Hybrid orbitals Hybridization of orbitals L Pauling the

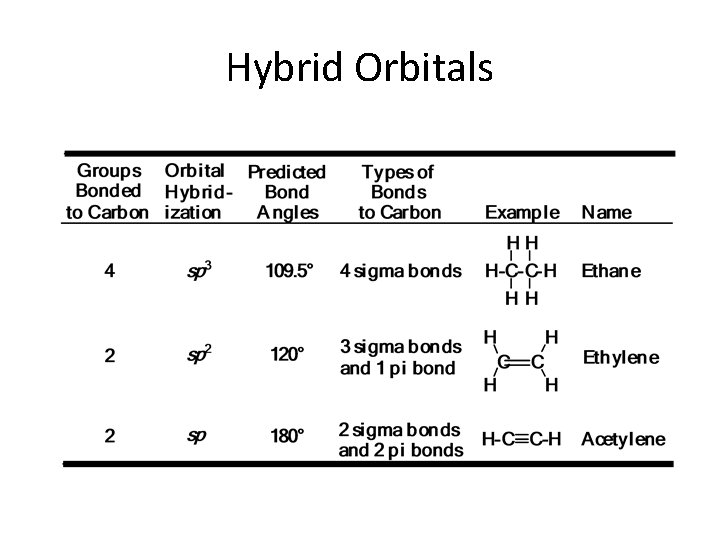
Hybrid orbitals Hybridization of orbitals (L. Pauling) the combination of two or more atomic orbitals forms a new set of atomic orbitals, called hybrid orbitals We deal with three types of hybrid orbitals sp 3 (one s orbital + three p orbitals) sp 2 (one s orbital + two p orbitals) sp (one s orbital + one p orbital) Overlap of hybrid orbitals can form two types of bonds depending on the geometry of overlap bonds are formed by “direct” overlap bonds are formed by “parallel” overlap
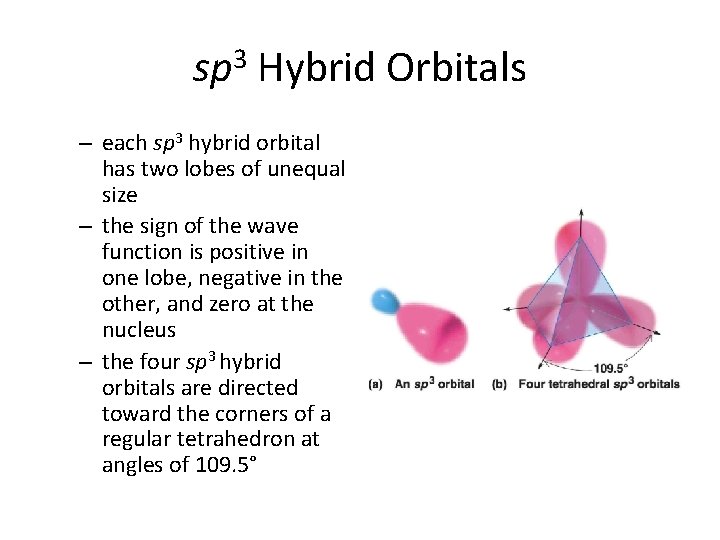
