Hybridized Atomic Orbitals CH 4 methane C needs
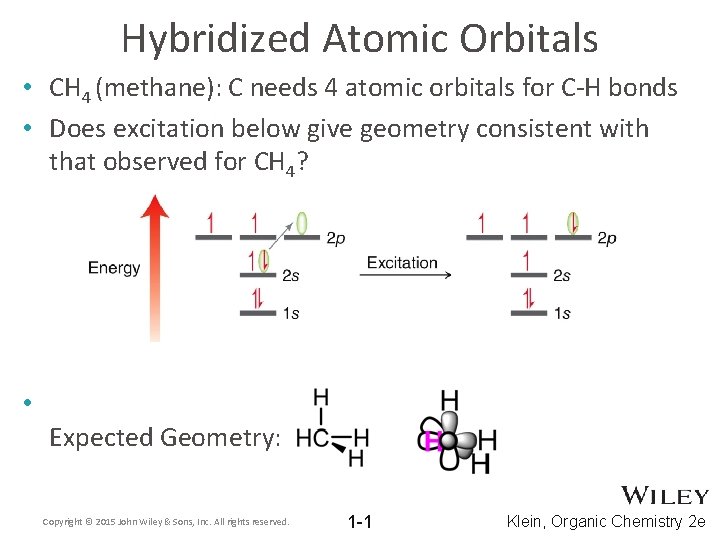
Hybridized Atomic Orbitals • CH 4 (methane): C needs 4 atomic orbitals for C-H bonds • Does excitation below give geometry consistent with that observed for CH 4? • Expected Geometry: Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -1 Klein, Organic Chemistry 2 e
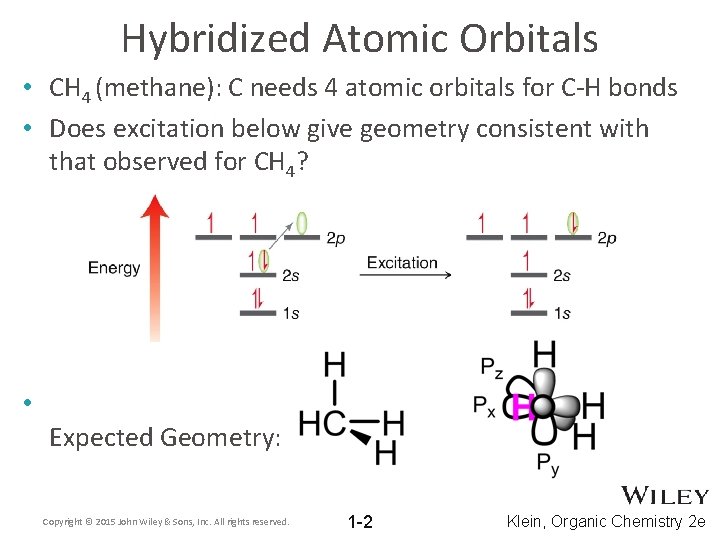
Hybridized Atomic Orbitals • CH 4 (methane): C needs 4 atomic orbitals for C-H bonds • Does excitation below give geometry consistent with that observed for CH 4? • Expected Geometry: Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -2 Klein, Organic Chemistry 2 e
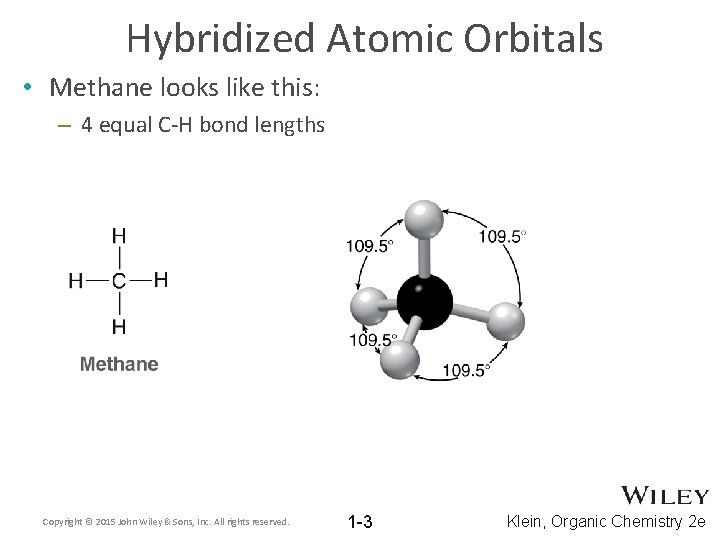
Hybridized Atomic Orbitals • Methane looks like this: – 4 equal C-H bond lengths Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -3 Klein, Organic Chemistry 2 e
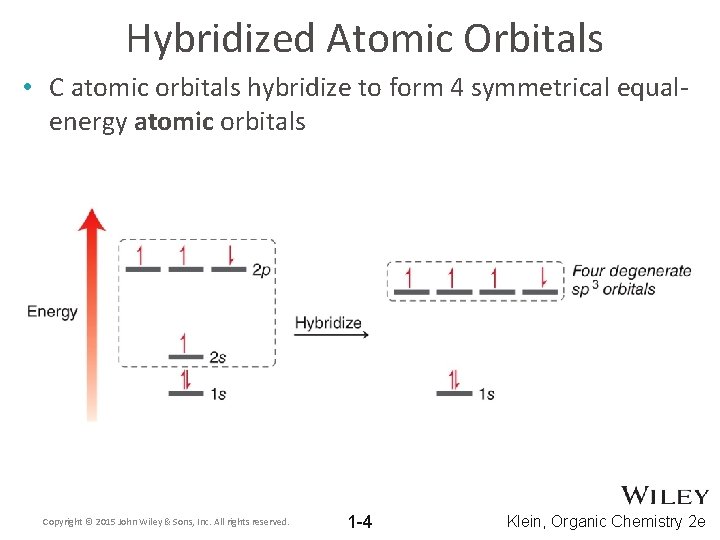
Hybridized Atomic Orbitals • C atomic orbitals hybridize to form 4 symmetrical equalenergy atomic orbitals Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -4 Klein, Organic Chemistry 2 e
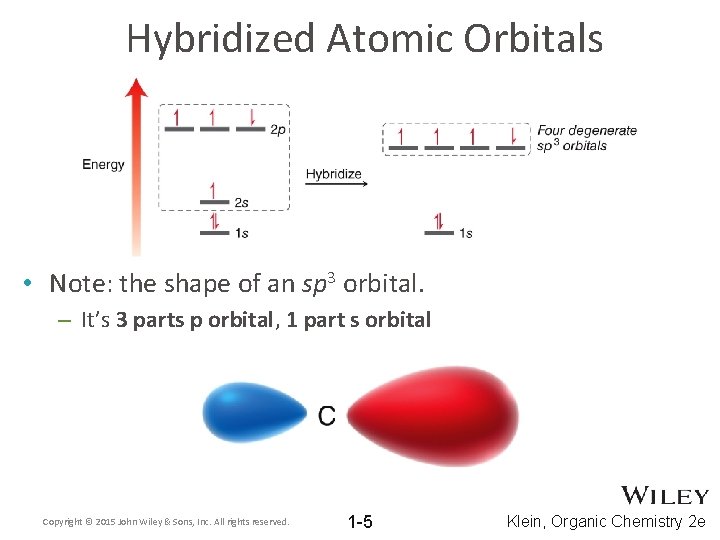
Hybridized Atomic Orbitals • Note: the shape of an sp 3 orbital. – It’s 3 parts p orbital, 1 part s orbital Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -5 Klein, Organic Chemistry 2 e
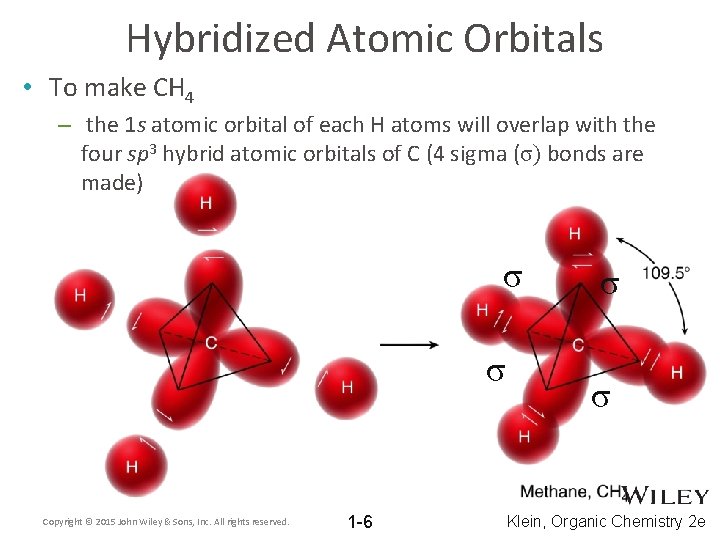
Hybridized Atomic Orbitals • To make CH 4 – the 1 s atomic orbital of each H atoms will overlap with the four sp 3 hybrid atomic orbitals of C (4 sigma (σ) bonds are made) σ σ Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -6 σ σ Klein, Organic Chemistry 2 e
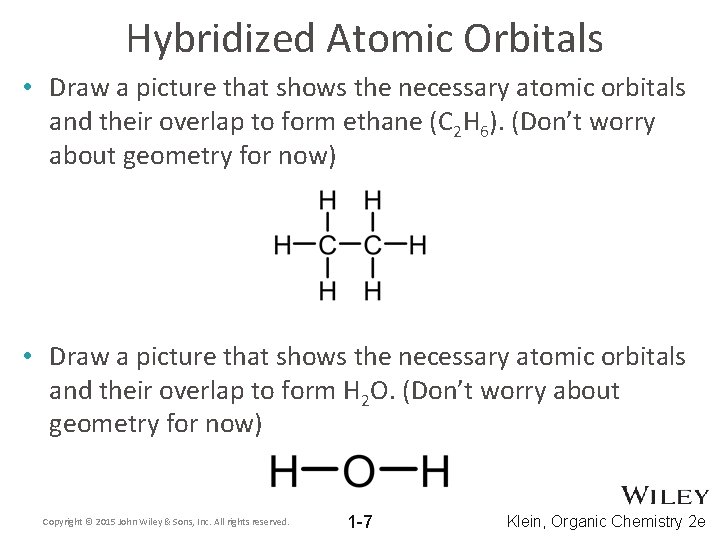
