Hydrocarbons Chemistry ch 21 Objectives Explain the organic

Hydrocarbons Chemistry ch 21

Objectives ¢ Explain the organic compound and organic chemistry ¢ Identify hydrocarbons and the models used to represent them ¢ Distinguish between saturated and unsaturated hydrocarbons ¢ Name alkanes and alkenes by examining their structures. ¢ Draw the structure of an alkane and alkenes when given its name

Hydrocarbons ¢ Organic Chemistry – branch of chemistry that deals with carbon compounds l All living organisms have carbon in them ¢ Organic compound is applied to all carbon-containing compounds with the primary exceptions of carbon oxides, carbides, and carbonates, which are considered inorganic l In organic compounds, carbon nearly always shares its electrons and forms four covalent bonds l Carbon atoms are bonded to hydrogen atoms or other elements near carbon on the periodic table. l Carbon atoms also bond to other carbon atoms and form long chains
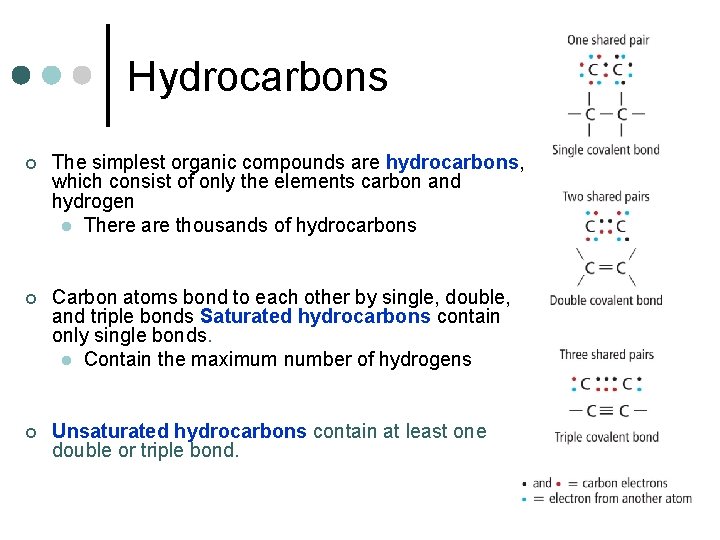
Hydrocarbons ¢ The simplest organic compounds are hydrocarbons, which consist of only the elements carbon and hydrogen l There are thousands of hydrocarbons ¢ Carbon atoms bond to each other by single, double, and triple bonds Saturated hydrocarbons contain only single bonds. l Contain the maximum number of hydrogens ¢ Unsaturated hydrocarbons contain at least one double or triple bond.
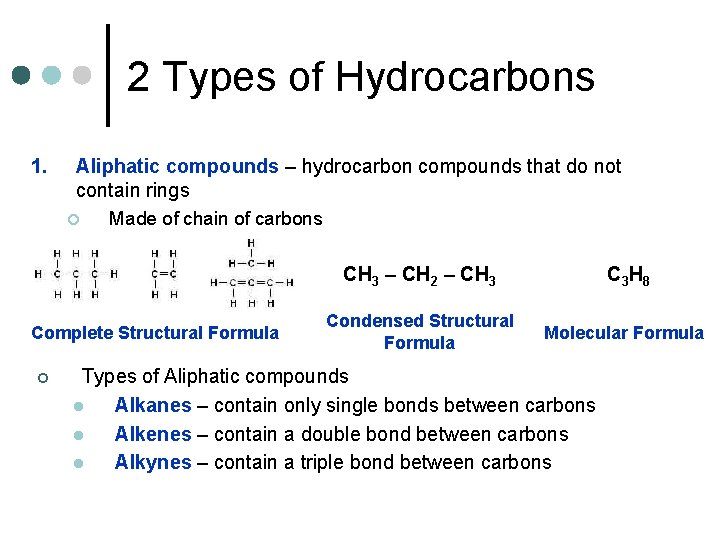
