Hydrocarbons Unsaturated hydrocarbons Double and triple bonds between

Hydrocarbons Unsaturated hydrocarbons – Double and triple bonds between carbons – Not every carbon has each of its 4 electrons bonded to 4 different atoms – More chemically reactive than saturated compounds, or alkanes – Unsaturated hydrocarbons include alkenes (double bonds) and alkynes (triple bonds) Mullis 1
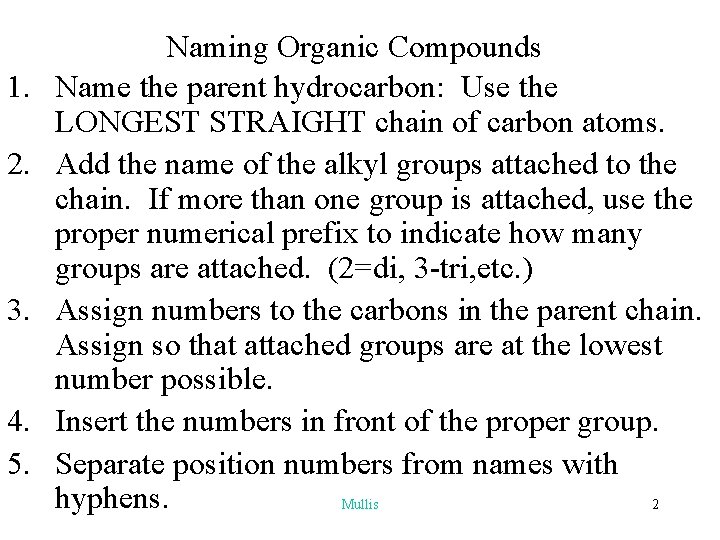
1. 2. 3. 4. 5. Naming Organic Compounds Name the parent hydrocarbon: Use the LONGEST STRAIGHT chain of carbon atoms. Add the name of the alkyl groups attached to the chain. If more than one group is attached, use the proper numerical prefix to indicate how many groups are attached. (2=di, 3 -tri, etc. ) Assign numbers to the carbons in the parent chain. Assign so that attached groups are at the lowest number possible. Insert the numbers in front of the proper group. Separate position numbers from names with hyphens. Mullis 2
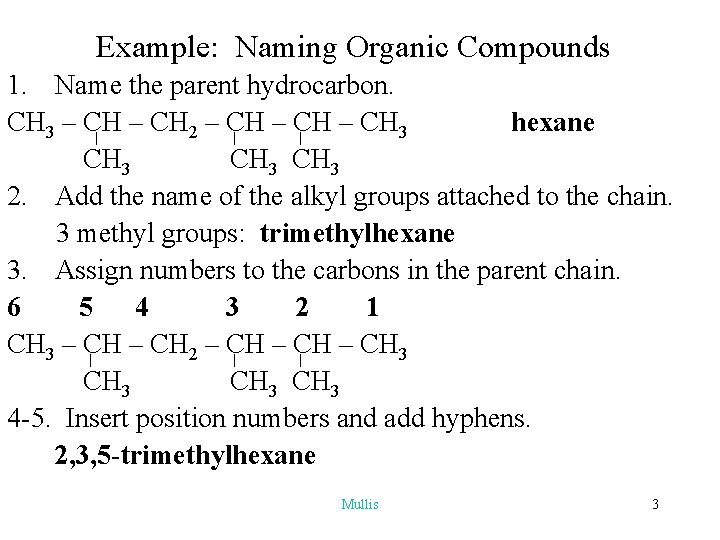
Example: Naming Organic Compounds 1. Name the parent hydrocarbon. CH 3 – CH 2 – CH 3 hexane CH 3 2. Add the name of the alkyl groups attached to the chain. 3 methyl groups: trimethylhexane 3. Assign numbers to the carbons in the parent chain. 6 5 4 3 2 1 CH 3 – CH 2 – CH 3 CH 3 4 -5. Insert position numbers and add hyphens. 2, 3, 5 -trimethylhexane Mullis 3
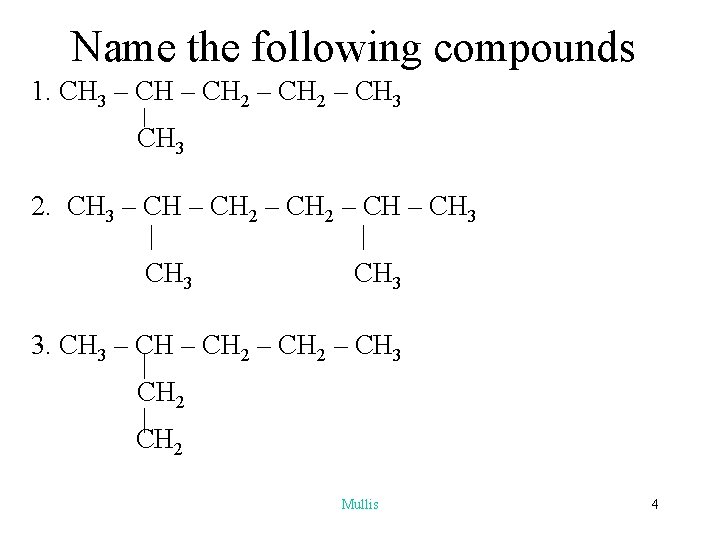