Hybridized Atomic Orbitals • Draw a picture that shows the necessary atomic orbitals and their overlap to form ethane (C 2 H 6). (Don’t worry about geometry for now) • Draw a picture that shows the necessary atomic orbitals and their overlap to form H 2 O. (Don’t worry about geometry for now) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -7 Klein, Organic Chemistry 2 e
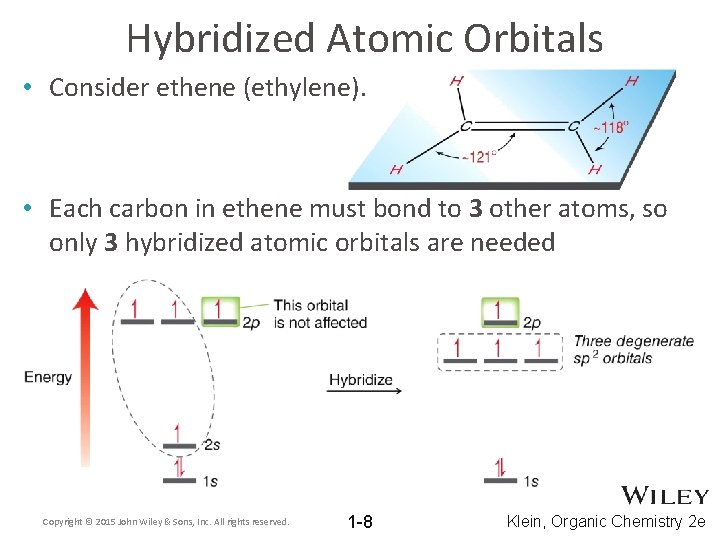
Hybridized Atomic Orbitals • Consider ethene (ethylene). • Each carbon in ethene must bond to 3 other atoms, so only 3 hybridized atomic orbitals are needed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -8 Klein, Organic Chemistry 2 e
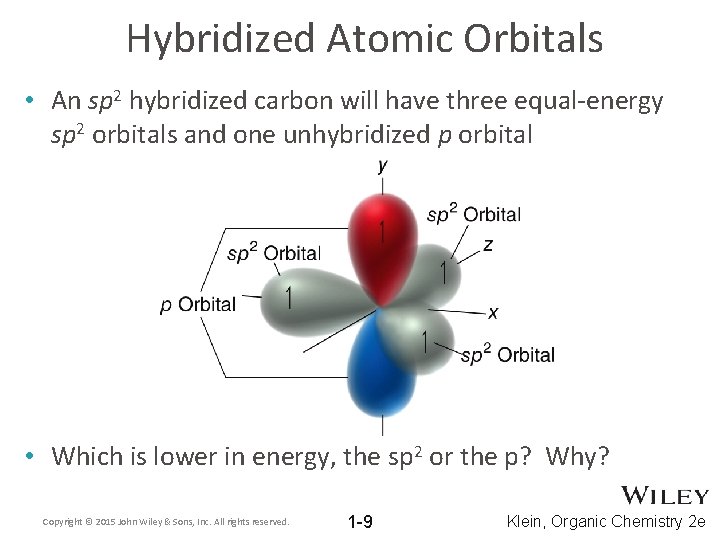
Hybridized Atomic Orbitals • An sp 2 hybridized carbon will have three equal-energy sp 2 orbitals and one unhybridized p orbital • Which is lower in energy, the sp 2 or the p? Why? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -9 Klein, Organic Chemistry 2 e
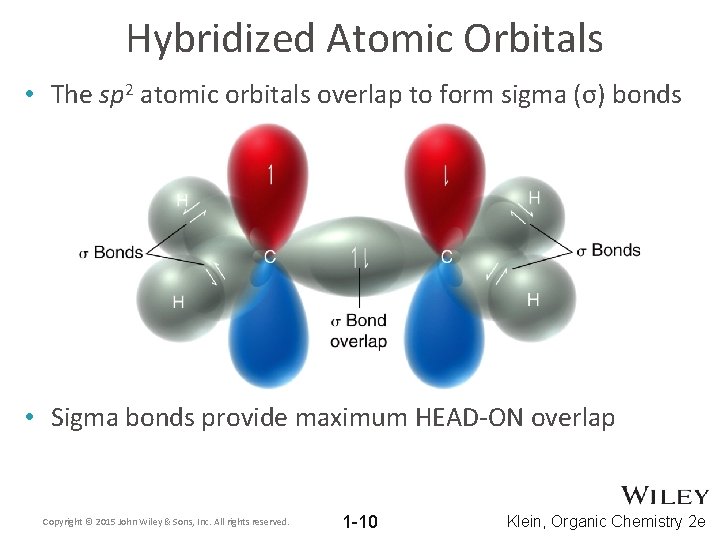
Hybridized Atomic Orbitals • The sp 2 atomic orbitals overlap to form sigma (σ) bonds • Sigma bonds provide maximum HEAD-ON overlap Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -10 Klein, Organic Chemistry 2 e
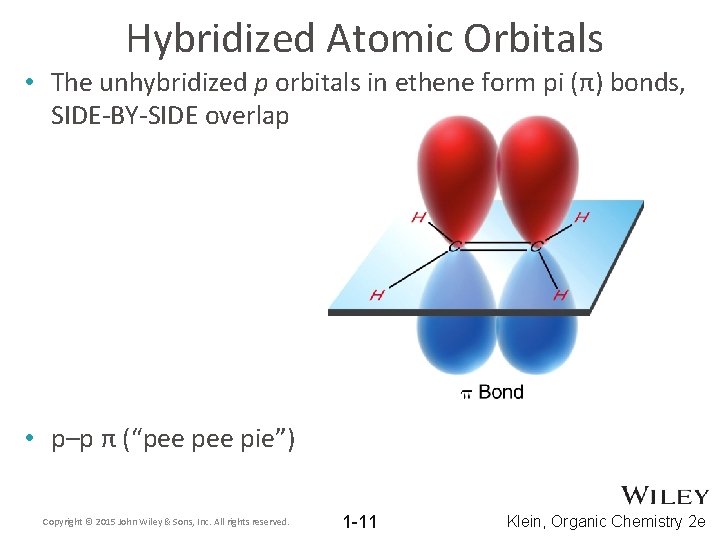
Hybridized Atomic Orbitals • The unhybridized p orbitals in ethene form pi (π) bonds, SIDE-BY-SIDE overlap • p–p π (“pee pie”) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -11 Klein, Organic Chemistry 2 e
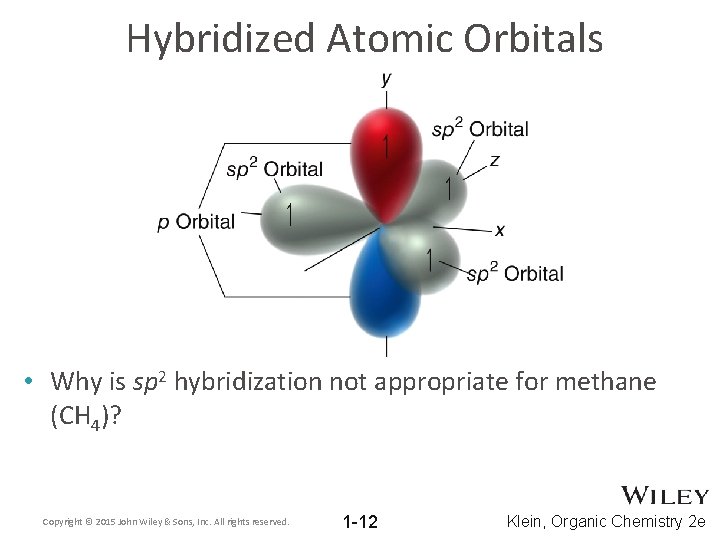
Hybridized Atomic Orbitals • Why is sp 2 hybridization not appropriate for methane (CH 4)? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -12 Klein, Organic Chemistry 2 e
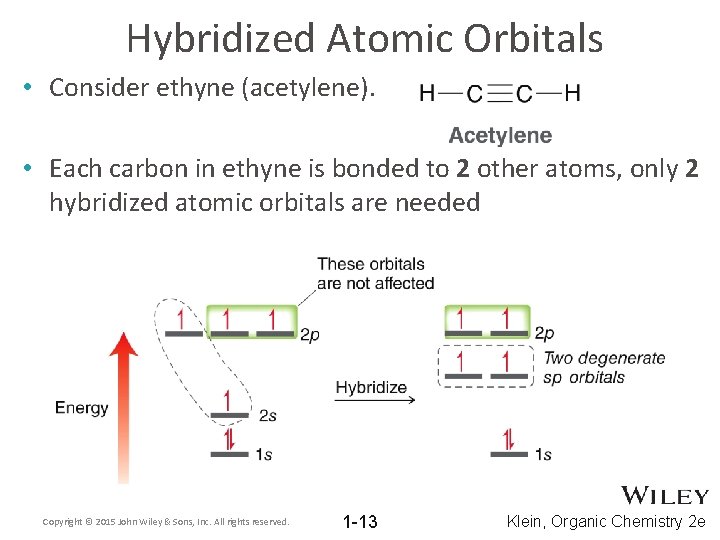
Hybridized Atomic Orbitals • Consider ethyne (acetylene). • Each carbon in ethyne is bonded to 2 other atoms, only 2 hybridized atomic orbitals are needed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -13 Klein, Organic Chemistry 2 e
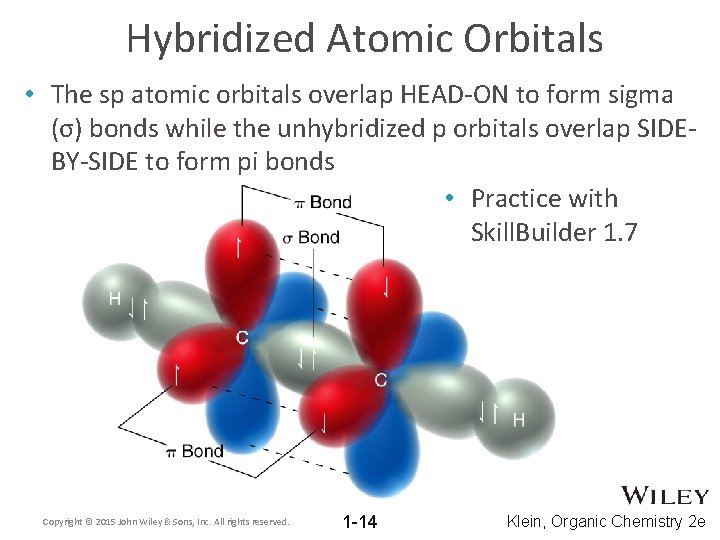