2 Types of Hydrocarbons 1. Aliphatic compounds – hydrocarbon compounds that do not contain rings ¢ Made of chain of carbons Complete Structural Formula ¢ CH 3 – CH 2 – CH 3 C 3 H 8 Condensed Structural Formula Molecular Formula Types of Aliphatic compounds l Alkanes – contain only single bonds between carbons l Alkenes – contain a double bond between carbons l Alkynes – contain a triple bond between carbons
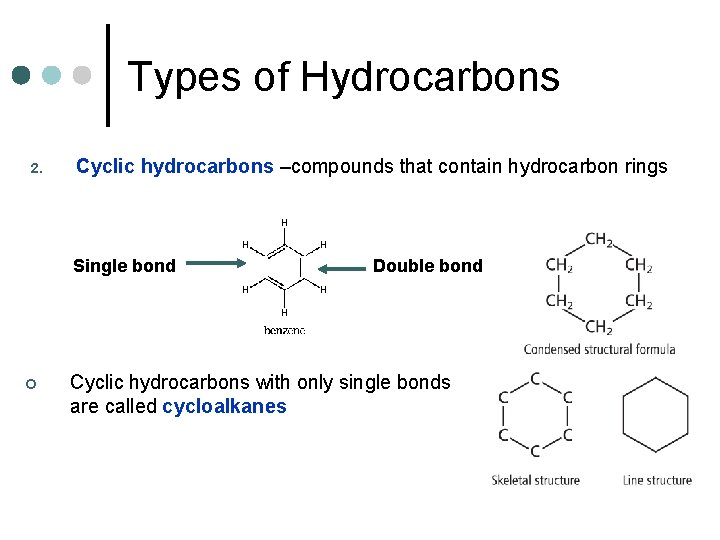
Types of Hydrocarbons 2. Cyclic hydrocarbons –compounds that contain hydrocarbon rings Single bond ¢ Double bond Cyclic hydrocarbons with only single bonds are called cycloalkanes
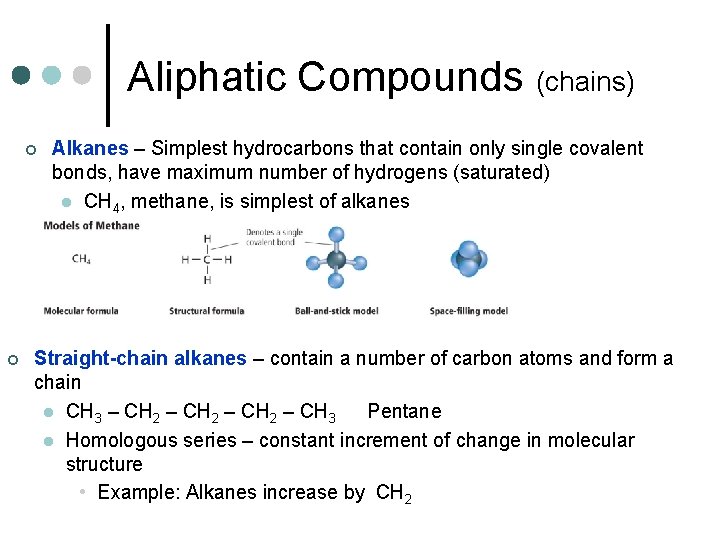
Aliphatic Compounds (chains) ¢ ¢ Alkanes – Simplest hydrocarbons that contain only single covalent bonds, have maximum number of hydrogens (saturated) l CH 4, methane, is simplest of alkanes Straight-chain alkanes – contain a number of carbon atoms and form a chain l CH 3 – CH 2 – CH 3 Pentane l Homologous series – constant increment of change in molecular structure • Example: Alkanes increase by CH 2
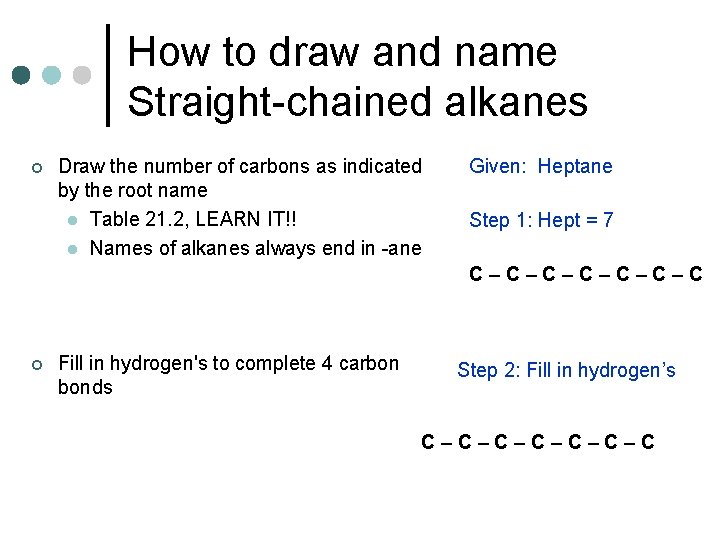
How to draw and name Straight-chained alkanes ¢ Draw the number of carbons as indicated by the root name l Table 21. 2, LEARN IT!! l Names of alkanes always end in -ane Given: Heptane Step 1: Hept = 7 C–C–C–C ¢ Fill in hydrogen's to complete 4 carbon bonds Step 2: Fill in hydrogen’s C–C–C–C

