Hybridization of Orbitals Sections 9 1 and 9

Hybridization of Orbitals Sections 9. 1 and 9. 5 March 14, 2007
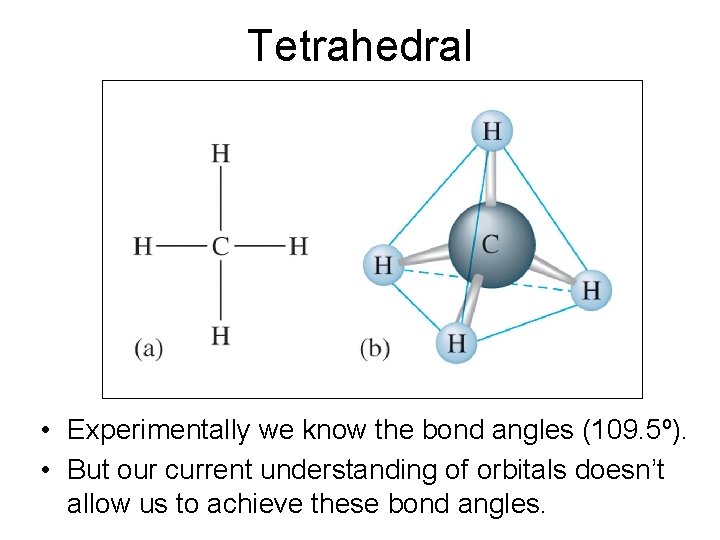
Tetrahedral • Experimentally we know the bond angles (109. 5º). • But our current understanding of orbitals doesn’t allow us to achieve these bond angles.
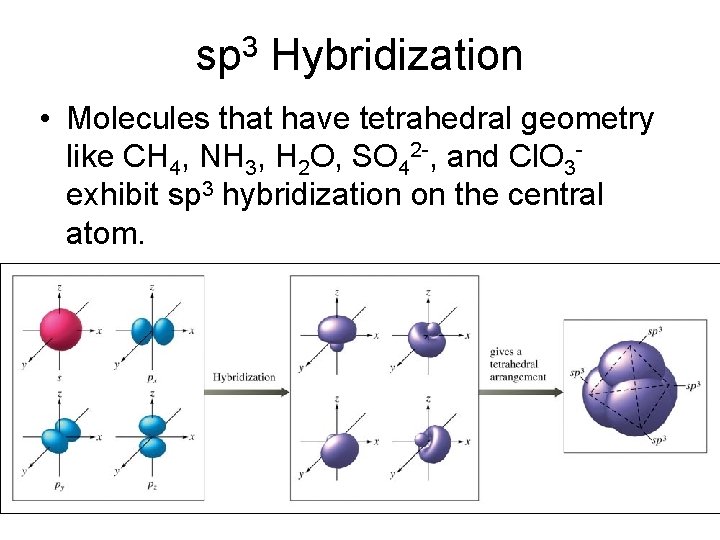
sp 3 Hybridization • Molecules that have tetrahedral geometry like CH 4, NH 3, H 2 O, SO 42 -, and Cl. O 3 exhibit sp 3 hybridization on the central atom.
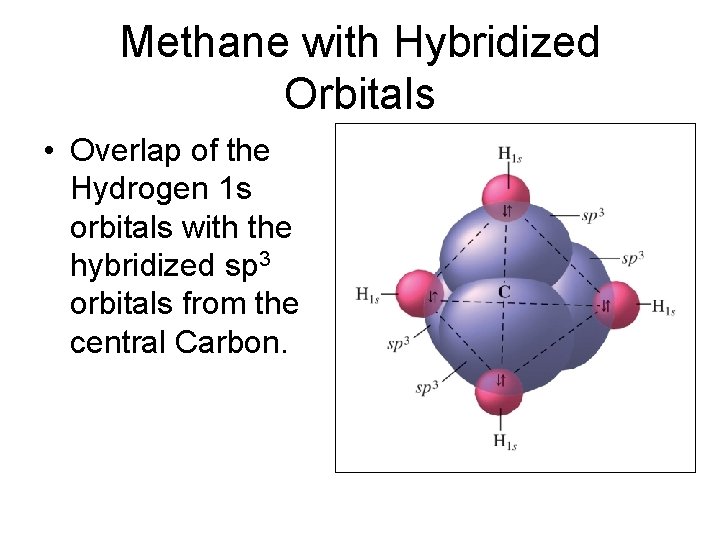
Methane with Hybridized Orbitals • Overlap of the Hydrogen 1 s orbitals with the hybridized sp 3 orbitals from the central Carbon.