Hybridized Atomic Orbitals • The sp atomic orbitals overlap HEAD-ON to form sigma (σ) bonds while the unhybridized p orbitals overlap SIDEBY-SIDE to form pi bonds • Practice with Skill. Builder 1. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -14 Klein, Organic Chemistry 2 e
Hybridized Atomic Orbitals • Which should be stronger, a pi bond or a sigma bond? WHY? – Pi bonds involve sideways overlap – sigma bonds involve head on (i. e. axial overlap) – Axial overlap is stronger than sideways overlap • Which should be longer, an sp 3 – sp 3 sigma bond overlap or an sp – sp sigma bond overlap? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -15 Klein, Organic Chemistry 2 e
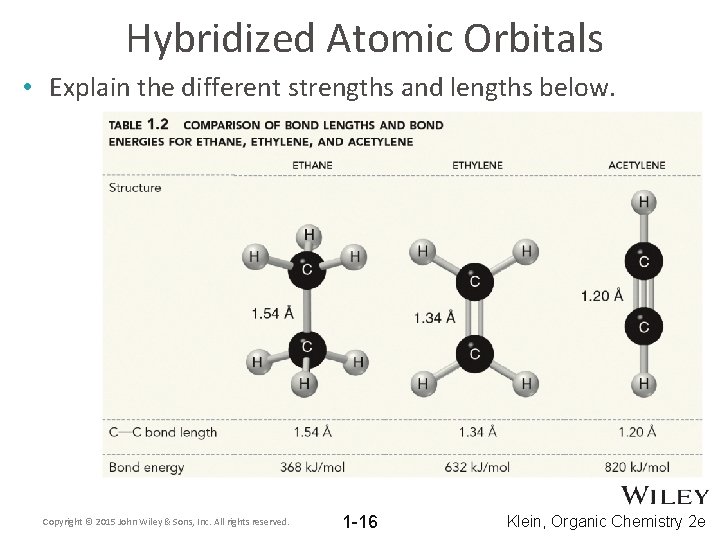
Hybridized Atomic Orbitals • Explain the different strengths and lengths below. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -16 Klein, Organic Chemistry 2 e
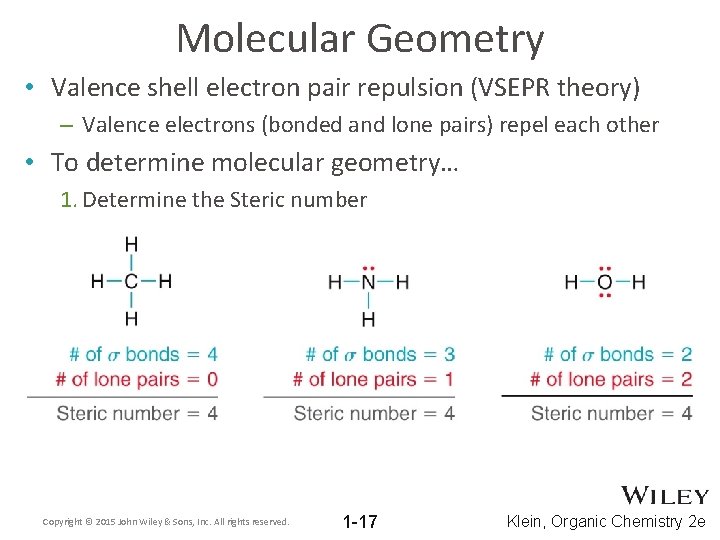
Molecular Geometry • Valence shell electron pair repulsion (VSEPR theory) – Valence electrons (bonded and lone pairs) repel each other • To determine molecular geometry… 1. Determine the Steric number Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -17 Klein, Organic Chemistry 2 e
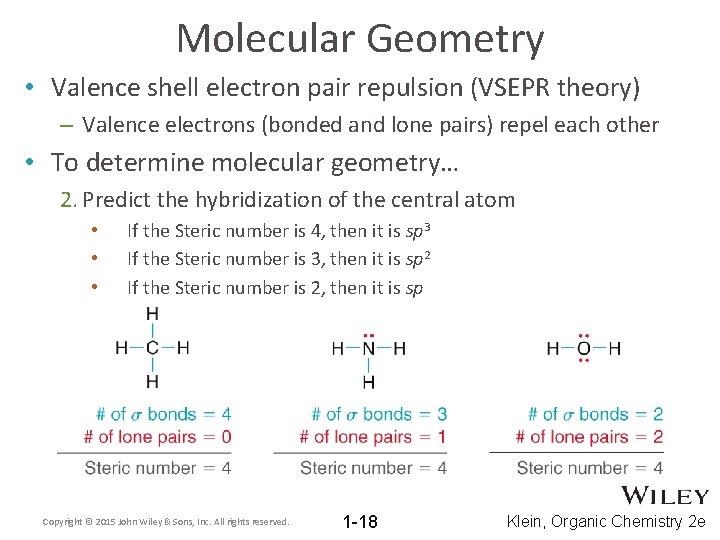
Molecular Geometry • Valence shell electron pair repulsion (VSEPR theory) – Valence electrons (bonded and lone pairs) repel each other • To determine molecular geometry… 2. Predict the hybridization of the central atom • • • If the Steric number is 4, then it is sp 3 If the Steric number is 3, then it is sp 2 If the Steric number is 2, then it is sp Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -18 Klein, Organic Chemistry 2 e
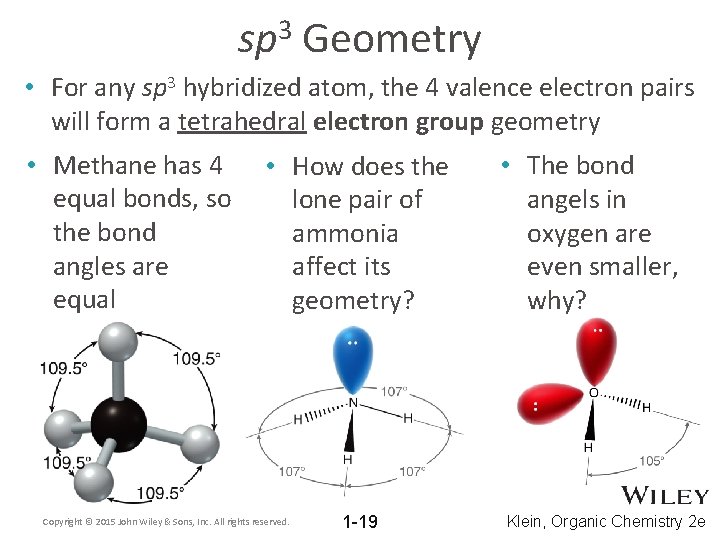