How to draw and name Straight-chained alkanes Given: CH 3 CH 2 CH 2 CH 3 Step 1: Count the carbons 5 carbons = Pent Step 2: add - ane Pentane
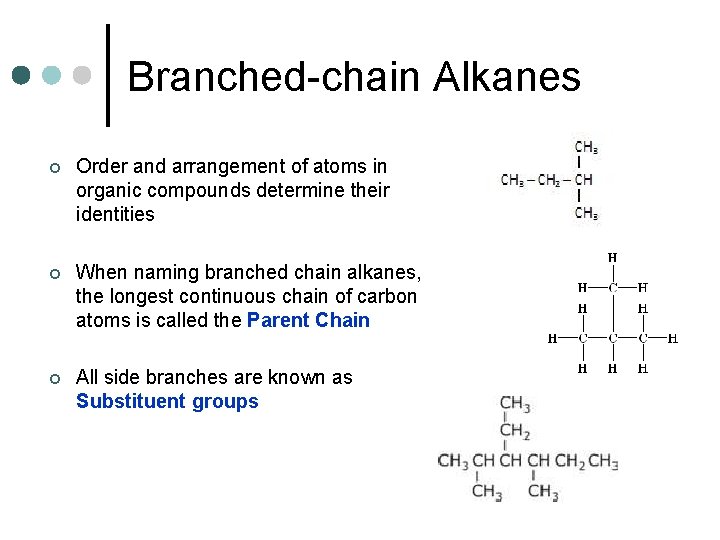
Branched-chain Alkanes ¢ Order and arrangement of atoms in organic compounds determine their identities ¢ When naming branched chain alkanes, the longest continuous chain of carbon atoms is called the Parent Chain ¢ All side branches are known as Substituent groups
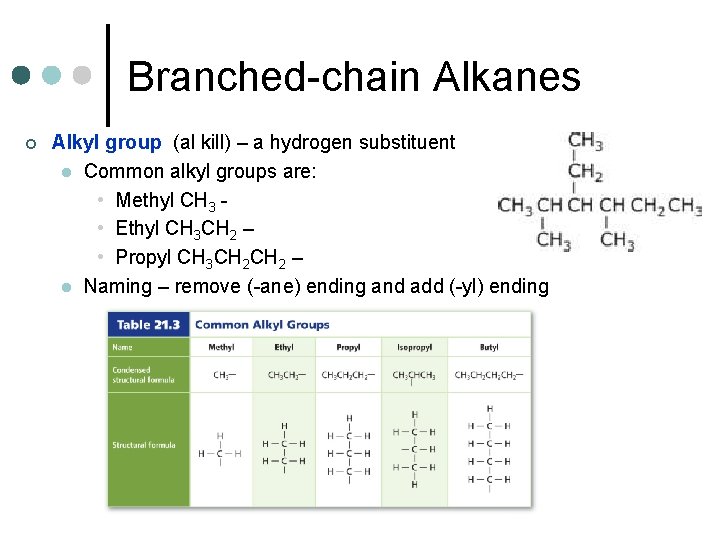
Branched-chain Alkanes ¢ Alkyl group (al kill) – a hydrogen substituent l Common alkyl groups are: • Methyl CH 3 • Ethyl CH 3 CH 2 – • Propyl CH 3 CH 2 – l Naming – remove (-ane) ending and add (-yl) ending

Naming Branched-chain Alkanes CH 3 Find the longest chain of carbons 4. Use prefixes if a substituent appears more | than. This onceisinthe theparent structure l chain CH 3–CH 2–CH–CH 2–CH 3 l Example: di-, tri-, tetra-, etc…. . 1. Number the carbons in the parent chain 5. List the names of the alkyl subsituents in sequence order alphabetical Start atdi-, thetri-, end that gives the attached ll Ignore etc… groups the smallest # 6. Proper punctuation Commas to names separate 3. ¢ Add numbersused to the of numbers the ¢ substituent Hyphensgroups used totoseparate and indentifynumbers their words positions on the chain ¢ Entire name is written without any l The numbers become prefixes to the spaces name of the parent alkane 2.