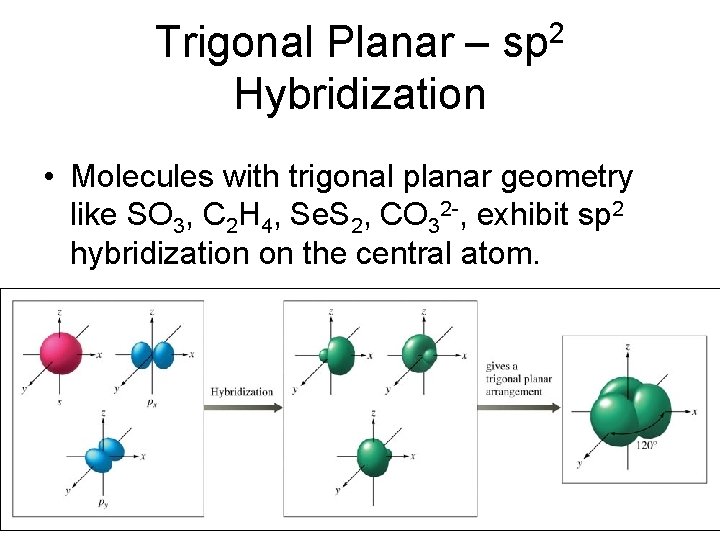
Trigonal Planar – Hybridization 2 sp • Molecules with trigonal planar geometry like SO 3, C 2 H 4, Se. S 2, CO 32 -, exhibit sp 2 hybridization on the central atom.
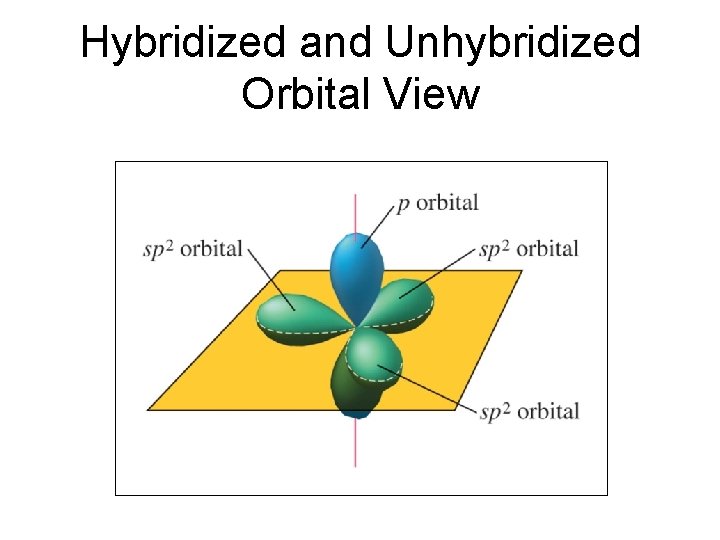
Hybridized and Unhybridized Orbital View
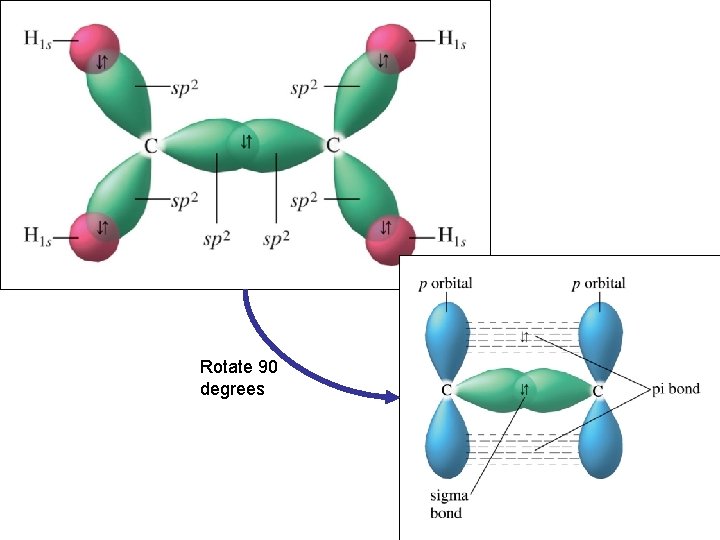
Rotate 90 degrees
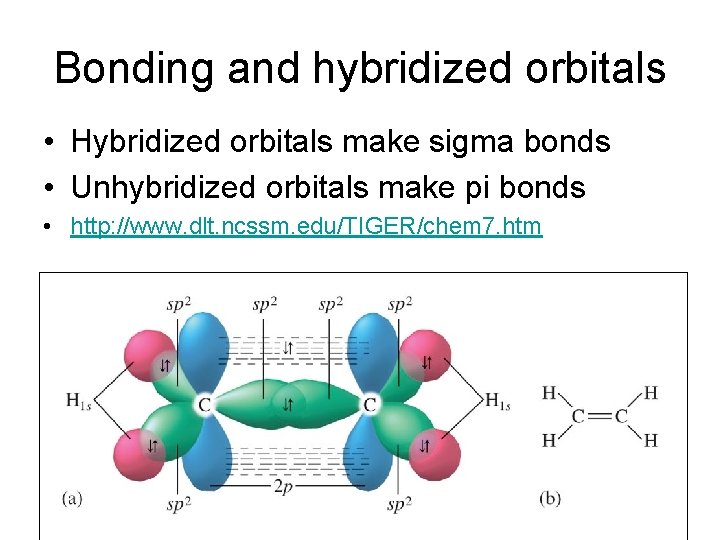