3 sp Geometry • For any sp 3 hybridized atom, the 4 valence electron pairs will form a tetrahedral electron group geometry • Methane has 4 equal bonds, so the bond angles are equal • How does the lone pair of ammonia affect its geometry? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -19 • The bond angels in oxygen are even smaller, why? Klein, Organic Chemistry 2 e
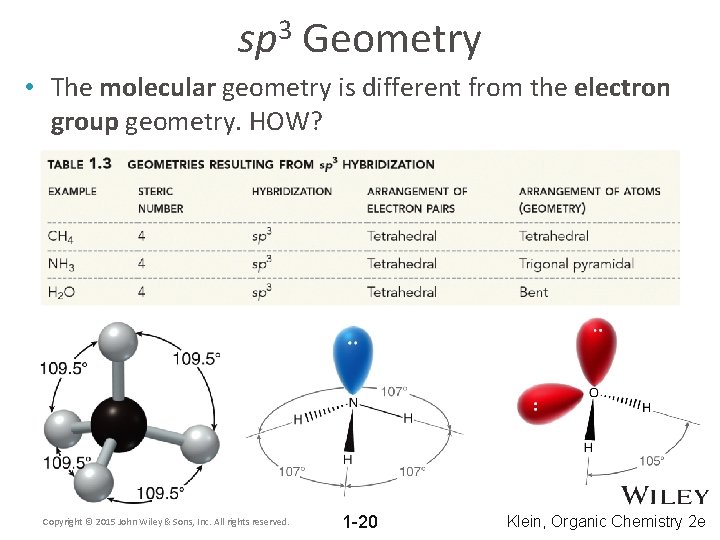
3 sp Geometry • The molecular geometry is different from the electron group geometry. HOW? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -20 Klein, Organic Chemistry 2 e
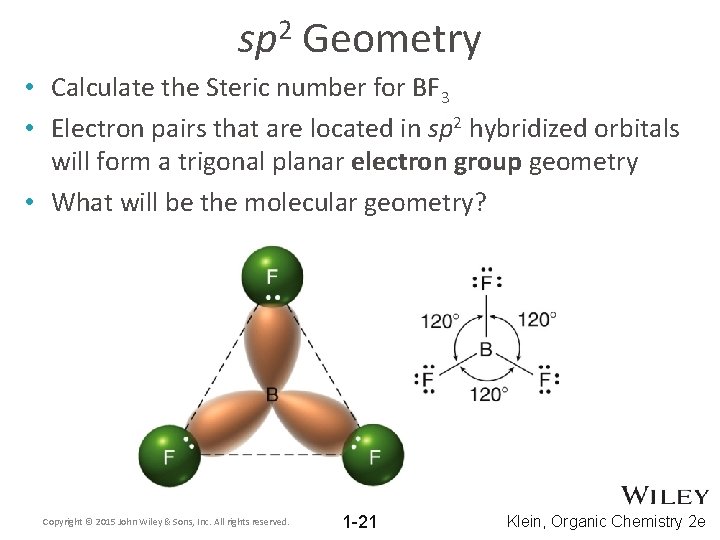
2 sp Geometry • Calculate the Steric number for BF 3 • Electron pairs that are located in sp 2 hybridized orbitals will form a trigonal planar electron group geometry • What will be the molecular geometry? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -21 Klein, Organic Chemistry 2 e
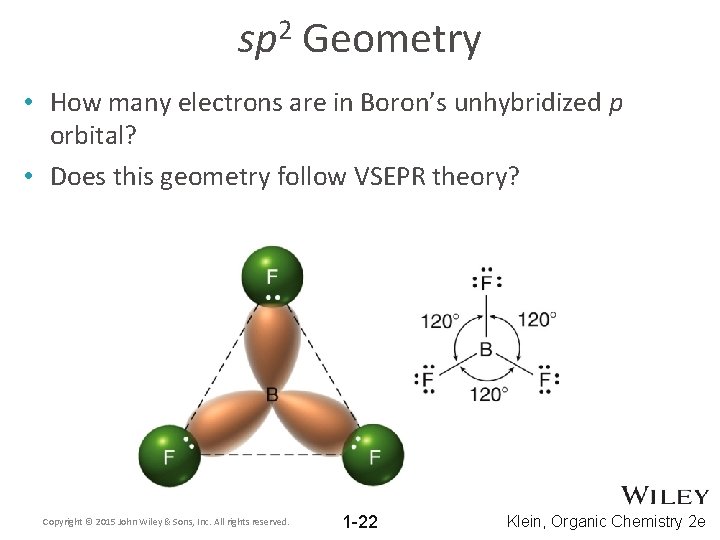
2 sp Geometry • How many electrons are in Boron’s unhybridized p orbital? • Does this geometry follow VSEPR theory? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -22 Klein, Organic Chemistry 2 e
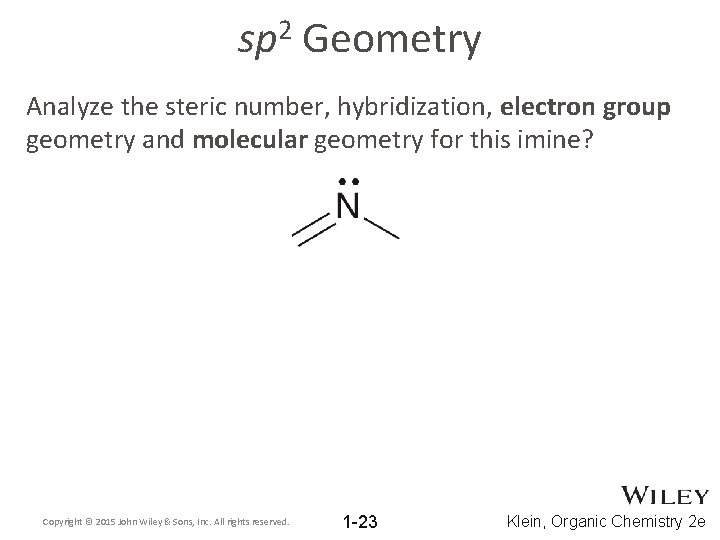
2 sp Geometry Analyze the steric number, hybridization, electron group geometry and molecular geometry for this imine? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -23 Klein, Organic Chemistry 2 e
1. 10 sp Geometry • Analyze the Steric number, the hybridization, the electron group geometry, and the molecular geometry for the following molecules • Can you draw the Lewis structures? – Be. H 2 – CO 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -24 Klein, Organic Chemistry 2 e
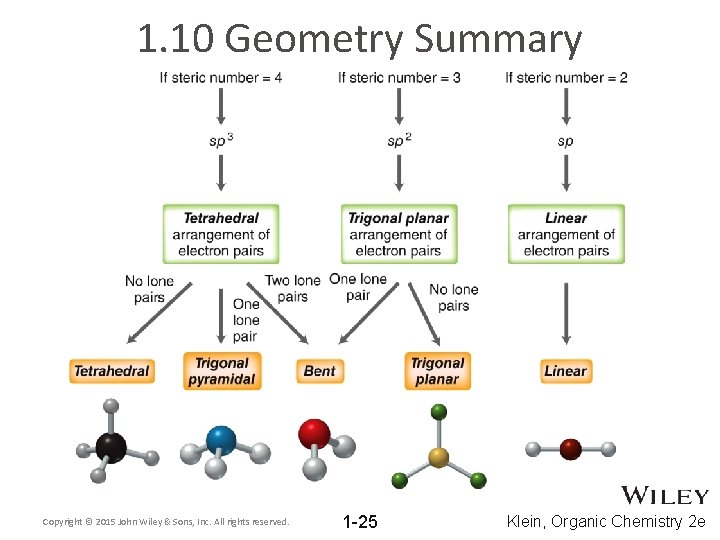
1. 10 Geometry Summary Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -25 Klein, Organic Chemistry 2 e
Molecular Polarity • Electronegativity Differences cause induction • Induction (shifting of electrons WITHIN a molecular orbitals) creates a dipole moment. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -26 Klein, Organic Chemistry 2 e