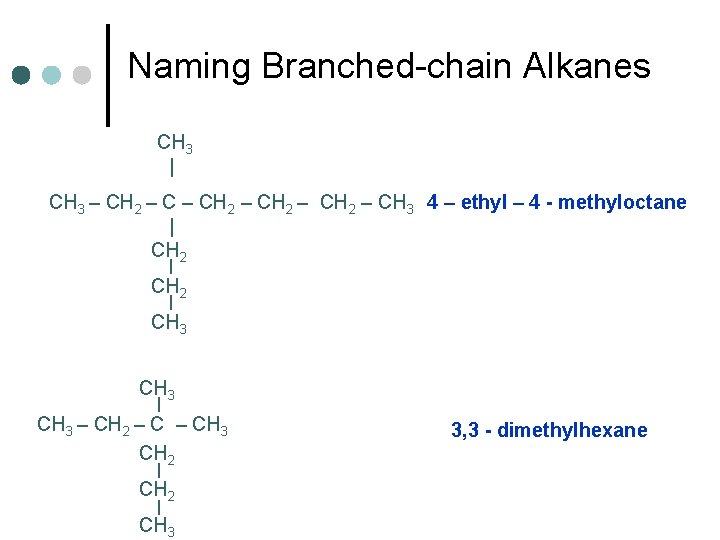
Naming Branched-chain Alkanes CH 3 | CH 3 – CH 2 – CH 3 4 – ethyl – 4 - methyloctane | CH 2 | CH 3 – CH 2 – CH 3 CH 2 | CH 3 3, 3 - dimethylhexane
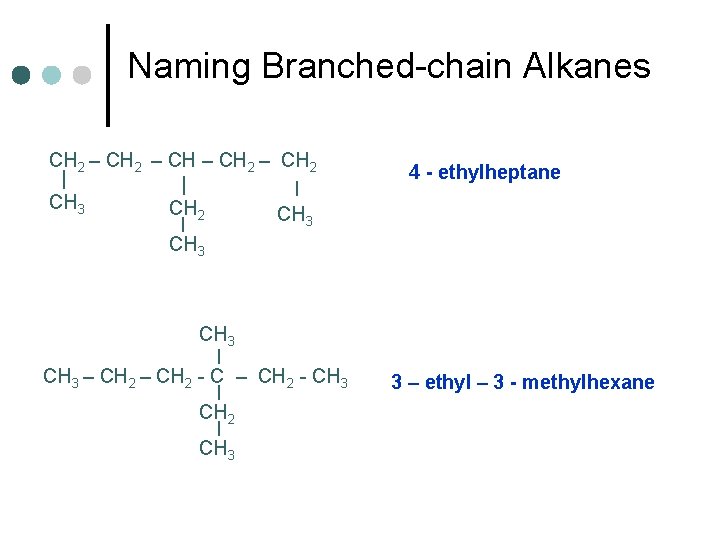
Naming Branched-chain Alkanes CH 2 – CH 2 | | | CH 3 CH 2 CH 3 | 4 - ethylheptane CH 3 | CH 3 – CH 2 - CH 3 | CH 2 | CH 3 3 – ethyl – 3 - methylhexane
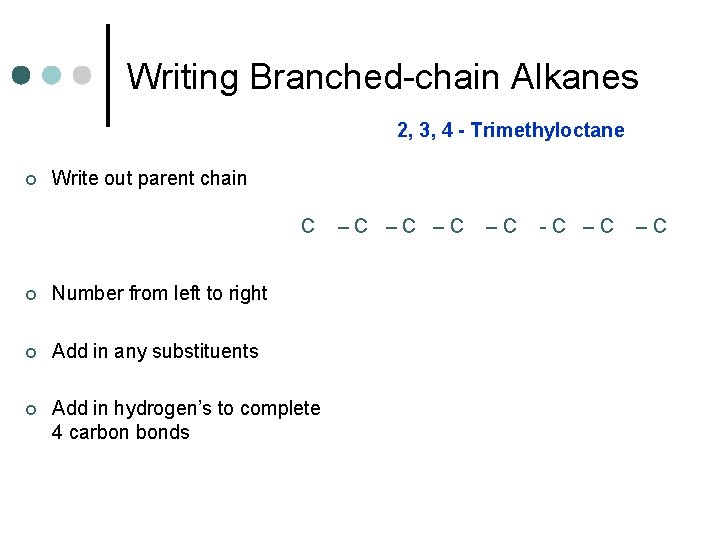
Writing Branched-chain Alkanes 2, 3, 4 - Trimethyloctane ¢ Write out parent chain C ¢ Number from left to right ¢ Add in any substituents ¢ Add in hydrogen’s to complete 4 carbon bonds –C –C -C –C –C
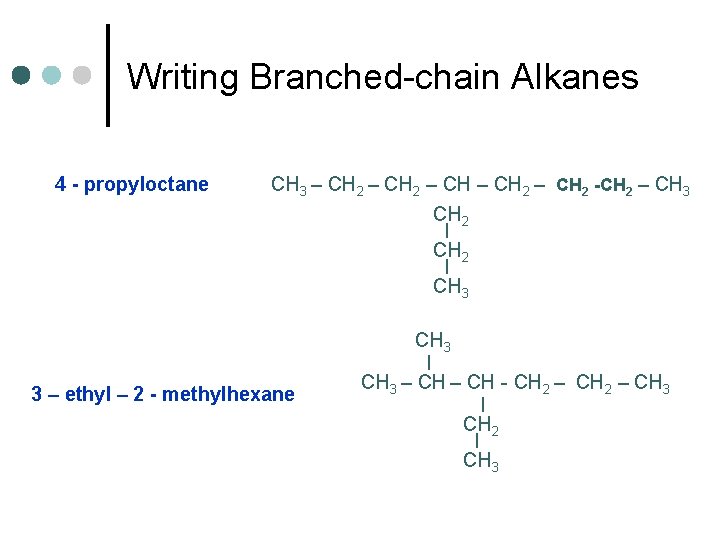
Writing Branched-chain Alkanes 4 - propyloctane CH 3 – CH 2 – CH 2 -CH 2 – CH 3 CH 2 | CH 3 | 3 – ethyl – 2 - methylhexane CH 3 – CH - CH 2 – CH 3 | CH 2 | CH 3

Aliphatic compounds ¢ Alkenes – hydrocarbons containing carbon-carbon double bonds l Alkenes do not have the maximum number of hydrogen’s due to the double bonds l Unsaturated hydrocarbon Naming Alkenes ¢ Find the