sp 3 Hybrid Orbitals – each sp 3 hybrid orbital has two lobes of unequal size – the sign of the wave function is positive in one lobe, negative in the other, and zero at the nucleus – the four sp 3 hybrid orbitals are directed toward the corners of a regular tetrahedron at angles of 109. 5°
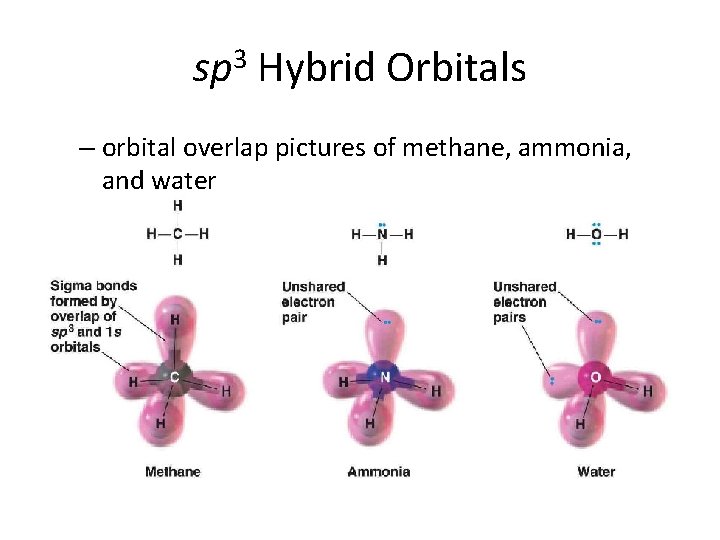
sp 3 Hybrid Orbitals – orbital overlap pictures of methane, ammonia, and water

Hydrocarbons • Hydrocarbons are said to be unsaturated when they contain multiple bonds (i. e C-C double bonds or triple bonds). Alkenes or alkynes are therefore known as unsaturated hydrocarbons.

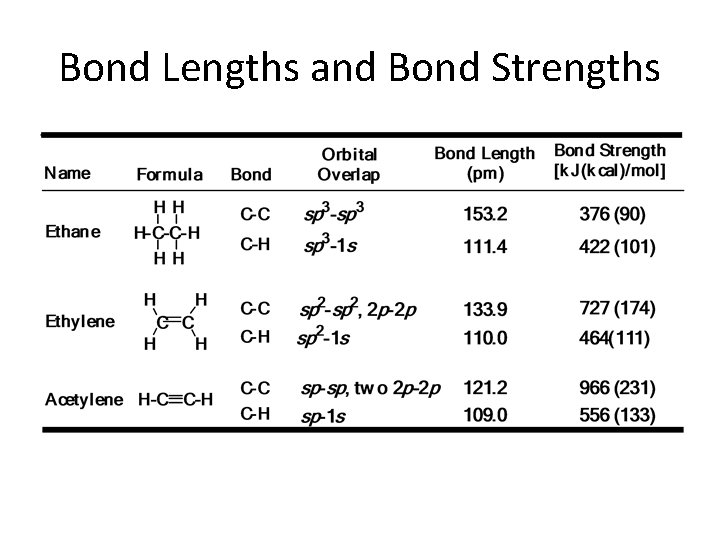