Bonding and hybridized orbitals • Hybridized orbitals make sigma bonds • Unhybridized orbitals make pi bonds • http: //www. dlt. ncssm. edu/TIGER/chem 7. htm
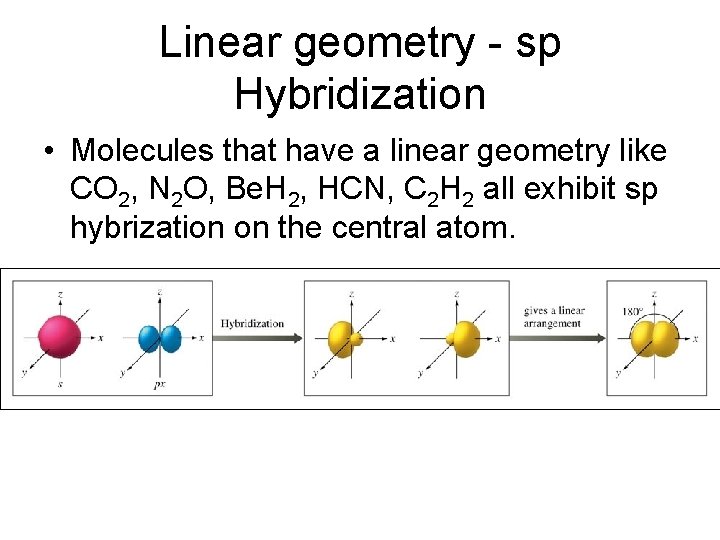
Linear geometry - sp Hybridization • Molecules that have a linear geometry like CO 2, N 2 O, Be. H 2, HCN, C 2 H 2 all exhibit sp hybrization on the central atom.