longest chain in molecule that contains double bond l This is the parent chain ¢ Chain is numbered so that double bond gets the lowest number possible ¢ Root name used, add –ene ending ¢ Substituents are named and numbered same way as alkanes
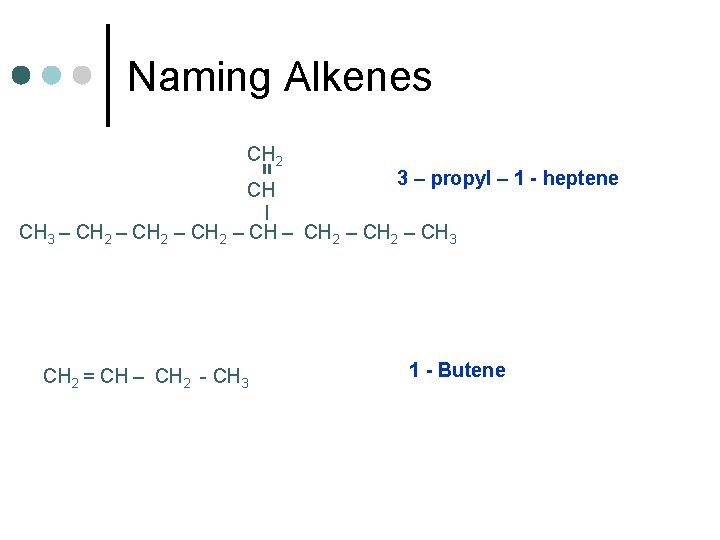
Naming Alkenes = CH 2 CH 3 – propyl – 1 - heptene | CH 3 – CH 2 – CH 2 – CH 3 CH 2 = CH – CH 2 - CH 3 1 - Butene
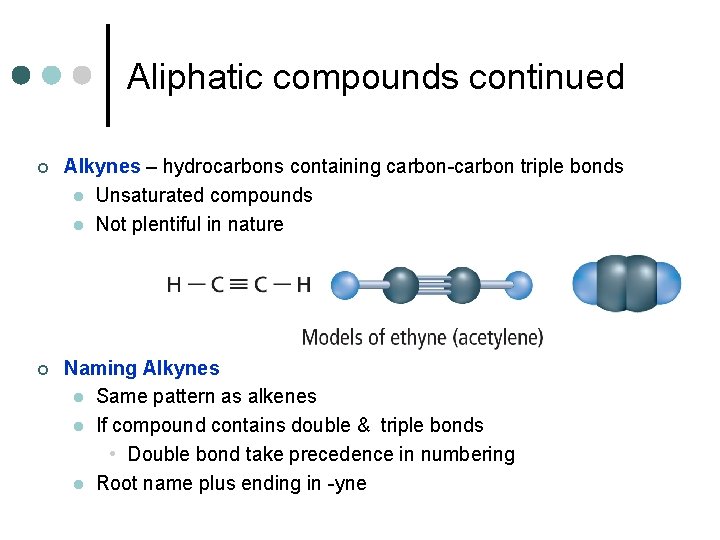
Aliphatic compounds continued ¢ Alkynes – hydrocarbons containing carbon-carbon triple bonds l Unsaturated compounds l Not plentiful in nature ¢ Naming Alkynes l Same pattern as alkenes l If compound contains double & triple bonds • Double bond take precedence in numbering l Root name plus ending in -yne
Isomers ¢ Isomers are two or more compounds that have the same molecular formula (chemical formula) but different molecular structures l The structure of a substance determines the properties of the hydrocarbon There are two main classes of isomers: 1. Structural isomers have the same chemical