Unsaturated compounds The double bond in alkenes, such as ethene (ethylene), and the triple bond in alkynes, such as ethyne (acetylene), are the result of the ability of the atomic orbitals of carbon to adopt sp 2 and sp hybridization, respectively. Thus, the s bonds in ethene are derived entirely from carbon-based sp 2 hybrid orbitals: Csp 2 – Csp 2 for the C – C bond, and Csp 2 – H 1 s for holding the four hydrogens
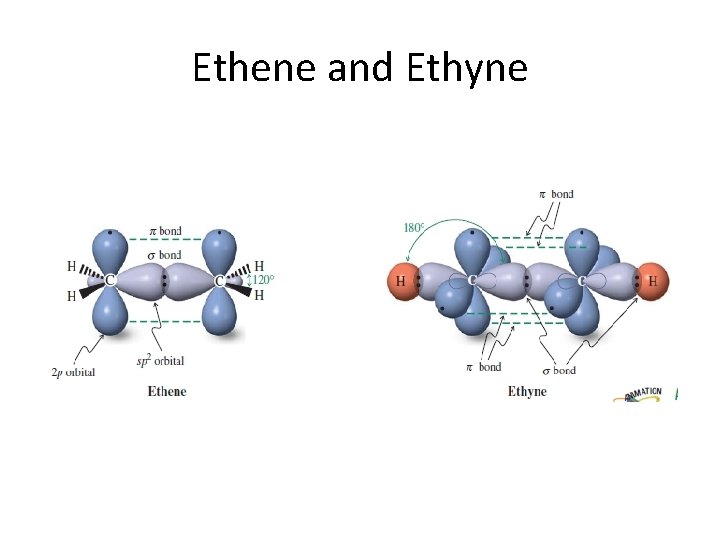
Ethene and Ethyne

Sp 2 Hybridization • Carbon atom may not use all its s- orbital and p-orbital for hybridization, it may use one sorbital and two p-orbital to form 3 Sp 2 hybrid orbitals • 2 s+ 2 px+ 2 py
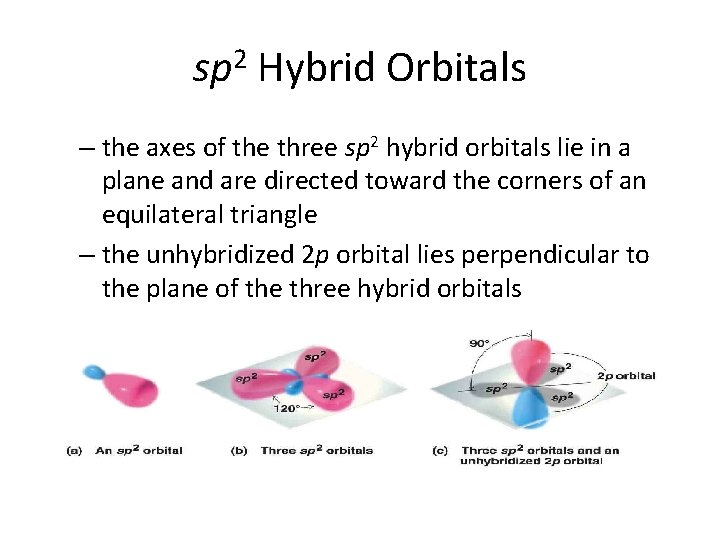
