Hybridization of Carbon Ground state orbital fill diagram

Hybridization of Carbon
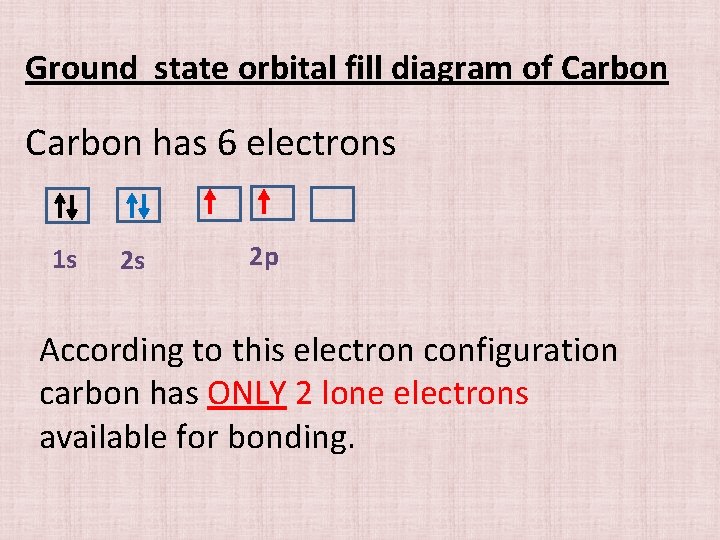
Ground state orbital fill diagram of Carbon has 6 electrons 1 s 2 s 2 p According to this electron configuration carbon has ONLY 2 lone electrons available for bonding.

However, it is known that when carbon has 4 single bonds in a compound , those bonds are identical and spaced evenly apart. Carbon will absorb energy when bonding and the electrons will move into an excited state.
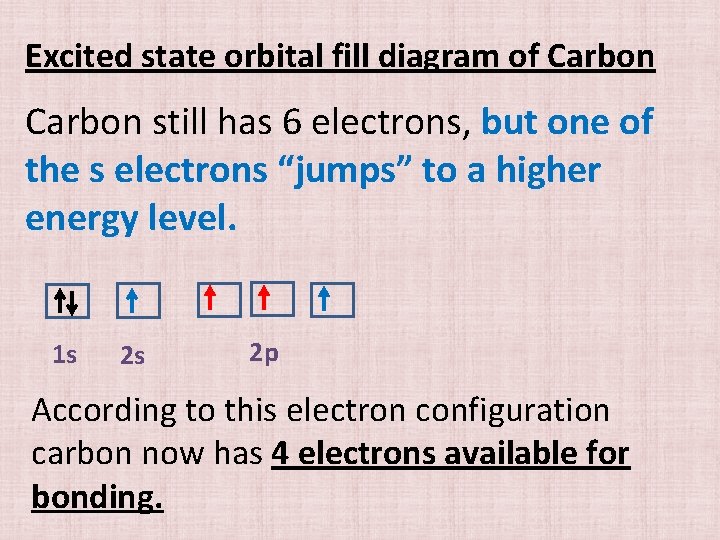
Excited state orbital fill diagram of Carbon still has 6 electrons, but one of the s electrons “jumps” to a higher energy level. 1 s 2 s 2 p According to this electron configuration carbon now has 4 electrons available for bonding.
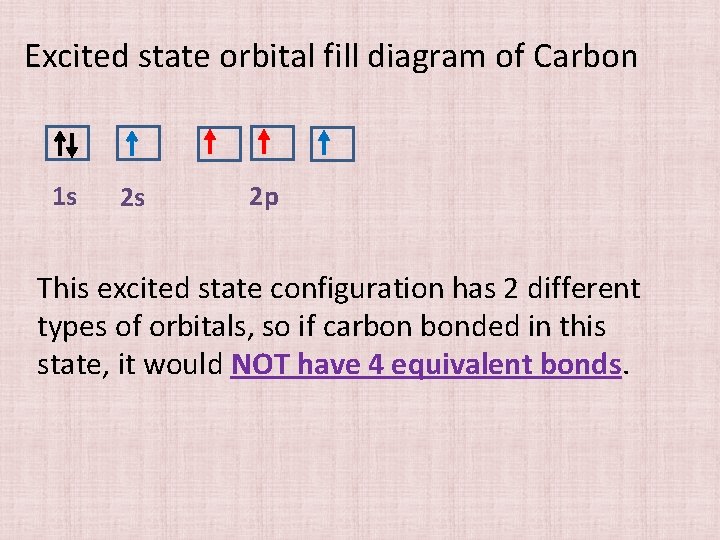
Excited state orbital fill diagram of Carbon 1 s 2 s 2 p This excited state configuration has 2 different types of orbitals, so if carbon bonded in this state, it would NOT have 4 equivalent bonds.
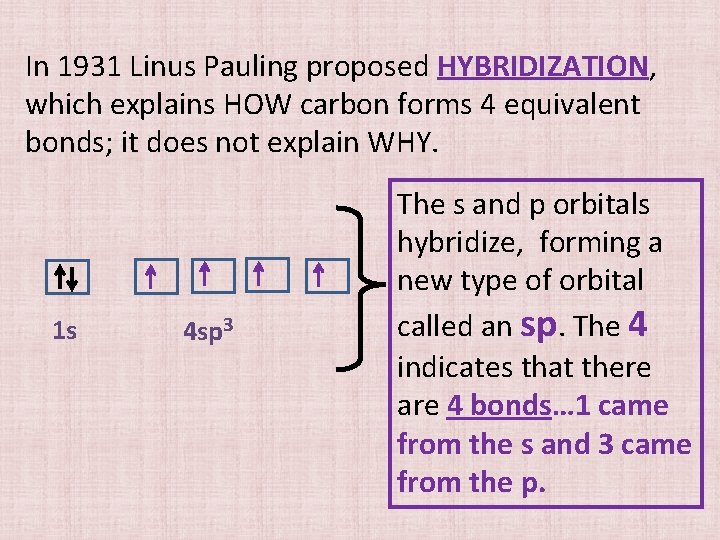
In 1931 Linus Pauling proposed HYBRIDIZATION, which explains HOW carbon forms 4 equivalent bonds; it does not explain WHY. 1 s 4 sp 3 The s and p orbitals hybridize, forming a new type of orbital called an sp. The 4 indicates that there are 4 bonds… 1 came from the s and 3 came from the p.

*Boron is another element that will hybridize. It will form 3 equivalent bonds. Its hybridization is 3 sp 2. *Write the ground state orbital fill diagram. *Write excited state orbital fill diagram. *Write the orbital fill diagram for 3 sp 2.
- Slides: 7