formula but their atoms are bonded in different orders Example: C 5 H 12 2. Stereoisomers are the atoms are bonded in the same order but are arranged differently in space
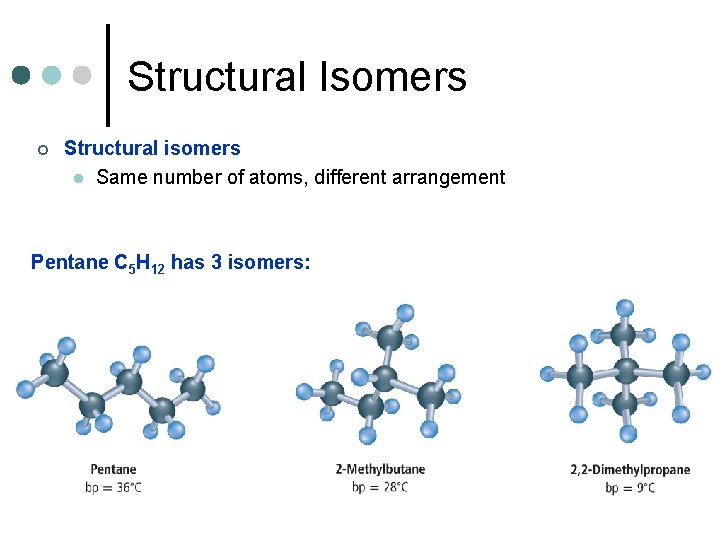
Structural Isomers ¢ Structural isomers l Same number of atoms, different arrangement Pentane C 5 H 12 has 3 isomers:
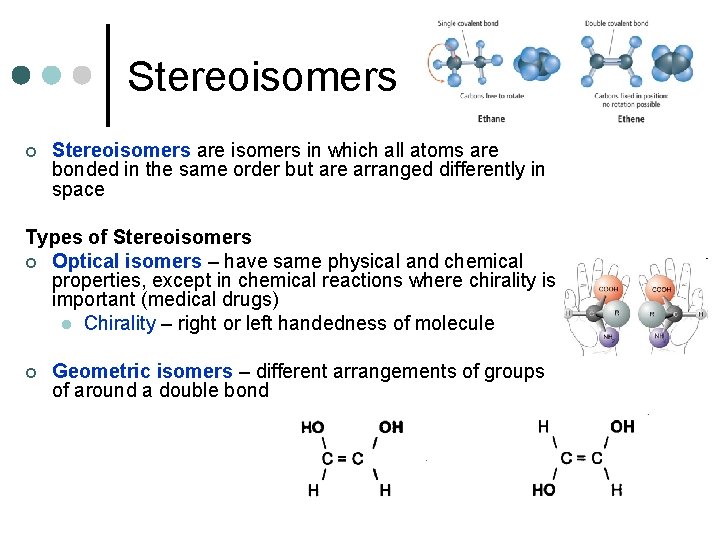
Stereoisomers ¢ Stereoisomers are isomers in which all atoms are bonded in the same order but are arranged differently in space Types of Stereoisomers ¢ Optical isomers – have same physical and chemical properties, except in chemical reactions where chirality is important (medical drugs) l Chirality – right or left handedness of molecule ¢ Geometric isomers – different arrangements of groups of around a double bond
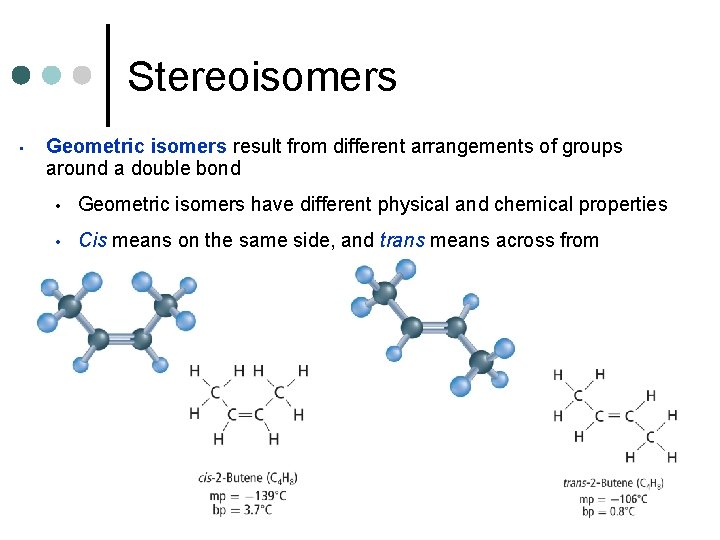
Stereoisomers • Geometric isomers result from different arrangements of groups around a double bond • Geometric isomers have different physical and chemical properties • Cis means on the same side, and trans means across from
