Human Subject Research Protections The Revised Common Rule

Human Subject Research Protections: The Revised Common Rule Office for Human Research Studies Emily Eldh, CIP January 2019
The (Forever Changing) Regulatory Landscape • Introductions • How did we get here? • Health and Human Services (HHS) Final Common Rule • 21 st Century Cures Act • What’s next? • Questions 2
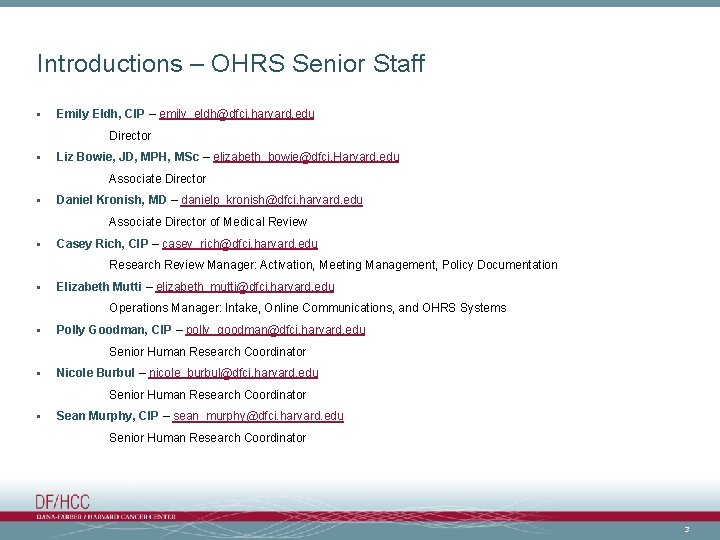
Introductions – OHRS Senior Staff • Emily Eldh, CIP – emily_eldh@dfci. harvard. edu Director • Liz Bowie, JD, MPH, MSc – elizabeth_bowie@dfci. Harvard. edu Associate Director • Daniel Kronish, MD – danielp_kronish@dfci. harvard. edu Associate Director of Medical Review • Casey Rich, CIP – casey_rich@dfci. harvard. edu Research Review Manager: Activation, Meeting Management, Policy Documentation • Elizabeth Mutti – elizabeth_mutti@dfci. harvard. edu Operations Manager: Intake, Online Communications, and OHRS Systems • Polly Goodman, CIP – polly_goodman@dfci. harvard. edu Senior Human Research Coordinator • Nicole Burbul – nicole_burbul@dfci. harvard. edu Senior Human Research Coordinator • Sean Murphy, CIP – sean_murphy@dfci. harvard. edu Senior Human Research Coordinator 3
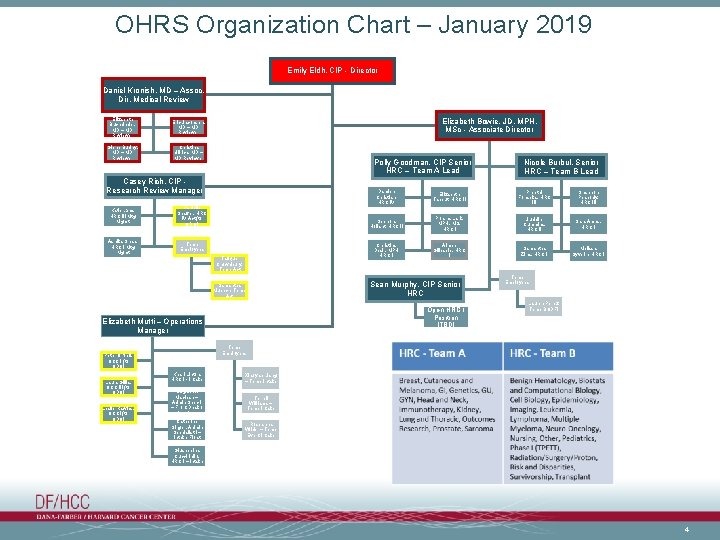
OHRS Organization Chart – January 2019 Emily Eldh, CIP - Director Daniel Kronish, MD – Assoc. Dir. Medical Review Elizabeth Buchbinder, MD – MD Reviewer Eric Jacobsen, MD – MD Reviewer Glenn Bubley, MD – MD Reviewer Christina Ullrich, MD – MD Reviewer Elizabeth Bowie, JD, MPH, MSc - Associate Director Polly Goodman, CIP Senior HRC – Team A Lead Casey Rich, CIP Research Review Manager Katie Lane, HRC III Mtg Mgmt Danielle Shaffer, HRC IV Act (to ODQ) Ashika Shah, HRC I Mtg Mgmt Temp Employees