Hydrocarbons Saturated Alkanes Chapter 2 Unsaturated Alkenes Chapters
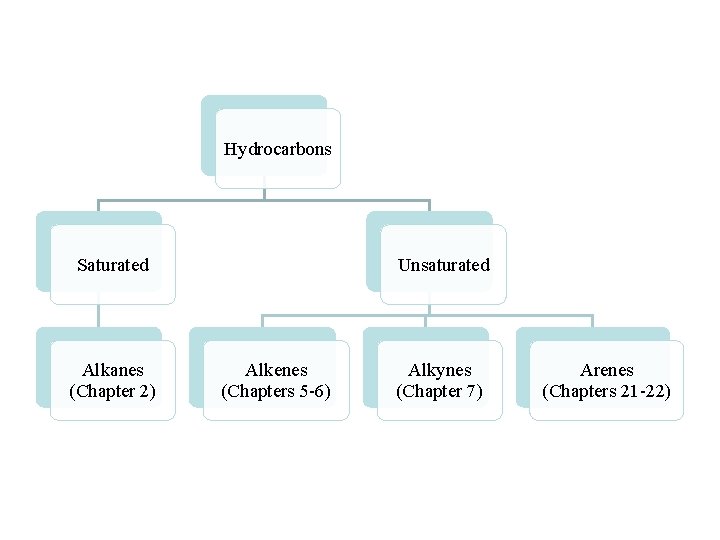
Hydrocarbons Saturated Alkanes (Chapter 2) Unsaturated Alkenes (Chapters 5 -6) Alkynes (Chapter 7) Arenes (Chapters 21 -22)
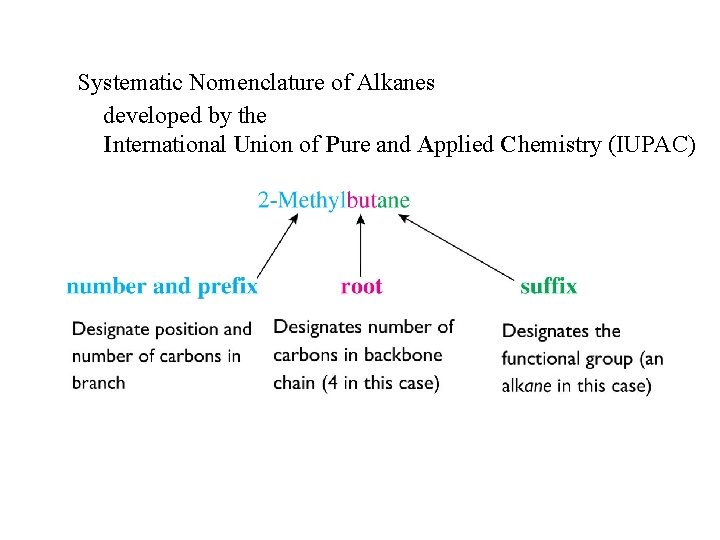
Systematic Nomenclature of Alkanes developed by the International Union of Pure and Applied Chemistry (IUPAC)
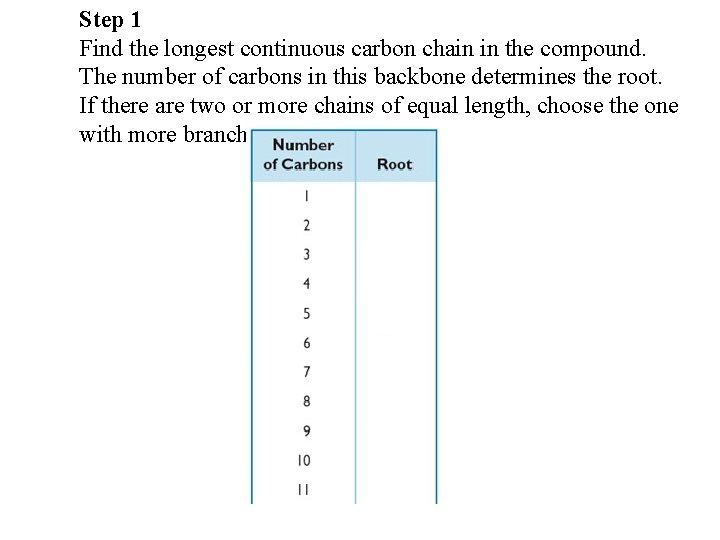
Step 1 Find the longest continuous carbon chain in the compound. The number of carbons in this backbone determines the root. If there are two or more chains of equal length, choose the one with more branches.
