Hydrochloric Acid Sodium Hydroxide Acids and Bases When

Hydrochloric Acid Sodium Hydroxide

Acids and Bases When we think of acids and bases we tend to think of science labs and chemicals…but did you know Acids cause: • Lemons to be sour • Acid rain to eat away at sculptures • Framed paintings to be damaged • Cavities in your teeth • Food to digest in your stomach • Ants and bees use it to sting

Acids and Bases

Acids and Bases

Properties of Acids and Bases ¡ Acids l turn blue litmus red l taste sour l Acids corrode metals l positively charged hydrogen ions (H+) ¡ Bases l turn red litmus blue l taste bitter l Negatively charged hydroxide ions (OH–) l Feel slippery l Most hand soaps and drain cleaners are bases l Strong bases are caustic
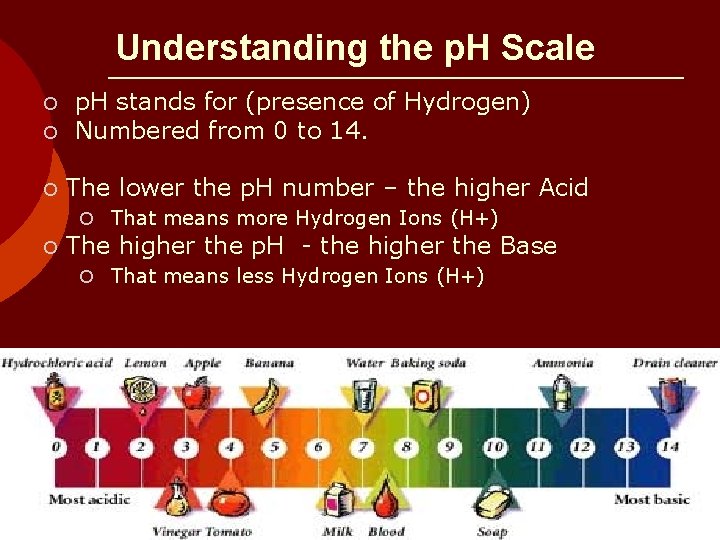
Understanding the p. H Scale o p. H stands for (presence of Hydrogen) o Numbered from 0 to 14. o The lower the p. H number – the higher Acid o That means more Hydrogen Ions (H+) o The higher the p. H - the higher the Base o That means less Hydrogen Ions (H+)

Acids and Bases ¡ Animated explanation

Electrolytes ¡ ¡ Mixture of chemicals that use H+ ions to conduct an electrical current. Fluid that regulates the flow of nutrients into and waste products out of cells. l l Where to find Electrolytes Car battery Lemon ¡ Pickle ¡

Several Types of Acids ¡ Hydrochloric Acid. l ¡ Acetic Acid l ¡ An ingredient in vinegar. Citric Acid l ¡ Stomach acid - has the sour taste of acid. Citrus fruits such as lemons, grapefruit, oranges, and limes have citric acid in the juice. Lactic Acid l Sour milk, sour cream, yogurt, and cottage cheese have lactic acid.

Strong Acids ¡ Nitric acid ¡ Hydrochloric acid ¡ Sulfuric acid ¡ Strong Acids l (HNO 3) (HCl) (H 2 SO 4) are acids that has many hydrogen ions (many H+).

Weak Acids ¡ Weak acids l ¡ Are acids that have fewer hydrogen ions (fewer H+). Examples l l l acetic acid boric acid ¡ sea-water, in plants and especially in fruits carbonic acid ¡ l Found in our blood citric acid

Properties of Bases ¡ ¡ Turn red litmus taste bitter ¡ Negatively charged hydroxide ions (OH–) ¡ Feel slippery ¡ Bases neutralize acids ¡ Poisonous and can cause severe burns ¡ Strong Bases are Caustic.

Some Common Bases ¡ Ammonia l l l ¡ Calcium hydroxide l l ¡ The most widely used base Used in household cleaning materials Used as fertilizer Used to make mortar and plaster Used to help neutralize acid soil Sodium hydroxide l l One of the strongest bases Used in oven cleaners and drain cleaners

Strong Bases ¡ Strong bases l l ¡ Are bases that produce many hydroxide ions (OH-) when it is dissolved in water. Are Caustic Examples l l l Lithium hydroxide (Li. OH) Sodium hydroxide (Na. OH) Potassium hydroxide (KOH)

Weak Bases ¡ Weak bases l ¡ Are bases that produce few hydroxide ions (OH-) when it is dissolved in water. . Examples l Aluminum hydroxide

Quiz - Acids and Bases 1. 2. 3. 4. 5. Please list two properties of Acids. Please list two properties of Bases. True or False A strong Acid has many OHPlease name one type of Acid Why should you wear gloves when using strong Bases?
- Slides: 16