Tafique Chowdhury, Temp Act Preethi Fonseka, HRC III Shannon Peabody, HRC III Sephora Hollant, HRC II Porsha Lark, MPH, MS, HRC I Judelle Cummins, HRC II Sara Ames, HRC I Christina Desir, MPH HRC I Aimee Gillespie, HRC I Samantha Zina, HRC I Melissa Syverin, HRC I Temp Employees Lauren Perez, Temp BODFI Temp Employees Peter O’Neil, OCC I (to ODQ) Leslie Rawles, OCCI (to ODQ) Elizabeth Tabbut, HRC II Open HRC I Position [TBD] Elizabeth Mutti – Operations Manager Laura Gillis, OCC III (to ODQ) Damien Christian, HRC IV Sean Murphy, CIP Senior HRC Samantha Muench, Temp Act Nicole Burbul, Senior HRC – Team B Lead Khari Linton, HRC I - Intake Xiaoyan Liang – Temp Intake Marguerite Maclean – Admin Spec I – Front Desk / Mtg. Asst. Terrell Williams – Temp Intake Catherine Singer, Admin Specialist I – Intake Float Roseanna Wilder – Temp Event Intake Giuseppina Cucciniello, HRC I – Intake 4

How Did We Get Here? 5

How did we get here? Health and Human Services (HHS) Common Rule & Food and Drug Administration (FDA) regulations governing Human Subjects Research detail: 1. What an Institutional Review Board (IRB) is charged with reviewing; 2. How an IRB should be organized; and 3. The Criteria for Approval of IRB reviewed research. Note: HHS & FDA regulations are currently aligned (pre-1/21/2019). Health Insurance Portability & Accountability Act (HIPAA) • The DFCI IRB also serves as the Privacy Board and determines whether waivers or alterations to the HIPAA Privacy Rule are appropriate in the context of human subjects research. 6
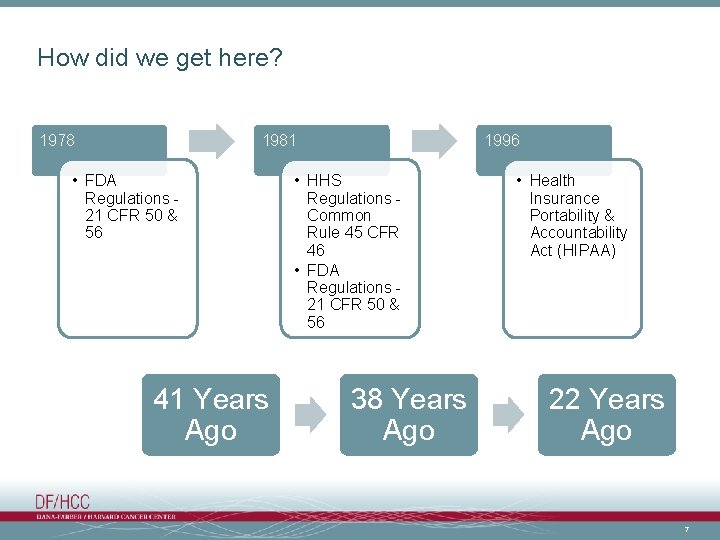
How did we get here? 1978 1981 • FDA Regulations 21 CFR 50 & 56 41 Years Ago 1996 • HHS Regulations Common Rule 45 CFR 46 • FDA Regulations 21 CFR 50 & 56 38 Years Ago • Health Insurance Portability & Accountability Act (HIPAA) 22 Years Ago 7