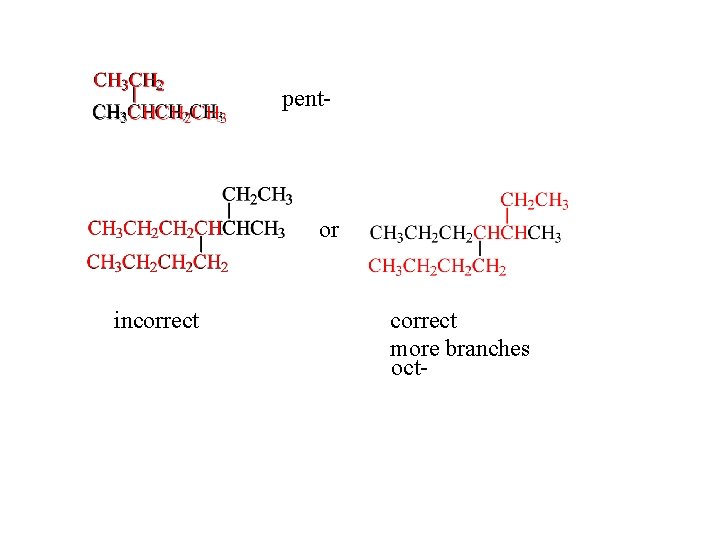
pent- or incorrect more branches oct-
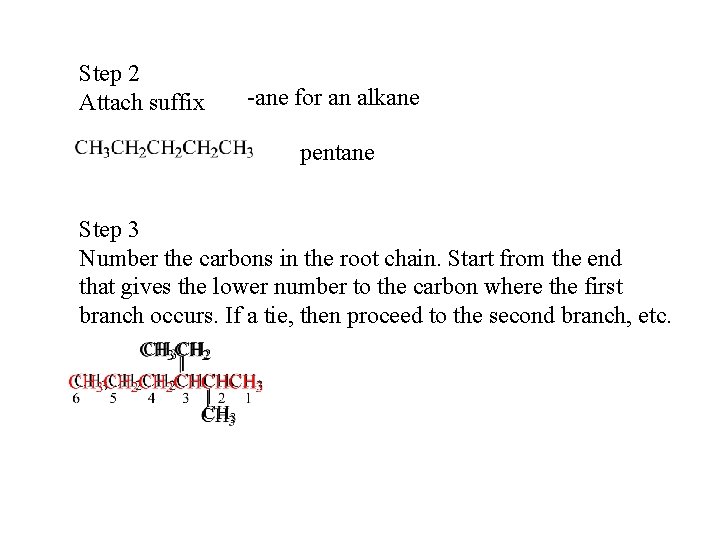
Step 2 Attach suffix -ane for an alkane pentane Step 3 Number the carbons in the root chain. Start from the end that gives the lower number to the carbon where the first branch occurs. If a tie, then proceed to the second branch, etc.
