Hydrocarbons Hydrocarbons are a class of compounds which

Hydrocarbons… Hydrocarbons are a class of compounds which contain ONLY hydrogen and carbon. There a number of different types but we will be concentrating on: • Alkanes • Alkenes

Hydrocarbons… Alkanes are a homologous series where: • No double bonds present (SATURATED) • General Formula = Cn. H 2 n+2 • Compounds are non-polar

Hydrocarbons… What shape does a carbon and its surrounding bonds have in an alkane (Use VSEPR) Tetrahedral 109. 5 o
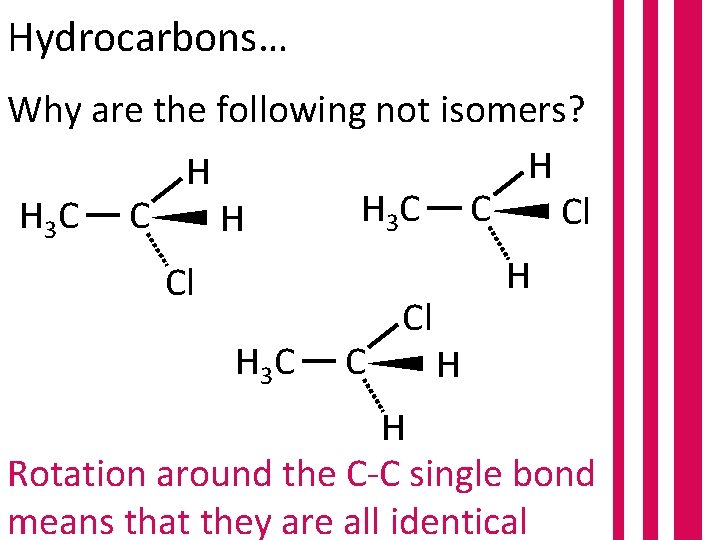
Hydrocarbons… Why are the following not isomers? H H H 3 C C Cl H 3 C C H H Cl Cl H 3 C C H H Rotation around the C-C single bond means that they are all identical

Hydrocarbons… What happens to the boiling point of alkanes as the chain length increases? [4 marks] • Increased contact between the hydrocarbon chains • Increased Instantaneous dipole – induced dipole • Increased energy required to overcome IM forces • Increased boiling point

Hydrocarbons… Which has the higher boiling point, heptane or 2, 4 -dimethylpentane? Explain your answer. • There is more contact between the chains of heptane as less BRANCHING • More contact means higher IM forces • Heptane has the higher b. p.

Hydrocarbons… Alkenes undergo the following types of reaction: • Combustion: • Complete • Incomplete • Radical Substitution: • Halogenation We will look at these in greater detail in a couple of lessons

Hydrocarbons… Alkenes are a homologous series where: • Double bonds present (UNSATURATED) • General Formula = Cn. H 2 n • Exhibit stereoisomerism