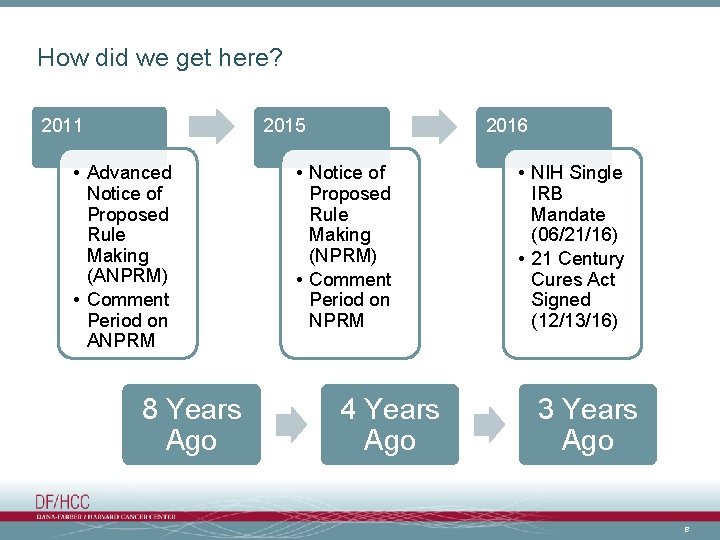
How did we get here? 2011 2015 • Advanced Notice of Proposed Rule Making (ANPRM) • Comment Period on ANPRM 8 Years Ago 2016 • Notice of Proposed Rule Making (NPRM) • Comment Period on NPRM 4 Years Ago • NIH Single IRB Mandate (06/21/16) • 21 Century Cures Act Signed (12/13/16) 3 Years Ago 8
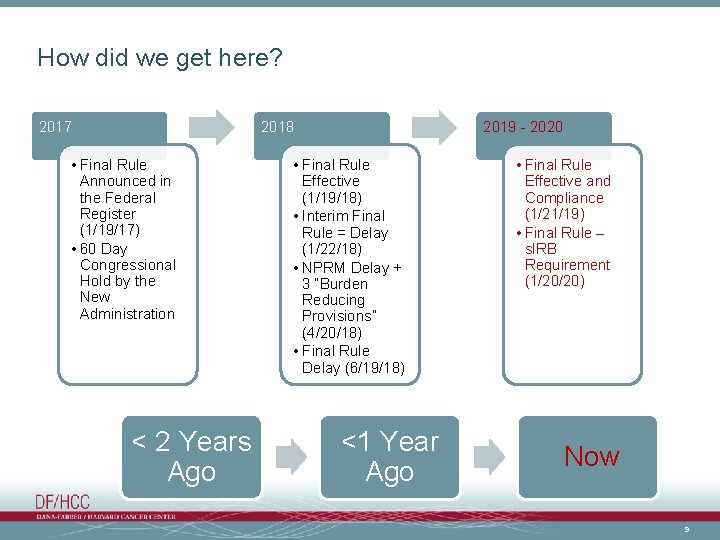
How did we get here? 2017 2018 • Final Rule Announced in the Federal Register (1/19/17) • 60 Day Congressional Hold by the New Administration < 2 Years Ago 2019 - 2020 • Final Rule Effective (1/19/18) • Interim Final Rule = Delay (1/22/18) • NPRM Delay + 3 “Burden Reducing Provisions” (4/20/18) • Final Rule Delay (6/19/18) <1 Year Ago • Final Rule Effective and Compliance (1/21/19) • Final Rule – s. IRB Requirement (1/20/20) Now 9
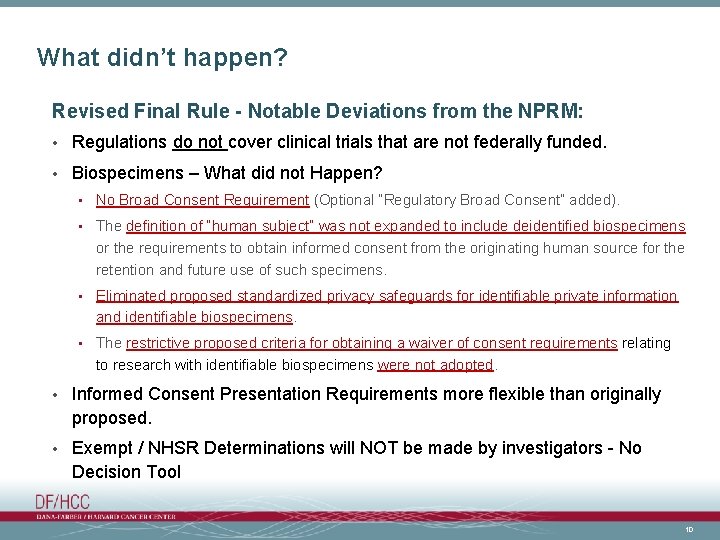
What didn’t happen? Revised Final Rule - Notable Deviations from the NPRM: • Regulations do not cover clinical trials that are not federally funded. • Biospecimens – What did not Happen? • No Broad Consent Requirement (Optional “Regulatory Broad Consent” added). • The definition of “human subject” was not expanded to include deidentified biospecimens or the requirements to obtain informed consent from the originating human source for the retention and future use of such specimens. • Eliminated proposed standardized privacy safeguards for identifiable private information and identifiable biospecimens. • The restrictive proposed criteria for obtaining a waiver of consent requirements relating to research with identifiable biospecimens were not adopted. • Informed Consent Presentation Requirements more flexible than originally proposed. • Exempt / NHSR Determinations will NOT be made by investigators - No Decision Tool 10