![Molecular Polarity • Dipole moment = [amount of partial charge] x (distance charges are Molecular Polarity • Dipole moment = [amount of partial charge] x (distance charges are](http://slidetodoc.com/presentation_image_h2/8f5c2adc3c50ffced28754f4809f1c90/image-27.jpg)
Molecular Polarity • Dipole moment = [amount of partial charge] x (distance charges are separated] • Dipole moment (μ) is reported in units of Debye (D) • 1 debye = 10 -18 esu ∙ cm – esu (electrostatic unit) – 1 e- charge = 4. 80 x 10 -10 esu Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -27 Klein, Organic Chemistry 2 e
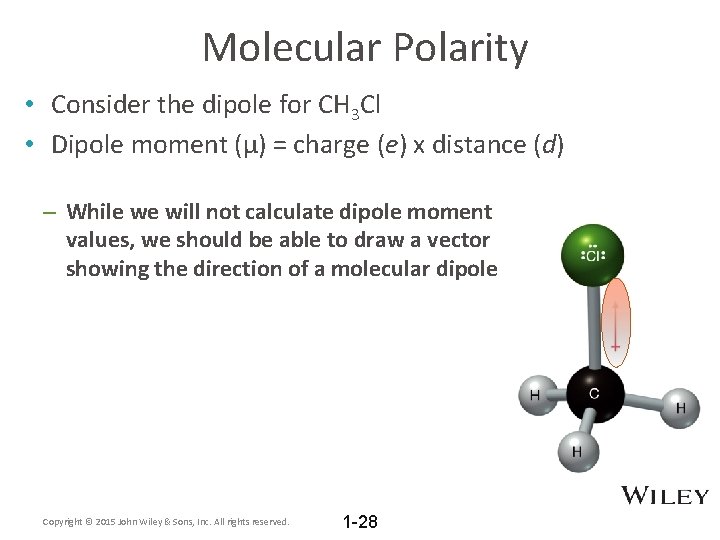
Molecular Polarity • Consider the dipole for CH 3 Cl • Dipole moment (μ) = charge (e) x distance (d) – While we will not calculate dipole moment values, we should be able to draw a vector showing the direction of a molecular dipole Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -28
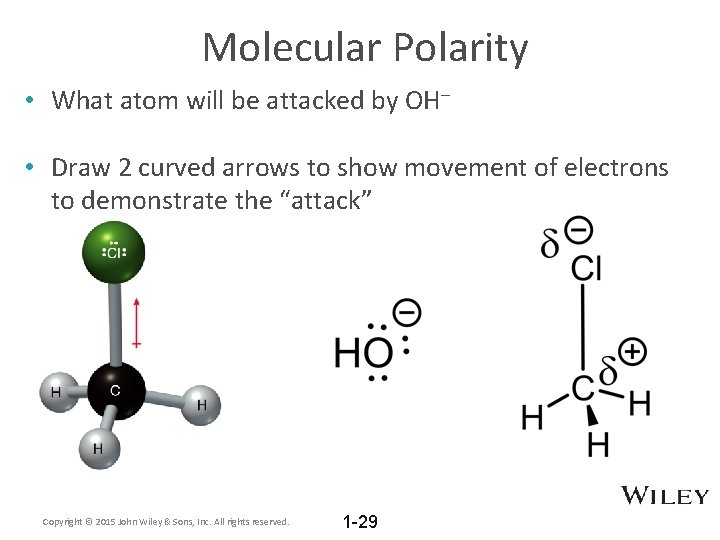
Molecular Polarity • What atom will be attacked by OH– • Draw 2 curved arrows to show movement of electrons to demonstrate the “attack” Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -29
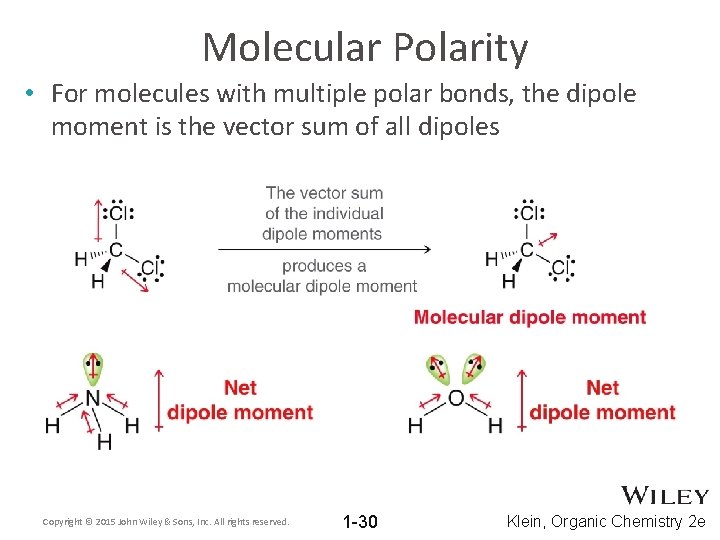
Molecular Polarity • For molecules with multiple polar bonds, the dipole moment is the vector sum of all dipoles Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -30 Klein, Organic Chemistry 2 e
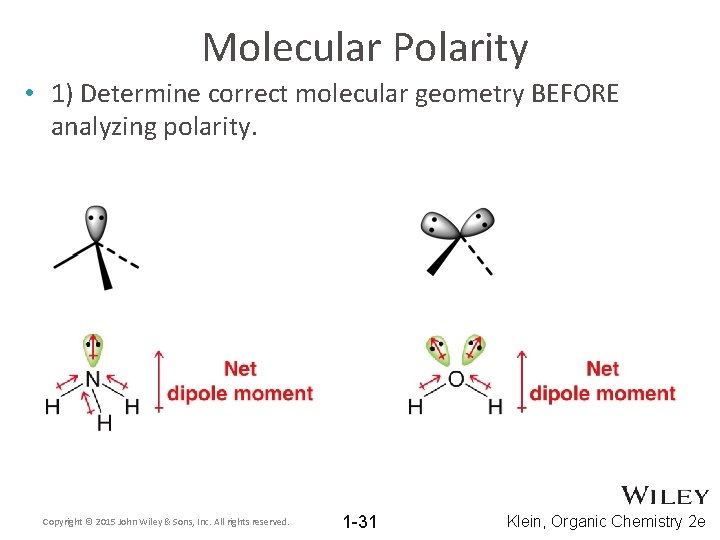
Molecular Polarity • 1) Determine correct molecular geometry BEFORE analyzing polarity. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -31 Klein, Organic Chemistry 2 e
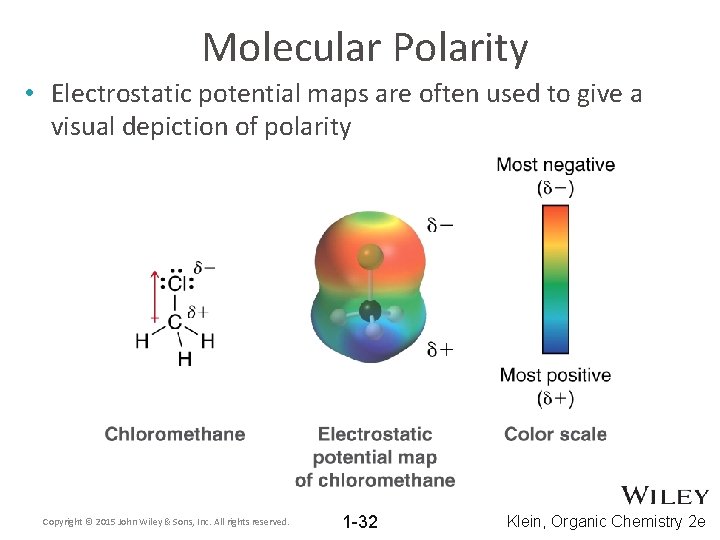
Molecular Polarity • Electrostatic potential maps are often used to give a visual depiction of polarity Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -32 Klein, Organic Chemistry 2 e