sp 2 Hybrid Orbitals – the axes of the three sp 2 hybrid orbitals lie in a plane and are directed toward the corners of an equilateral triangle – the unhybridized 2 p orbital lies perpendicular to the plane of the three hybrid orbitals
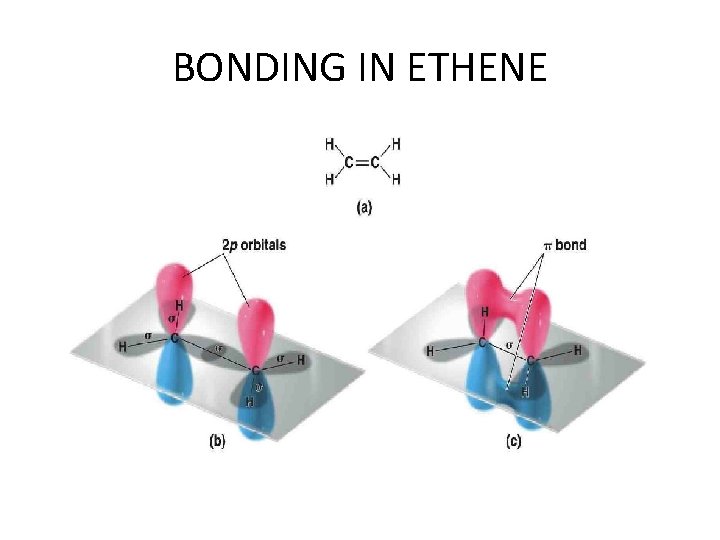
BONDING IN ETHENE
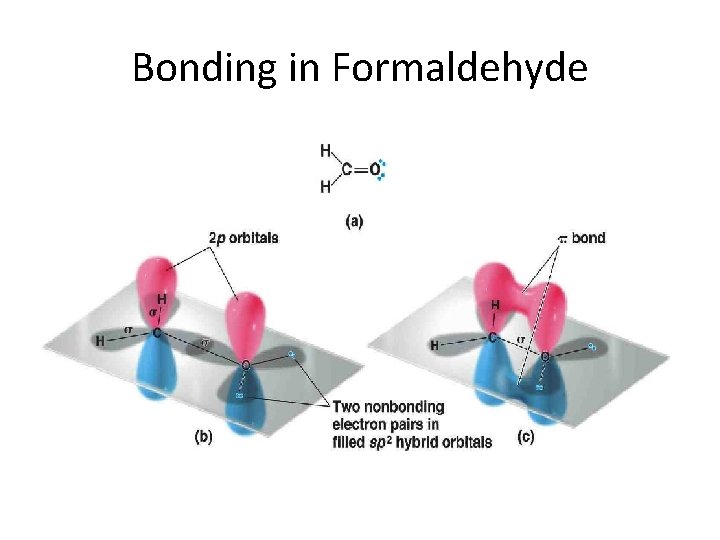
Bonding in Formaldehyde
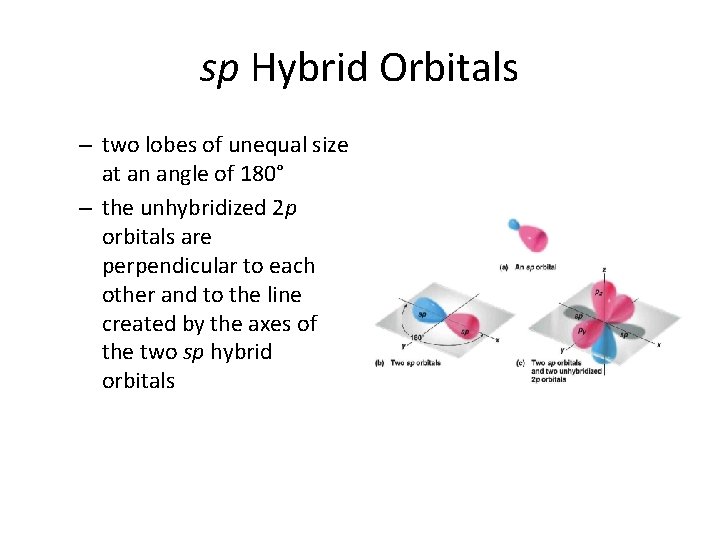
sp Hybrid Orbitals – two lobes of unequal size at an angle of 180° – the unhybridized 2 p orbitals are perpendicular to each other and to the line created by the axes of the two sp hybrid orbitals
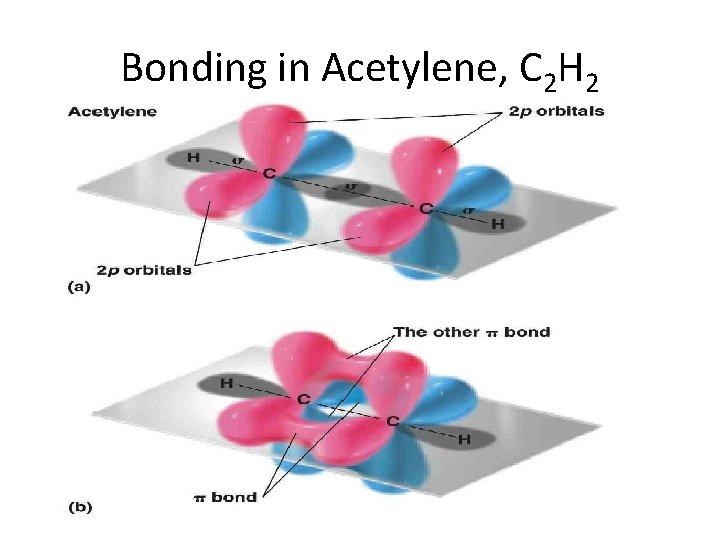
Bonding in Acetylene, C 2 H 2
Hybrid Orbitals
Bond Lengths and Bond Strengths
- Slides: 14