Final Revised Common Rule What is changing? • Definitions (Scope) • IRB Operations • Informed Consent Content • New Guidelines for Exemptions • Other Major Changes for Investigators are noted with an asterisk. 11
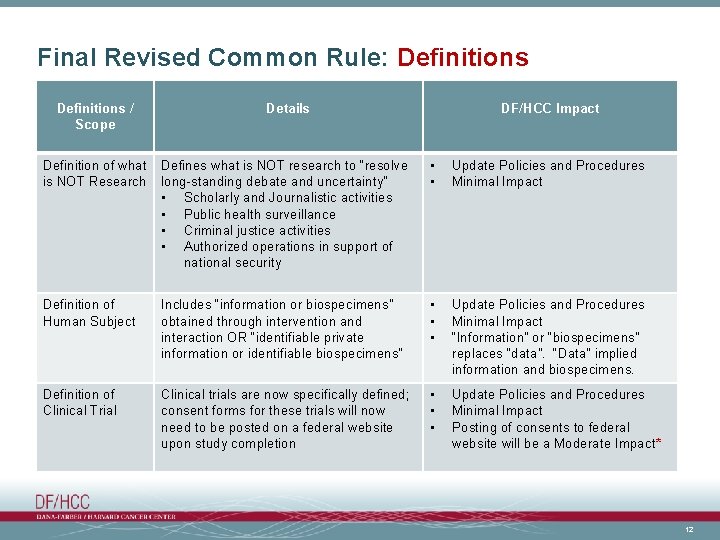
Final Revised Common Rule: Definitions / Scope Details DF/HCC Impact Definition of what is NOT Research Defines what is NOT research to “resolve long-standing debate and uncertainty” • Scholarly and Journalistic activities • Public health surveillance • Criminal justice activities • Authorized operations in support of national security • • Update Policies and Procedures Minimal Impact Definition of Human Subject Includes “information or biospecimens” obtained through intervention and interaction OR “identifiable private information or identifiable biospecimens” • • • Update Policies and Procedures Minimal Impact “Information” or “biospecimens” replaces “data”. “Data” implied information and biospecimens. Definition of Clinical Trial Clinical trials are now specifically defined; consent forms for these trials will now need to be posted on a federal website upon study completion • • • Update Policies and Procedures Minimal Impact Posting of consents to federal website will be a Moderate Impact* 12
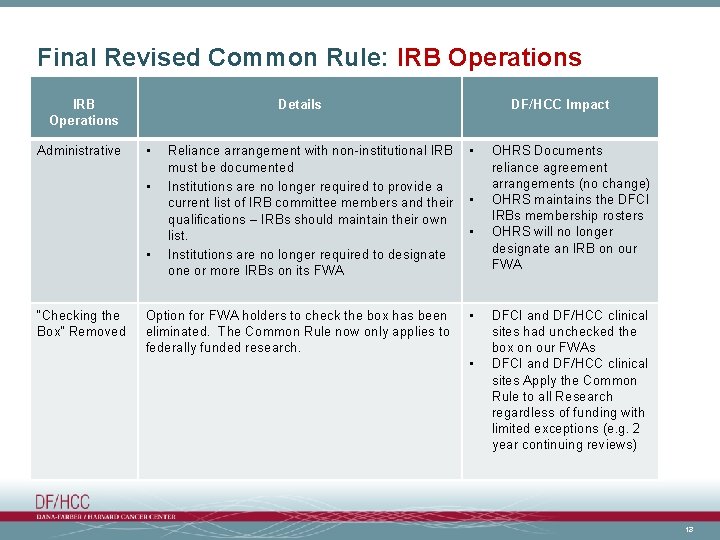
Final Revised Common Rule: IRB Operations Administrative Details • Reliance arrangement with non-institutional IRB must be documented Institutions are no longer required to provide a current list of IRB committee members and their qualifications – IRBs should maintain their own list. Institutions