Name the following compounds 1. CH 3 – CH 2 – CH 3 2. CH 3 – CH 2 – CH 3 | | CH 3 3. CH 3 – CH 2 – CH 3 CH 2 Mullis 4
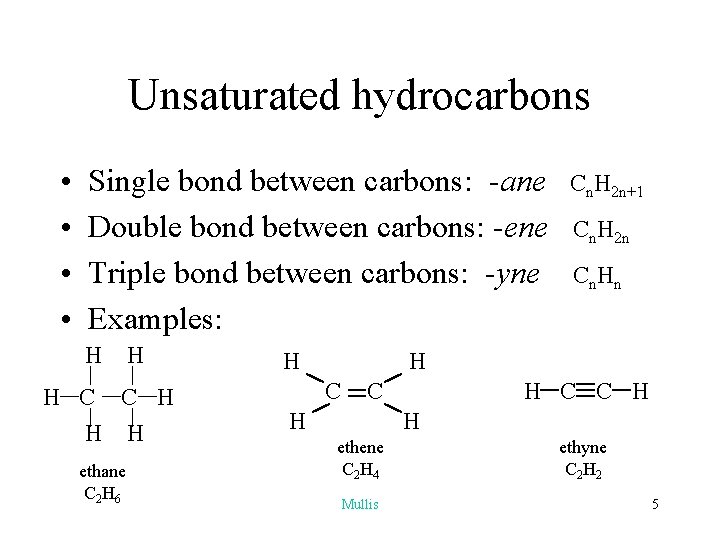
Unsaturated hydrocarbons • • Single bond between carbons: -ane Double bond between carbons: -ene Triple bond between carbons: -yne Examples: H H C H H ethane C 2 H 6 H H Cn. H 2 n+1 Cn. H 2 n Cn. Hn H C C H H ethene C 2 H 4 Mullis ethyne C 2 H 2 5

Other Functional Groups Compound Formula Example Alcohol R-OH hydroxyl group 1 -propanol Alkyl Halide -X X = any halide 1, 2 -dibromopropane Ether R-O-R’ one oxygen bonded to 2 hydrocarbon groups diethyl ether CH 3 -CH 2 –O--CH 2 --CH 3 Aldehyde O || R-C-H Carbonyl group attached to end carbon Ethanal Ketone O || R-C-R’ Carbonyl group attached to a middle carbon 2 -propanone O || CH 3—C-- CH 3 Carboxylic Acid O || R-C-OH Carboxyl group Ester O || R-C-O-R’ Carboxyl group without the H Mullis O || CH 3—C--H ethanoic acid O || CH 3—C—OH methyl ethanoate O || CH 3—C—O-- CH 3 6
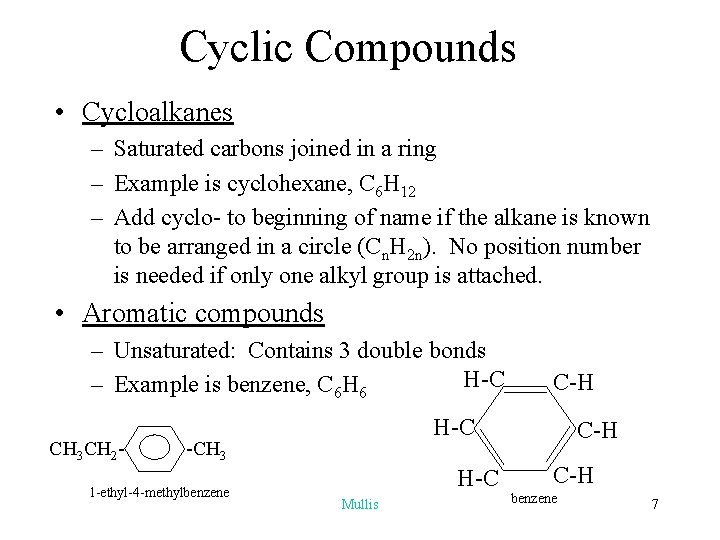
Cyclic Compounds • Cycloalkanes – Saturated carbons joined in a ring – Example is cyclohexane, C 6 H 12 – Add cyclo- to beginning of name if the alkane is known to be arranged in a circle (Cn. H 2 n). No position number is needed if only one alkyl group is attached. • Aromatic compounds – Unsaturated: Contains 3 double bonds H-C – Example is benzene, C 6 H 6 CH 3 CH 2 - H-C -CH 3 1 -ethyl-4 -methylbenzene C-H H-C Mullis C-H benzene 7