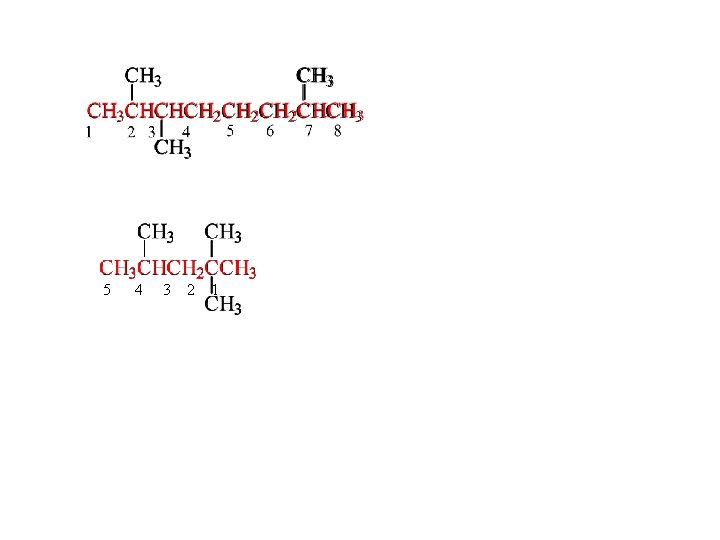
5 4 3 2 1

Step 4 Name the groups. root + -yl
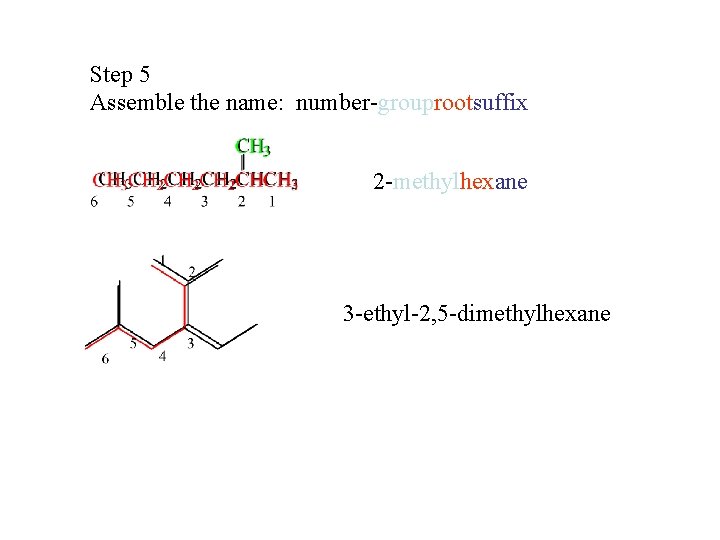
Step 5 Assemble the name: number-grouprootsuffix 2 -methylhexane 3 -ethyl-2, 5 -dimethylhexane
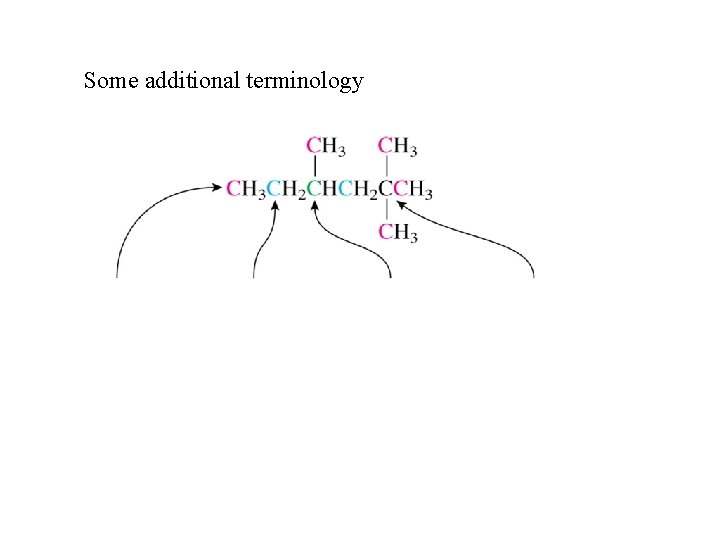