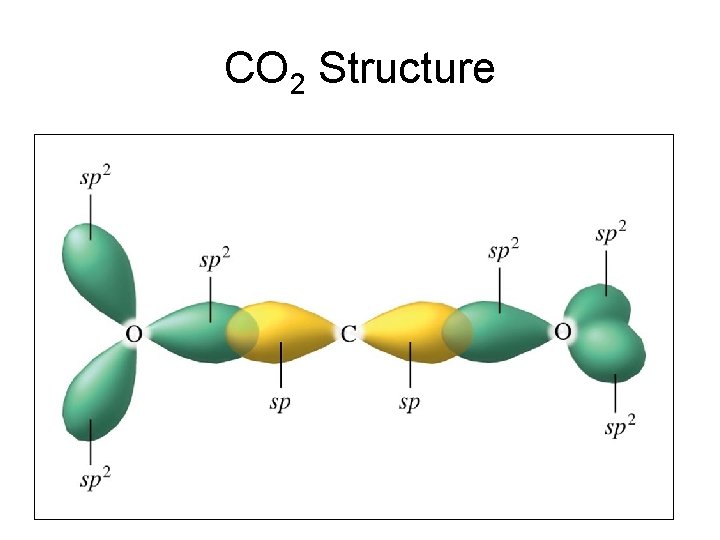
CO 2 Structure
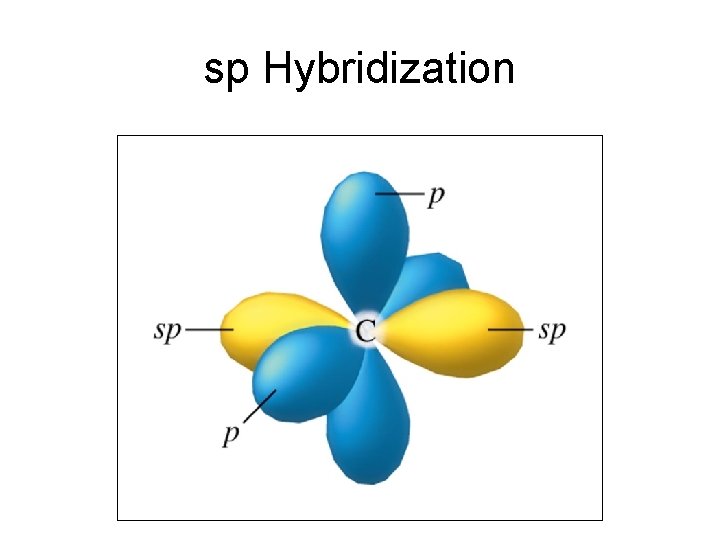
sp Hybridization
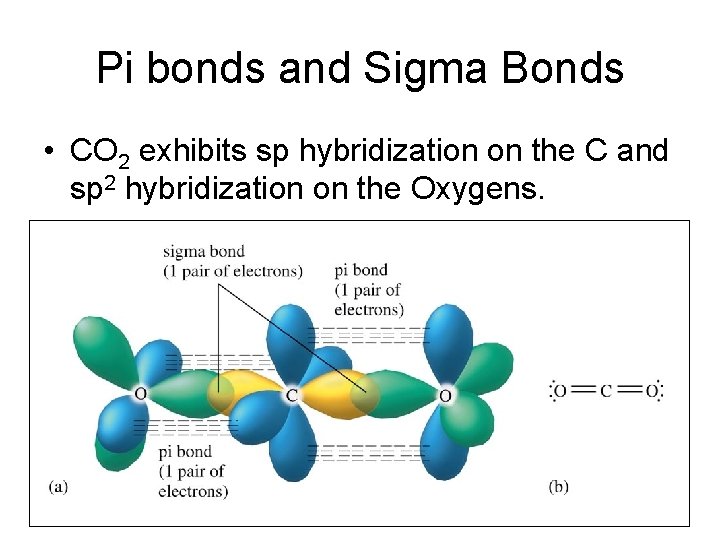
Pi bonds and Sigma Bonds • CO 2 exhibits sp hybridization on the C and sp 2 hybridization on the Oxygens.