Intermolecular Forces • Many physical properties such as: – – – Solubility Boiling Point Density State of matter Melting Point are affected by the attractions BETWEEN molecules Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -33 Klein, Organic Chemistry 2 e
Intermolecular Forces • Neutral molecules (polar and nonpolar) are attracted to one another through… – Dipole-dipole interactions – Hydrogen bonding – Dispersion forces (a. k. a. London forces or fleeting dipole forces) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -34 Klein, Organic Chemistry 2 e
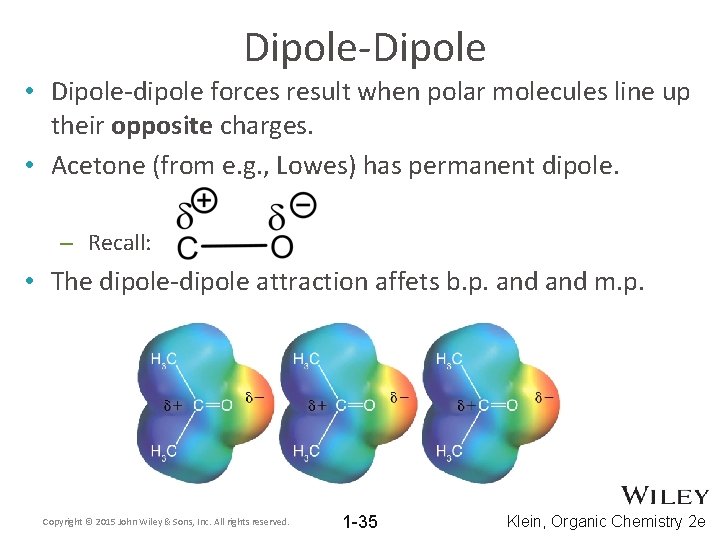
Dipole-Dipole • Dipole-dipole forces result when polar molecules line up their opposite charges. • Acetone (from e. g. , Lowes) has permanent dipole. – Recall: • The dipole-dipole attraction affets b. p. and m. p. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -35 Klein, Organic Chemistry 2 e
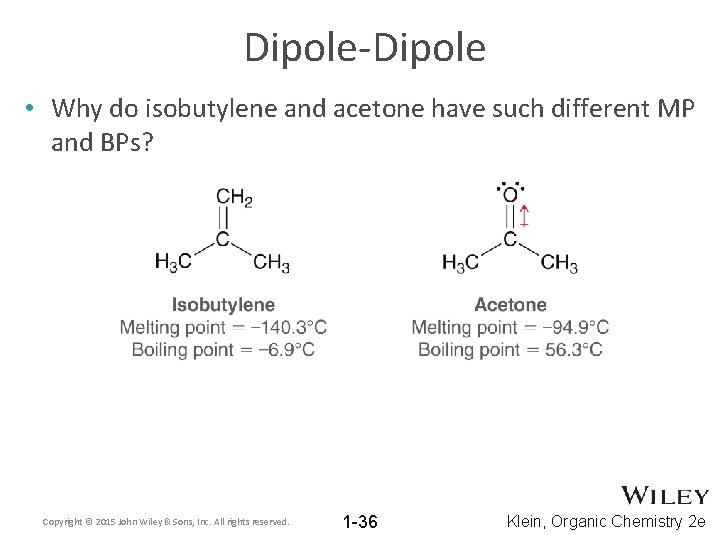
Dipole-Dipole • Why do isobutylene and acetone have such different MP and BPs? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -36 Klein, Organic Chemistry 2 e
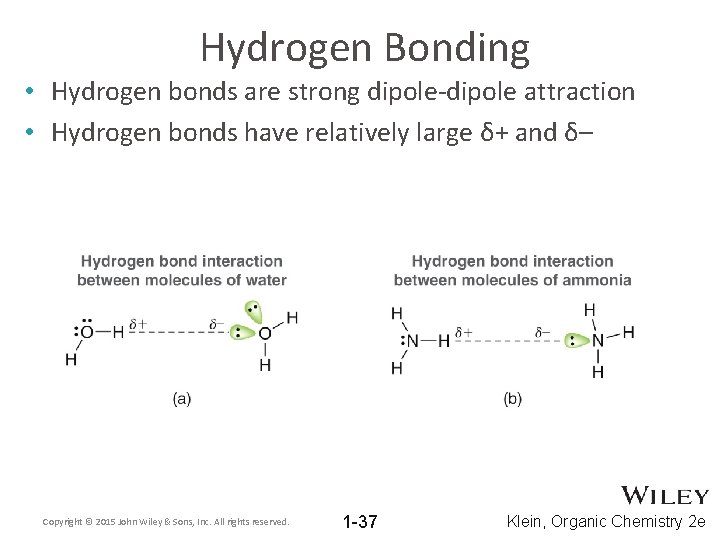
Hydrogen Bonding • Hydrogen bonds are strong dipole-dipole attraction • Hydrogen bonds have relatively large δ+ and δ– Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -37 Klein, Organic Chemistry 2 e
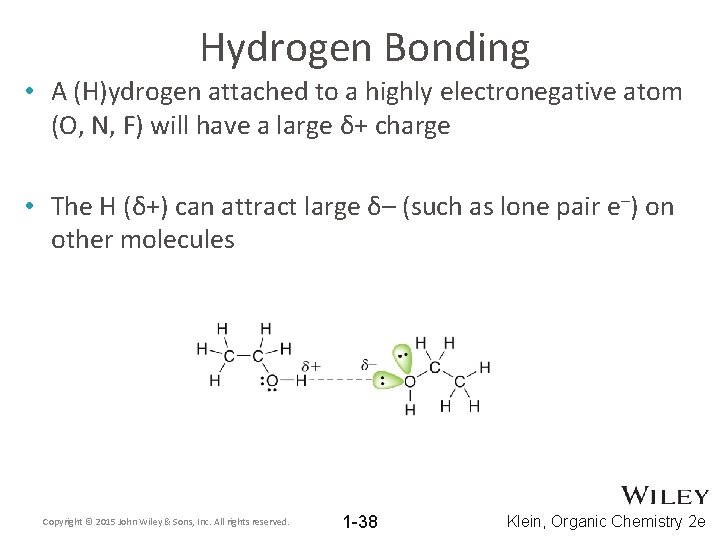
Hydrogen Bonding • A (H)ydrogen attached to a highly electronegative atom (O, N, F) will have a large δ+ charge • The H (δ+) can attract large δ– (such as lone pair e–) on other molecules Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -38 Klein, Organic Chemistry 2 e
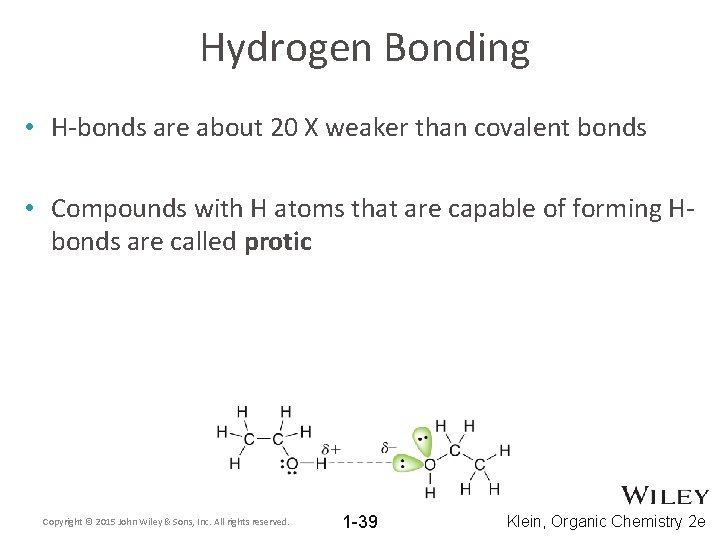