Some additional terminology
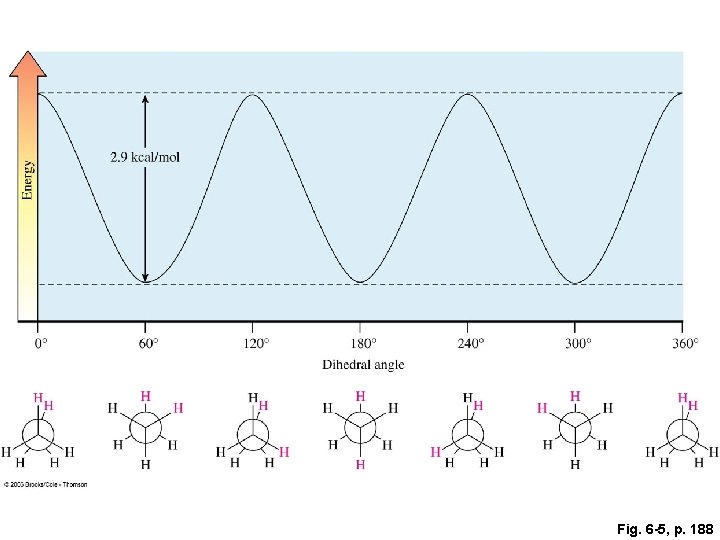
Fig. 6 -5, p. 188
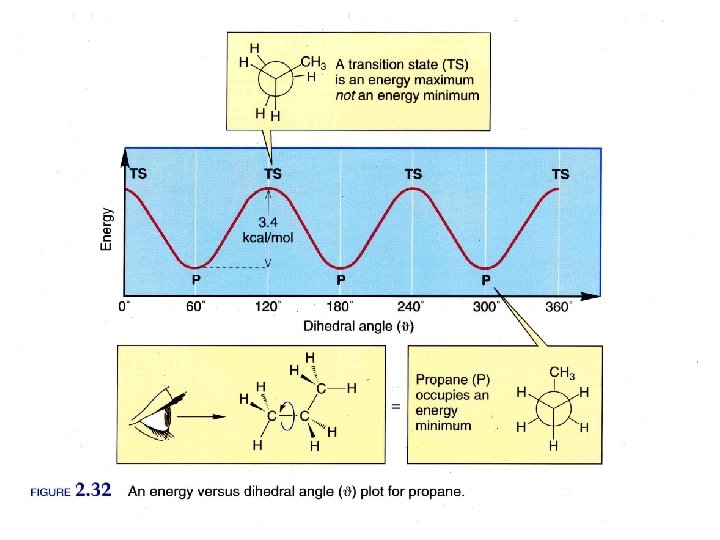
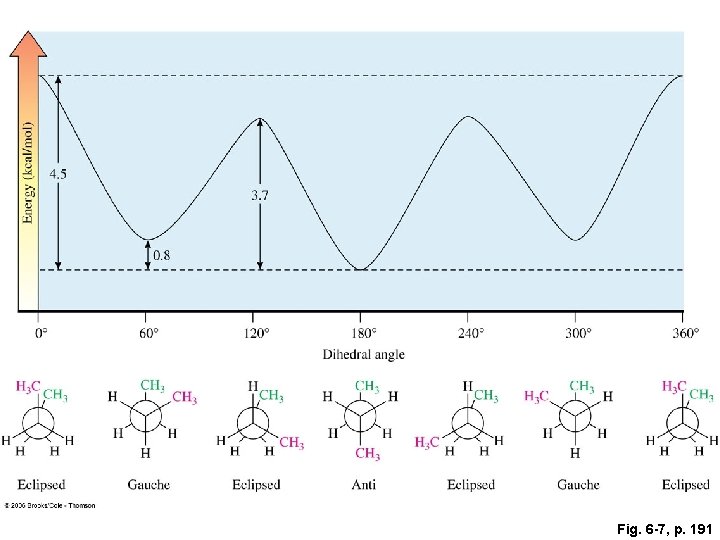
Fig. 6 -7, p. 191
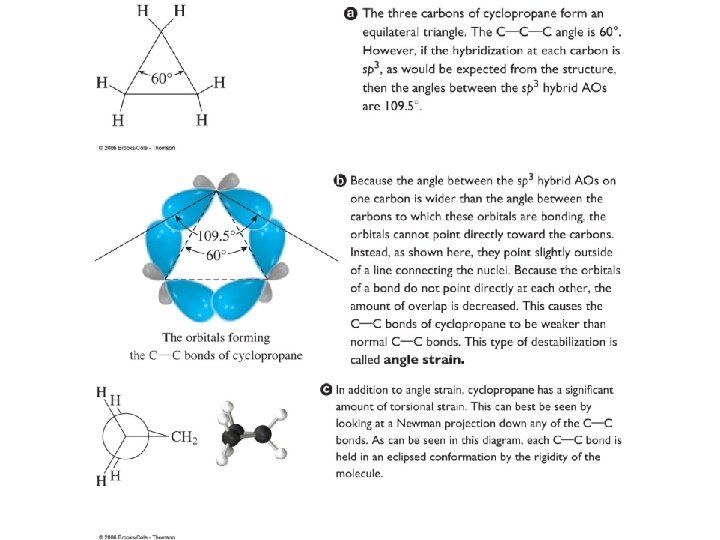
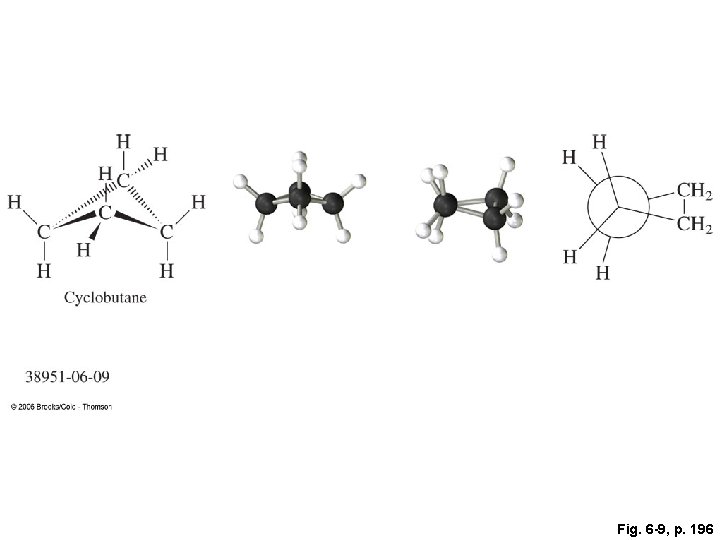
Fig. 6 -9, p. 196
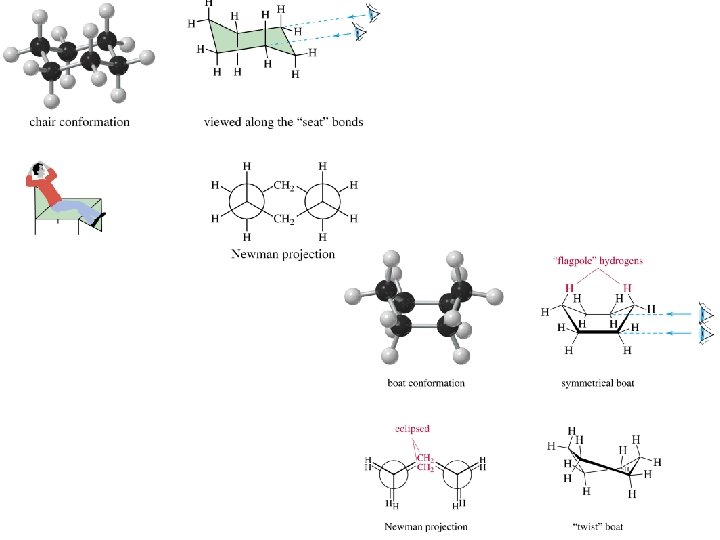