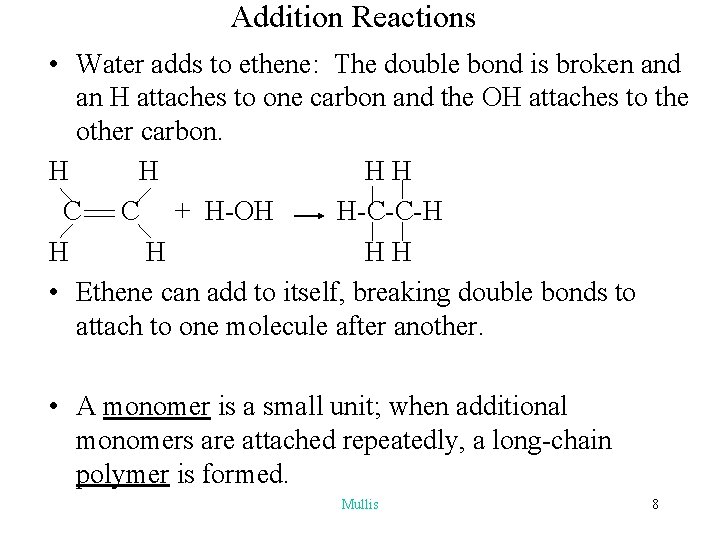
Addition Reactions • Water adds to ethene: The double bond is broken and an H attaches to one carbon and the OH attaches to the other carbon. H H HH C C + H-OH H-C-C-H H H HH • Ethene can add to itself, breaking double bonds to attach to one molecule after another. • A monomer is a small unit; when additional monomers are attached repeatedly, a long-chain polymer is formed. Mullis 8
Addition Polymers • Polyethylene is a polymer made from the monomer ethene. Zip-lock bags are usually made from lowdensity polyethylene. • Common polymer variations replace one of ethene’s hydrogens with another unit, such as a halogen atom (F or Cl), Cyanide (CN), or benzene (C 6 H 6). – Vinyl chloride – Acrylonitrile – Styrene polyvinyl chloride polyacrylnitrile polystyrene • Atoms that compose the monomers determine the properties of the polymer. Mullis 9
Petrochemicals • Petrochemicals are compounds produced from oil or natural gas. • Most are used to produce other synthetic products, especially plastics. • Builder molecules are those small-molecule compounds such as ethene. • Ethene is a 2 -carbon hydrocarbon with a double bond (2 pairs of shared electrons). • Compounds with double and triple bonds are more reactive than those with single bonds. Mullis 10
- Slides: 10