Hydrocarbons… What shape does a carbon and its surrounding bonds have in a double bond (Use VSEPR) Trigonal Planar 120 o
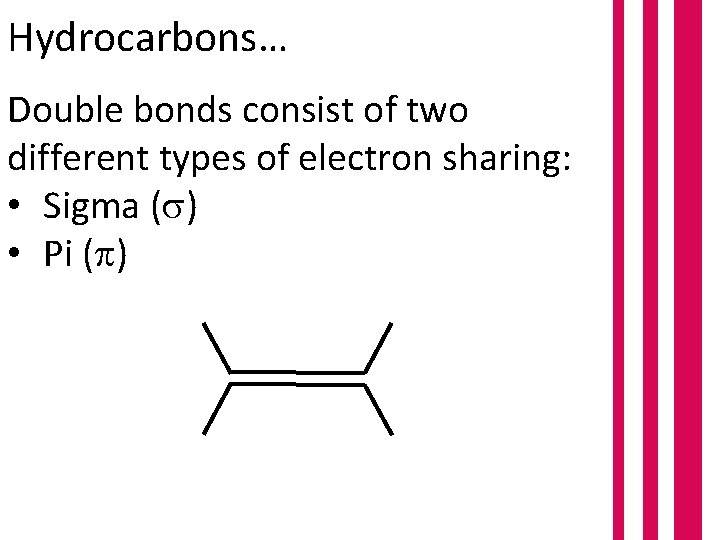
Hydrocarbons… Double bonds consist of two different types of electron sharing: • Sigma ( ) • Pi ( )
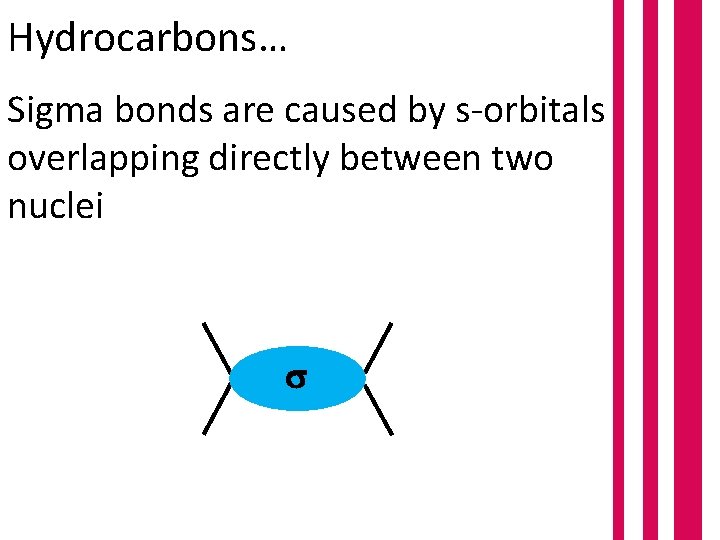
Hydrocarbons… Sigma bonds are caused by s-orbitals overlapping directly between two nuclei
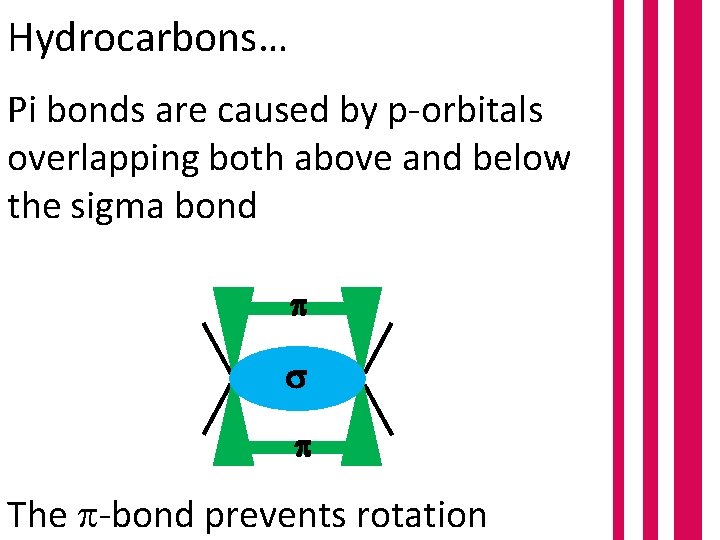
Hydrocarbons… Pi bonds are caused by p-orbitals overlapping both above and below the sigma bond The -bond prevents rotation

Hydrocarbons… Alkenes undergo the following reactions: • Hydrogenation • Electrophilic addition • Halogenation • Hydrogen halides • Hydration • Polymerisation
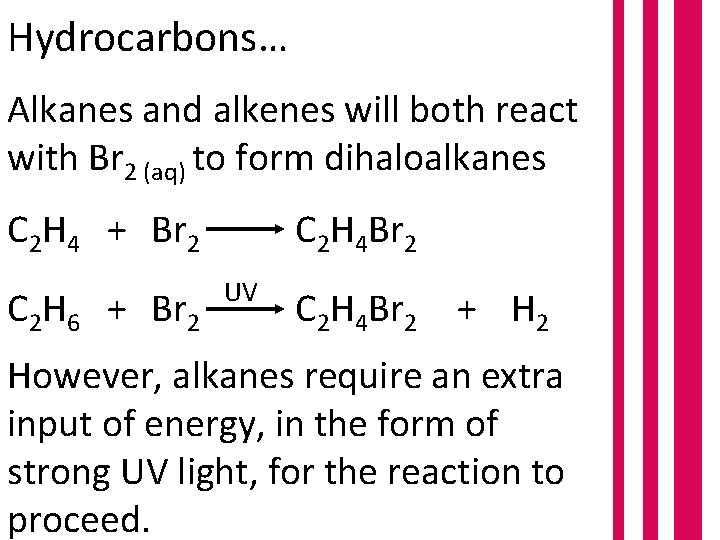
Hydrocarbons… Alkanes and alkenes will both react with Br 2 (aq) to form dihaloalkanes C 2 H 4 + Br 2 C 2 H 6 + Br 2 C 2 H 4 Br 2 UV C 2 H 4 Br 2 + H 2 However, alkanes require an extra input of energy, in the form of strong UV light, for the reaction to proceed.

Hydrocarbons… Use this information to design an experiment to determine which samples are alkenes. You will be given: • • Reference Alkane Reference Alkene Bromine water (Br 2 (aq)) Unlabelled hydrocarbons You need to produce: • • Method Results table
- Slides: 15