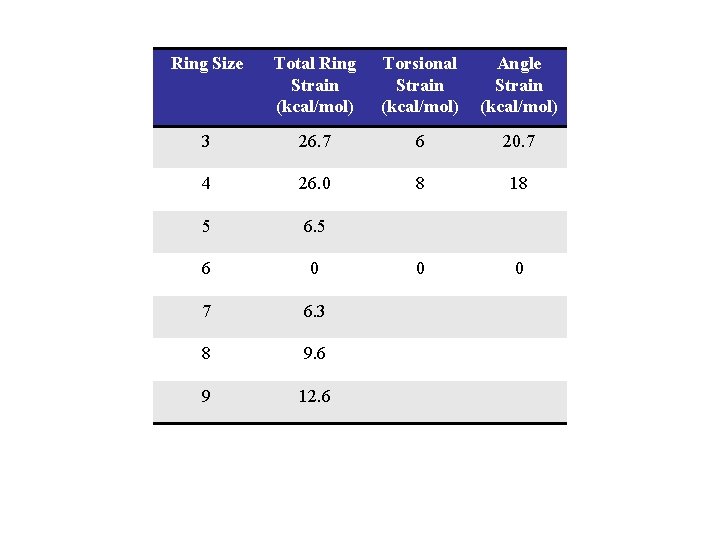
Ring Size Total Ring Strain (kcal/mol) Torsional Strain (kcal/mol) Angle Strain (kcal/mol) 3 26. 7 6 20. 7 4 26. 0 8 18 5 6 0 0 0 7 6. 3 8 9. 6 9 12. 6
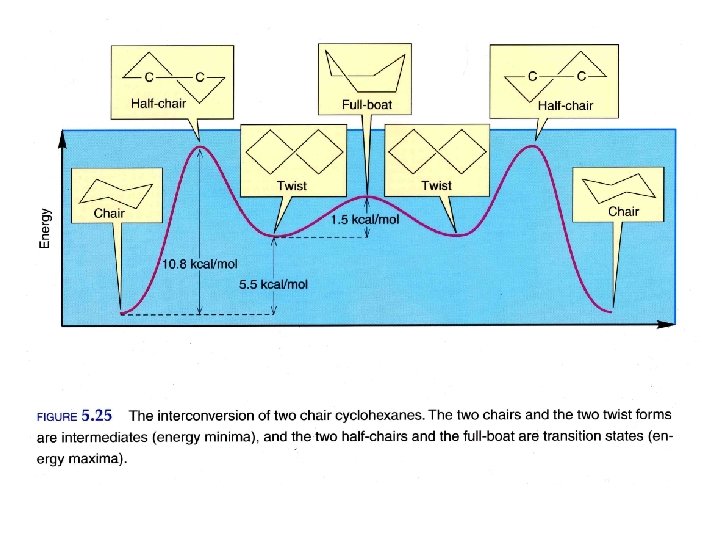
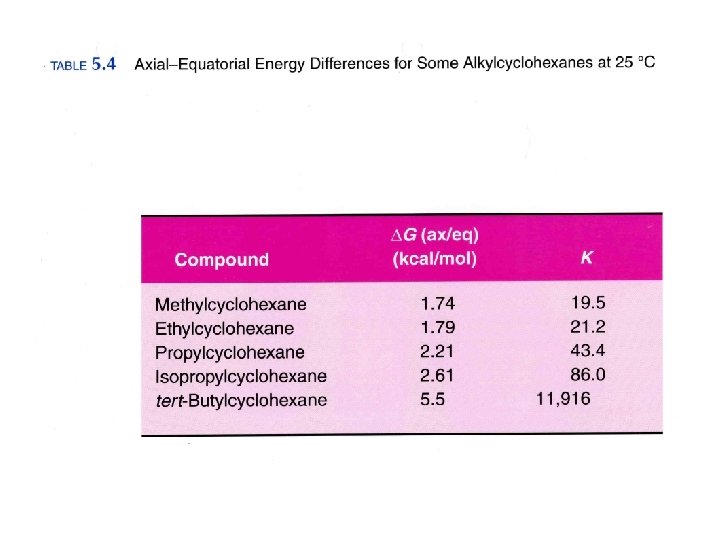
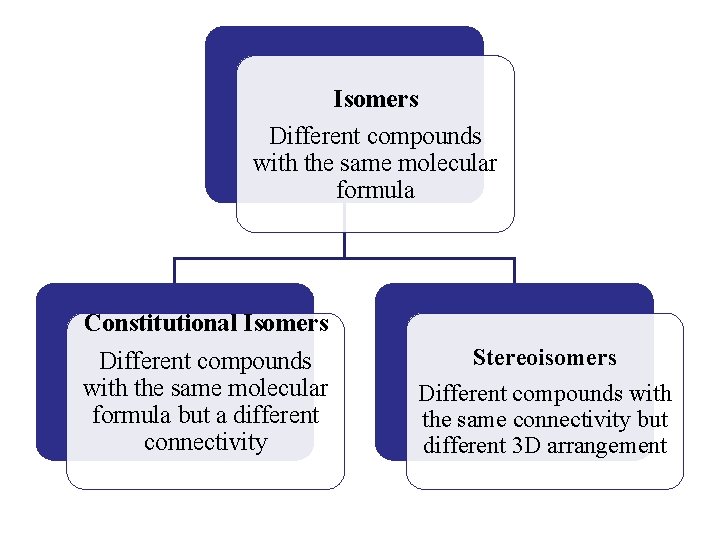
