Hybridization Atomic Orbits Hybridization the concept of mixing

Hybridization Atomic Orbits

Hybridization ¡ the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties

The sp hybrid atomic orbitals ¡ The sp hybrid atomic orbitals are possible states of electron in an atom, especially when it is bonded to others. These electron states have half 2 s and half 2 p characters
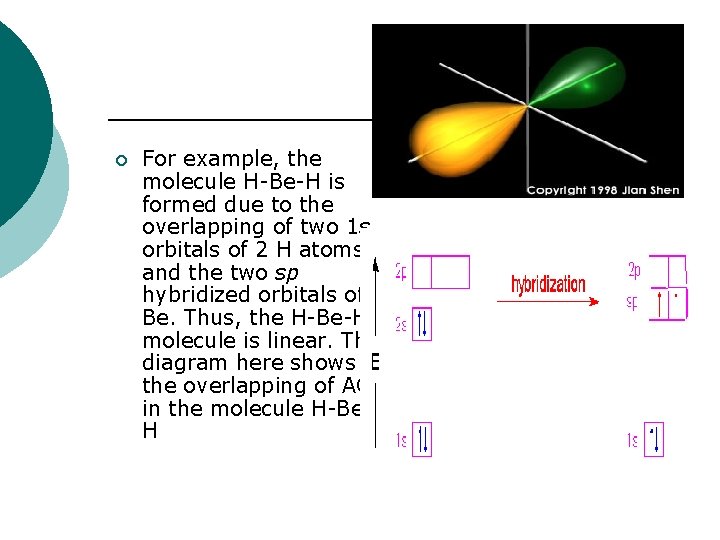
¡ For example, the molecule H-Be-H is formed due to the overlapping of two 1 s orbitals of 2 H atoms and the two sp hybridized orbitals of Be. Thus, the H-Be-H molecule is linear. The diagram here shows the overlapping of AOs in the molecule H-Be. H

The sp 2 hybrid orbitals ¡ The energy states of the valence electrons in atoms of the second period are in the 2 s and 2 p orbitals. If we mix two of the 2 p orbitals with a 2 s orbital, we end up with three sp 2 hybridized orbitals. These three orbitals lie on a plane, and they point to the vertices of a equilateral triangle as shown here.
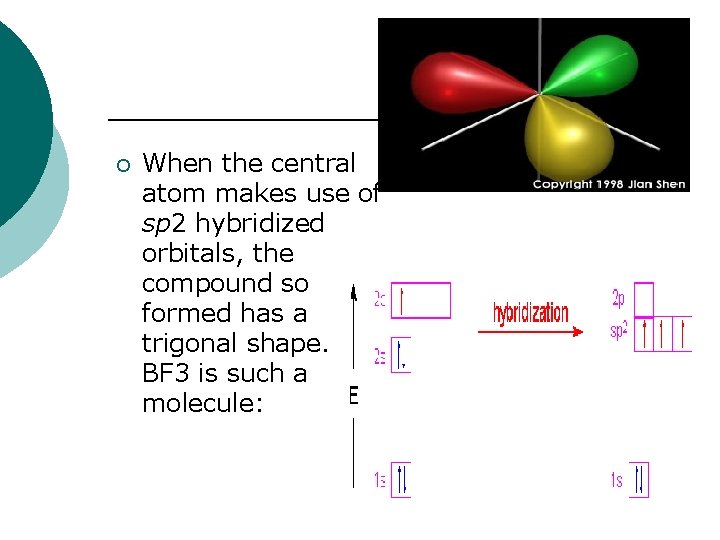
¡ When the central atom makes use of sp 2 hybridized orbitals, the compound so formed has a trigonal shape. BF 3 is such a molecule:
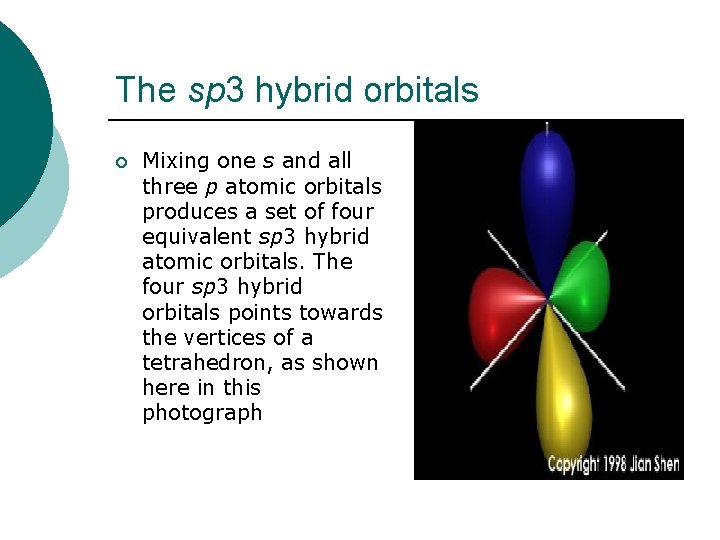
The sp 3 hybrid orbitals ¡ Mixing one s and all three p atomic orbitals produces a set of four equivalent sp 3 hybrid atomic orbitals. The four sp 3 hybrid orbitals points towards the vertices of a tetrahedron, as shown here in this photograph
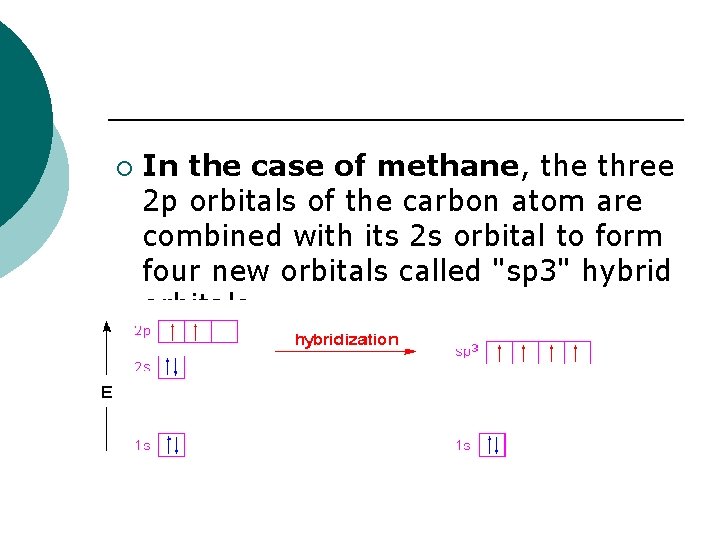
In the case of methane, the three 2 p orbitals of the carbon atom are combined with its 2 s orbital to form four new orbitals called "sp 3" hybrid orbitals. ¡. ¡
Hybridization Involving Multiple Bonds ¡ Only a maximum of two electrons can occupy any orbital whether it is an atomic orbital or a molecular orbital due to electron-electron repulsion.

When we draw a double or a triplebond between two atoms, we imply that either four or six electrons are directly between these two atoms. ¡ Since this is impossible, we must have these extra electrons off to the side in what we refer to as pi bonds. ¡

¡ Therefore, all multiple bonds are composed of two different kinds of molecular bonds called pi-bonds and sigma-bonds.

¡ The sigma-bond is defined as the linear overlap of atomic orbitals (hybrids except for hydrogen) in which two electrons are directly between the two bonded nuclei.
¡ Pi-bonds are defined as the parallel overlap of p-orbitals. A double bond has one sigma-bond and one pibond. A triple bond thus consists of a sigma-bond and two pi-bonds with the pi-bonds in different planes
- Slides: 13