Hydrogen Bonding • H-bonds are about 20 X weaker than covalent bonds • Compounds with H atoms that are capable of forming Hbonds are called protic Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -39 Klein, Organic Chemistry 2 e
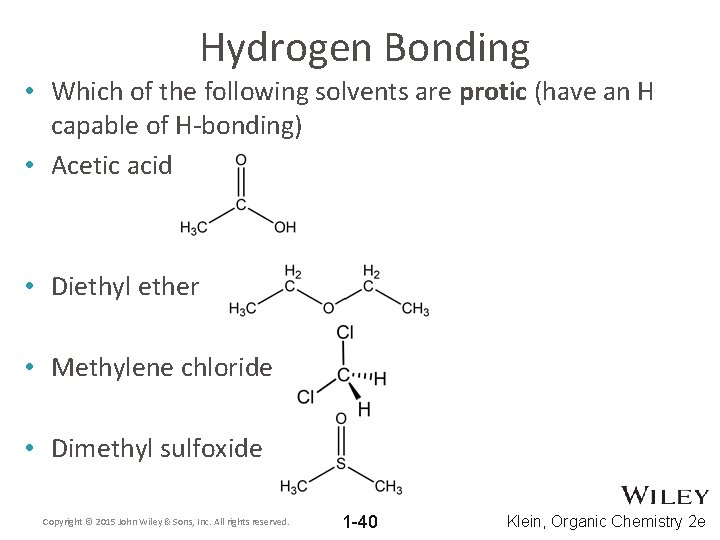
Hydrogen Bonding • Which of the following solvents are protic (have an H capable of H-bonding) • Acetic acid • Diethyl ether • Methylene chloride • Dimethyl sulfoxide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -40 Klein, Organic Chemistry 2 e
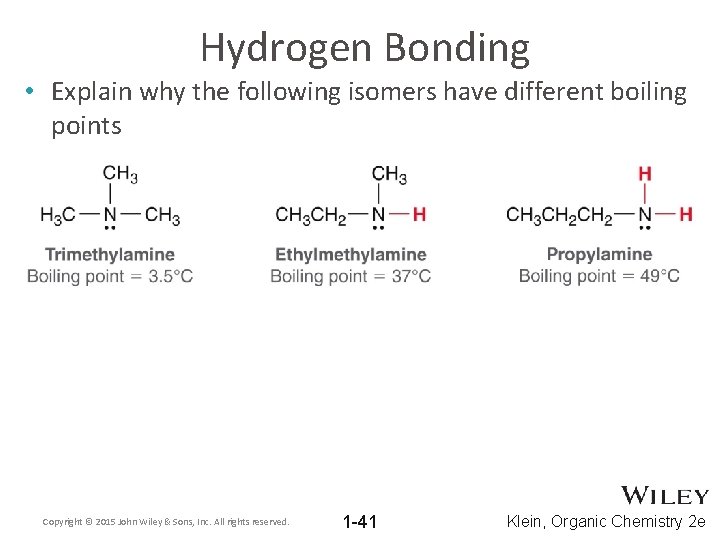
Hydrogen Bonding • Explain why the following isomers have different boiling points Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -41 Klein, Organic Chemistry 2 e
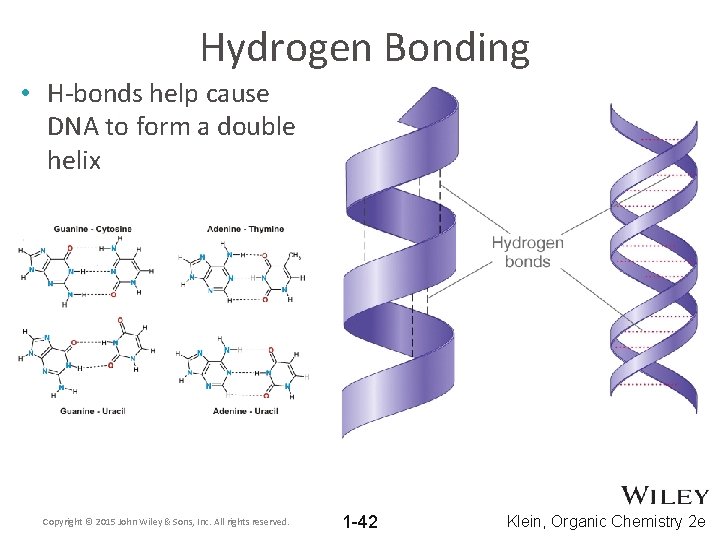
Hydrogen Bonding • H-bonds help cause DNA to form a double helix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -42 Klein, Organic Chemistry 2 e

London Dispersion Forces • Nonpolar molecules (dipole = 0 D) will they attract one another – HOW? • Since nonpolar molecules normally have their e– spread out evenly around the nuclei (+) completely balancing the charge Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -43 Klein, Organic Chemistry 2 e
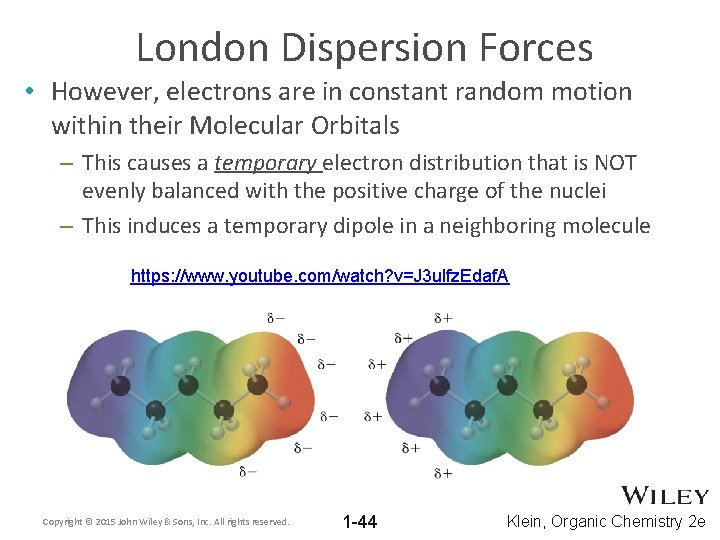
London Dispersion Forces • However, electrons are in constant random motion within their Molecular Orbitals – This causes a temporary electron distribution that is NOT evenly balanced with the positive charge of the nuclei – This induces a temporary dipole in a neighboring molecule https: //www. youtube. com/watch? v=J 3 ulfz. Edaf. A Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -44 Klein, Organic Chemistry 2 e
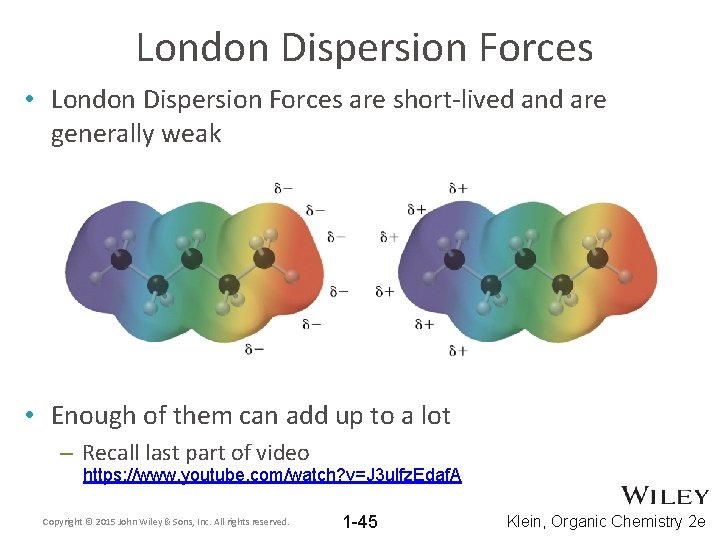
London Dispersion Forces • London Dispersion Forces are short-lived and are generally weak • Enough of them can add up to a lot – Recall last part of video https: //www. youtube. com/watch? v=J 3 ulfz. Edaf. A Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -45 Klein, Organic Chemistry 2 e