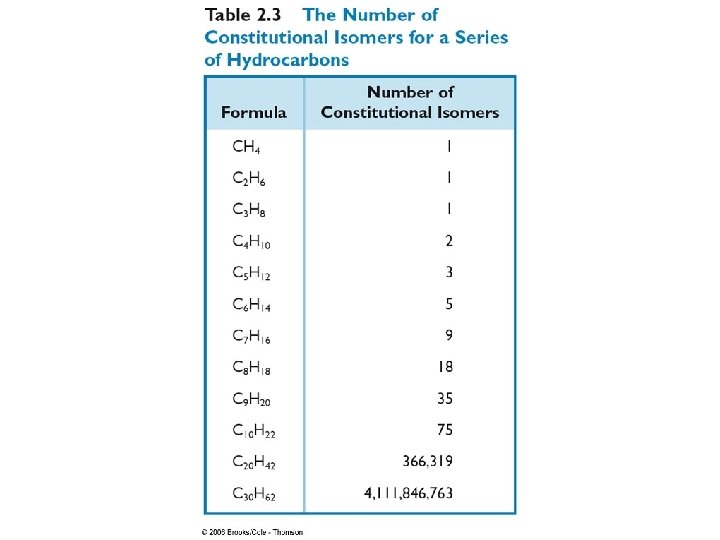
Isomers Different compounds with the same molecular formula Constitutional Isomers Different compounds with the same molecular formula but a different connectivity Stereoisomers Different compounds with the same connectivity but different 3 D arrangement
- Slides: 20