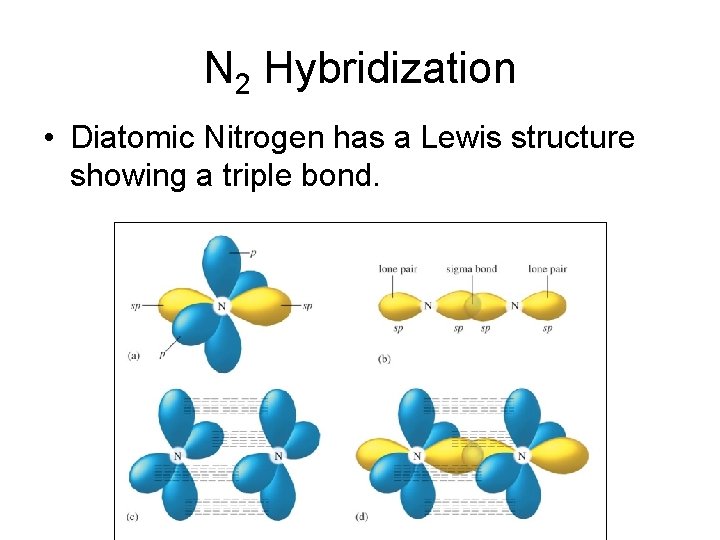
N 2 Hybridization • Diatomic Nitrogen has a Lewis structure showing a triple bond.
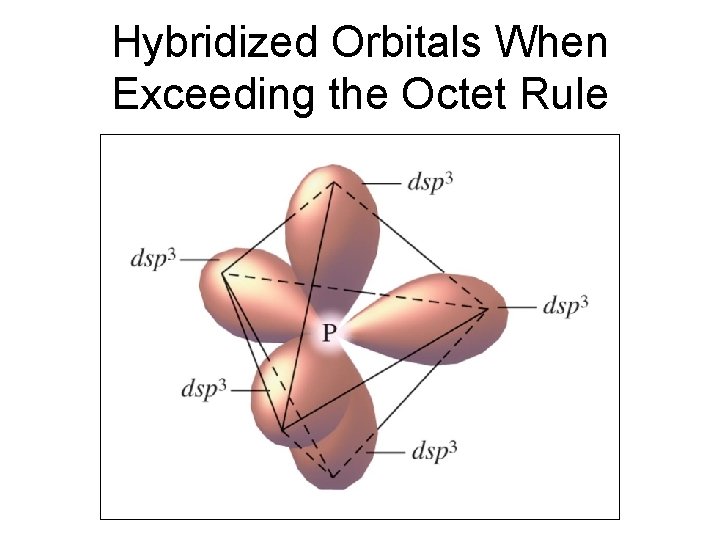
Hybridized Orbitals When Exceeding the Octet Rule
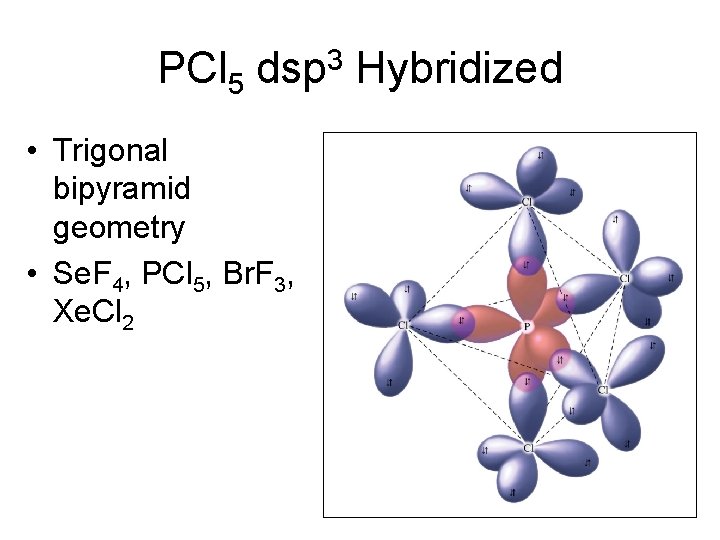
PCl 5 dsp 3 Hybridized • Trigonal bipyramid geometry • Se. F 4, PCl 5, Br. F 3, Xe. Cl 2
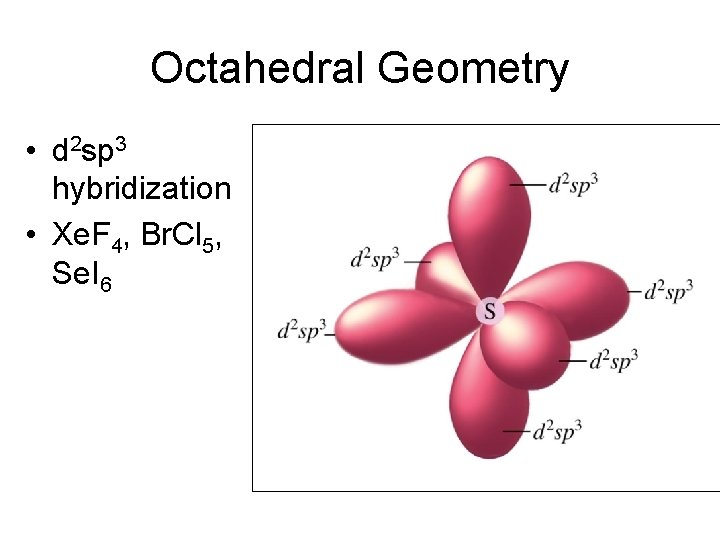