Properties of Aliphatic hydrocarbons ¢ STRUCTURE AFFECTS MOLECULAR PROPERTIES ¢ Alkanes are not polar and are good solvents for other nonpolar molecules Alkanes have low reactivity because they are nonpolar and have no charge, and because they have strong single bonds between carbon atoms. Alkenes are nonpolar and have low solubility in water. Alkenes are more reactive than alkanes because the double bond increases electron density between the two carbon atoms, providing a good site for chemical reactivity Alkynes have physical and chemical properties similar to alkenes but are generally more reactive because the triple bonds cause even larger electron densities than double bonds ¢ ¢ ¢
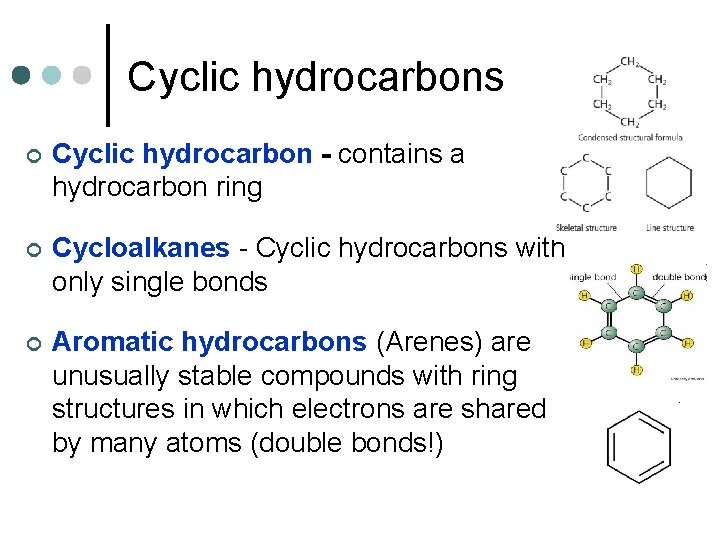
Cyclic hydrocarbons ¢ Cyclic hydrocarbon - contains a hydrocarbon ring ¢ Cycloalkanes - Cyclic hydrocarbons with only single bonds ¢ Aromatic hydrocarbons (Arenes) are unusually stable compounds with ring structures in which electrons are shared by many atoms (double bonds!)