London Dispersion Forces • London dispersion forces are strong enough to support the weight of the Gecko Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -46 Klein, Organic Chemistry 2 e
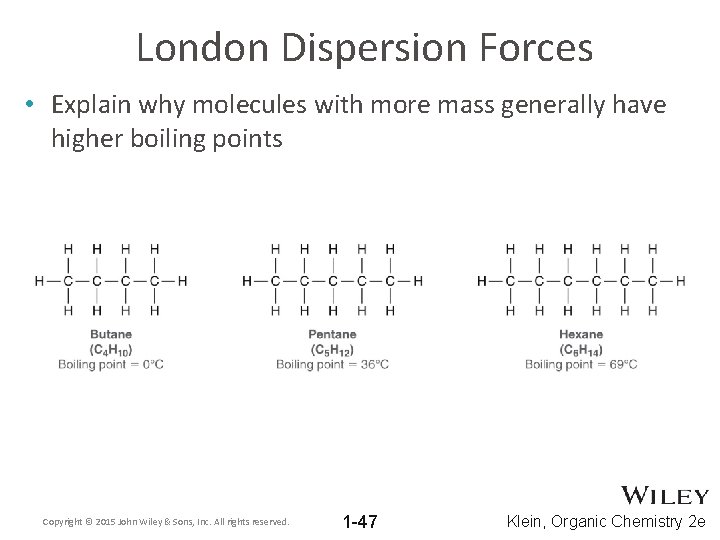
London Dispersion Forces • Explain why molecules with more mass generally have higher boiling points Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -47 Klein, Organic Chemistry 2 e
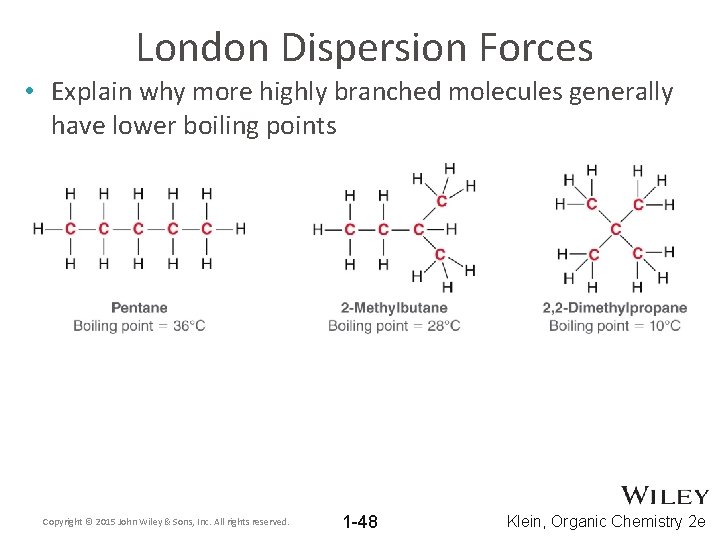
London Dispersion Forces • Explain why more highly branched molecules generally have lower boiling points Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -48 Klein, Organic Chemistry 2 e

Solubility • We use the principle, like-dissolves-like • Polar compounds generally mix well with other polar compounds – If the compounds mixing are all capable of H-bonding and/or strong dipole-dipole, then there is no reason why they shouldn’t mix • Nonpolar compounds generally mix well with other nonpolar compounds – If none of the compounds are capable of forming strong attractions, then no strong attractions would have to be broken to allow them to mix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -49 Klein, Organic Chemistry 2 e
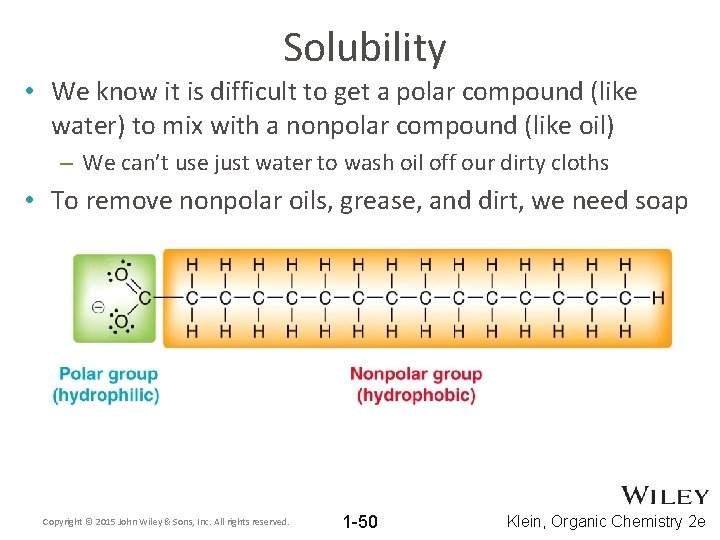
Solubility • We know it is difficult to get a polar compound (like water) to mix with a nonpolar compound (like oil) – We can’t use just water to wash oil off our dirty cloths • To remove nonpolar oils, grease, and dirt, we need soap Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -50 Klein, Organic Chemistry 2 e
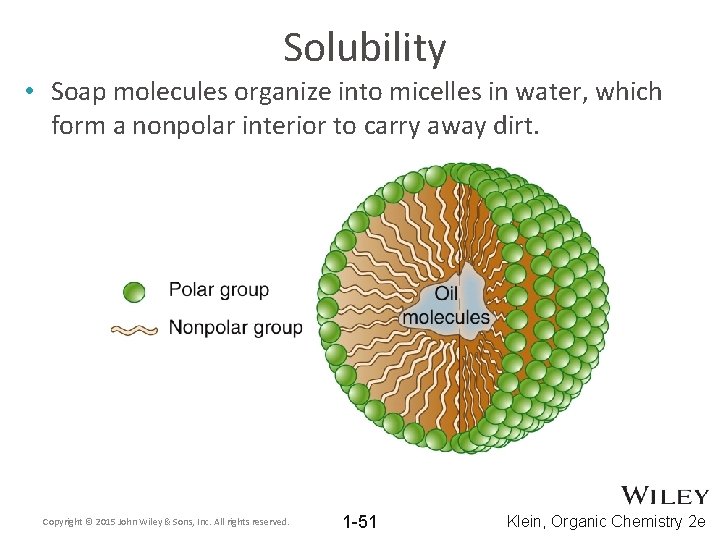
Solubility • Soap molecules organize into micelles in water, which form a nonpolar interior to carry away dirt. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -51 Klein, Organic Chemistry 2 e
- Slides: 51