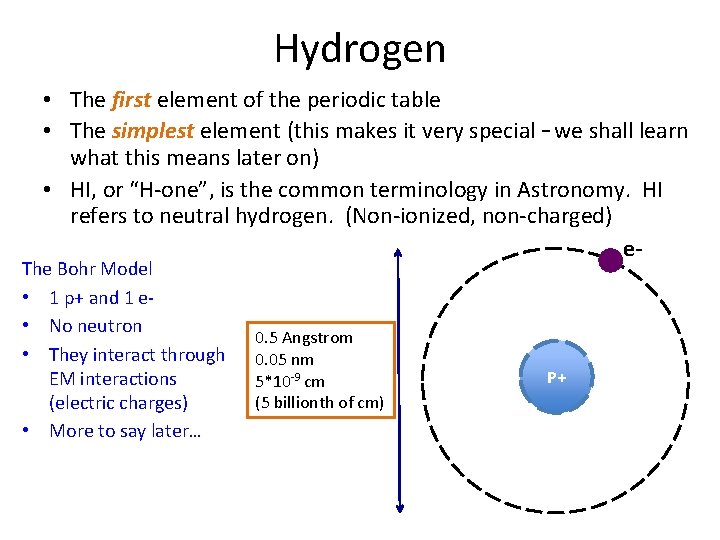
Hydrogen • The first element of the periodic table • The simplest element (this makes it very special – we shall learn what this means later on) • HI, or “H-one”, is the common terminology in Astronomy. HI refers to neutral hydrogen. (Non-ionized, non-charged) e- The Bohr Model • 1 p+ and 1 e • No neutron • They interact through EM interactions (electric charges) • More to say later… 0. 5 Angstrom 0. 05 nm 5*10 -9 cm (5 billionth of cm) P+

Hydrogen Isotopes • 99. 98% of what we call hydrogen is “Protium” • 0. 02% is Deuterium a. k. a “Heavy Water”. • Tritium: Very low abundances < 10 -18 (? ), can be produced by high energy cosmic rays. NOT STABLE. • We will not talk about higher isotopes https: //en. wikipedia. org/wiki/Isotopes_of_hydrogen
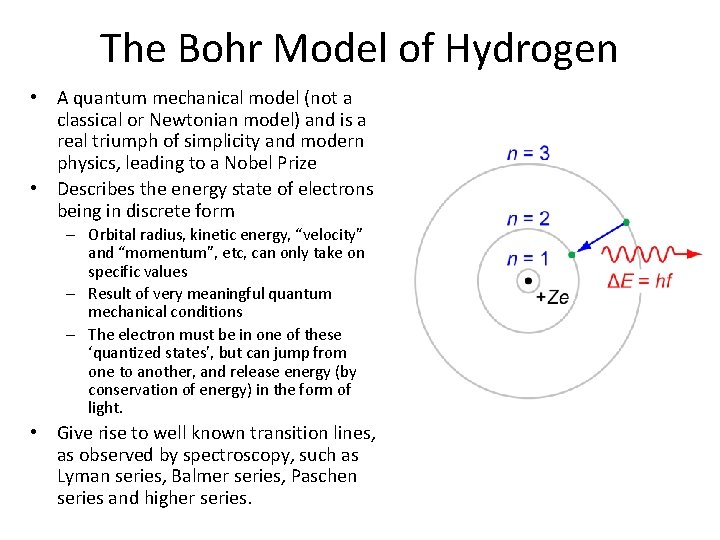
The Bohr Model of Hydrogen • A quantum mechanical model (not a classical or Newtonian model) and is a real triumph of simplicity and modern physics, leading to a Nobel Prize • Describes the energy state of electrons being in discrete form – Orbital radius, kinetic energy, “velocity” and “momentum”, etc, can only take on specific values – Result of very meaningful quantum mechanical conditions – The electron must be in one of these ‘quantized states’, but can jump from one to another, and release energy (by conservation of energy) in the form of light. • Give rise to well known transition lines, as observed by spectroscopy, such as Lyman series, Balmer series, Paschen series and higher series.

Helium • The second element of the periodic table • The second simplest element (also special) • Can also be described by a Borh Model-like configuration, with a nucleus (of 2 p+ and 2 n) with 2 e- revolving around

Higher Elements • Beyond Hydrogen and Helium atom, elements/atoms with more electrons (and more massive nucleus) are not (easily) describable by simple Bohr Model • Full quantum mechanical calculation is required. For example, you may learn about the s, p, d, f orbitals in Chemistry. • But we will not discuss about higher elements in detail. • Nevertheless, the big idea about “quantum mechanical state”, “discrete energy”, “atomic transition lines” remains the same.

The main points • The make up of atoms give rises to the various light, optical phenomena light emission lines that we we see. These are the transition lines of atoms coming from electrons jumping from one discrete energy state to another, release light (photons) in the process. • Absorption line is the reverse.
- Slides: 9