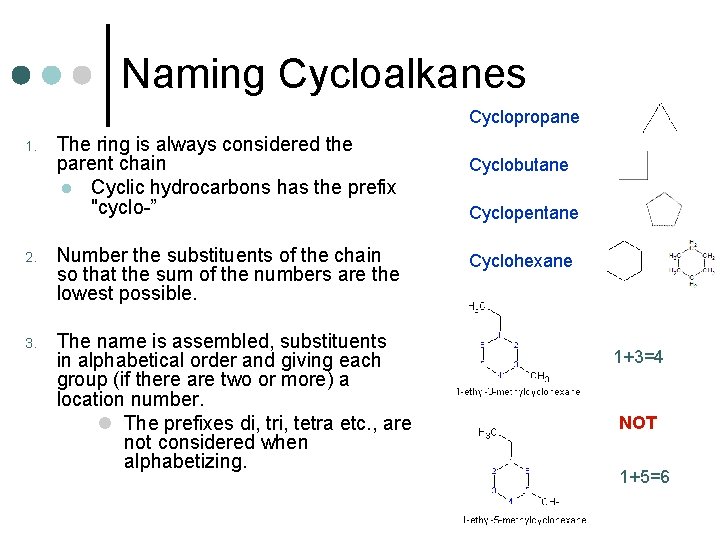
Naming Cycloalkanes Cyclopropane 1. The ring is always considered the parent chain l Cyclic hydrocarbons has the prefix "cyclo-” 2. Number the substituents of the chain so that the sum of the numbers are the lowest possible. 3. The name is assembled, substituents in alphabetical order and giving each group (if there are two or more) a location number. l The prefixes di, tri, tetra etc. , are not considered when alphabetizing. Cyclobutane Cyclopentane Cyclohexane 1+3=4 NOT 1+5=6
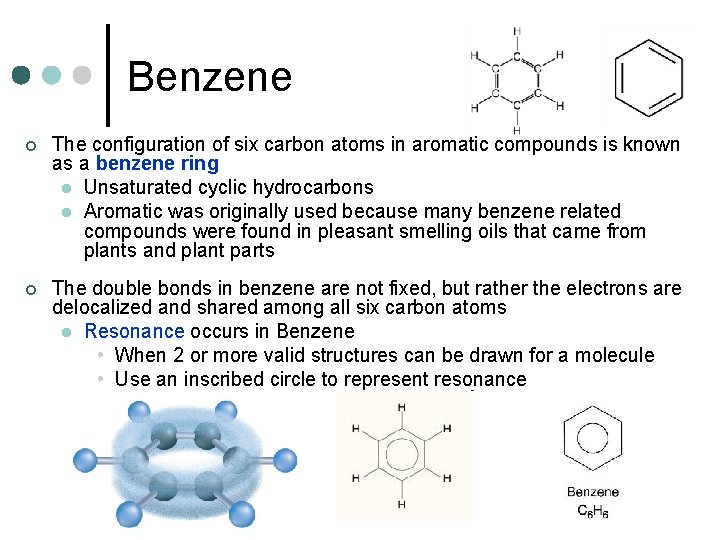
Benzene ¢ The configuration of six carbon atoms in aromatic compounds is known as a benzene ring l Unsaturated cyclic hydrocarbons l Aromatic was originally used because many benzene related compounds were found in pleasant smelling oils that came from plants and plant parts ¢ The double bonds in benzene are not fixed, but rather the electrons are delocalized and shared among all six carbon atoms l Resonance occurs in Benzene • When 2 or more valid structures can be drawn for a molecule • Use an inscribed circle to represent resonance

Key concepts • Organic compounds contain the element carbon, which is able to form straight chains and branched chains. ¢ Hydrocarbons are organic substances composed of carbon and hydrogen ¢ The major sources of hydrocarbons are petroleum and natural gas. ¢ Petroleum can be separated into components by the process of fractional distillation ¢ Alkanes contain only single bonds between carbon atoms. ¢ Alkanes and other organic compounds are best represented by structural formulas and can be named using systematic rules determined by the International Union of Pure and Applied Chemistry (IUPAC) ¢ Alkanes that contain hydrocarbon rings are called cyclic alkanes.
- Slides: 28