Octahedral Geometry • d 2 sp 3 hybridization • Xe. F 4, Br. Cl 5, Se. I 6
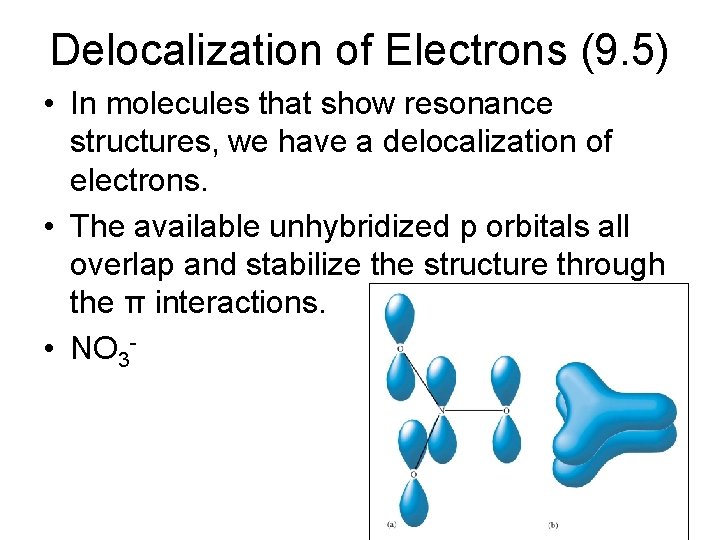
Delocalization of Electrons (9. 5) • In molecules that show resonance structures, we have a delocalization of electrons. • The available unhybridized p orbitals all overlap and stabilize the structure through the π interactions. • NO 3 -
- Slides: 17