are no longer required to designate one or more IRBs on its FWA • Option for FWA holders to check the box has been eliminated. The Common Rule now only applies to federally funded research. • • • “Checking the Box” Removed DF/HCC Impact • • • OHRS Documents reliance agreement arrangements (no change) OHRS maintains the DFCI IRBs membership rosters OHRS will no longer designate an IRB on our FWA DFCI and DF/HCC clinical sites had unchecked the box on our FWAs DFCI and DF/HCC clinical sites Apply the Common Rule to all Research regardless of funding with limited exceptions (e. g. 2 year continuing reviews) 13
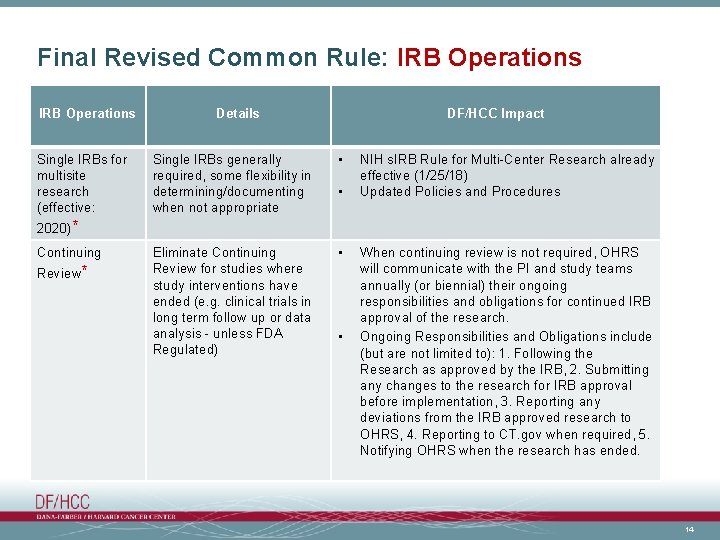
Final Revised Common Rule: IRB Operations Single IRBs for multisite research (effective: Details DF/HCC Impact Single IRBs generally required, some flexibility in determining/documenting when not appropriate • Eliminate Continuing Review for studies where study interventions have ended (e. g. clinical trials in long term follow up or data analysis - unless FDA Regulated) • • NIH s. IRB Rule for Multi-Center Research already effective (1/25/18) Updated Policies and Procedures 2020)* Continuing Review* • When continuing review is not required, OHRS will communicate with the PI and study teams annually (or biennial) their ongoing responsibilities and obligations for continued IRB approval of the research. Ongoing Responsibilities and Obligations include (but are not limited to): 1. Following the Research as approved by the IRB, 2. Submitting any changes to the research for IRB approval before implementation, 3. Reporting any deviations from the IRB approved research to OHRS, 4. Reporting to CT. gov when required, 5. Notifying OHRS when the research has ended. 14
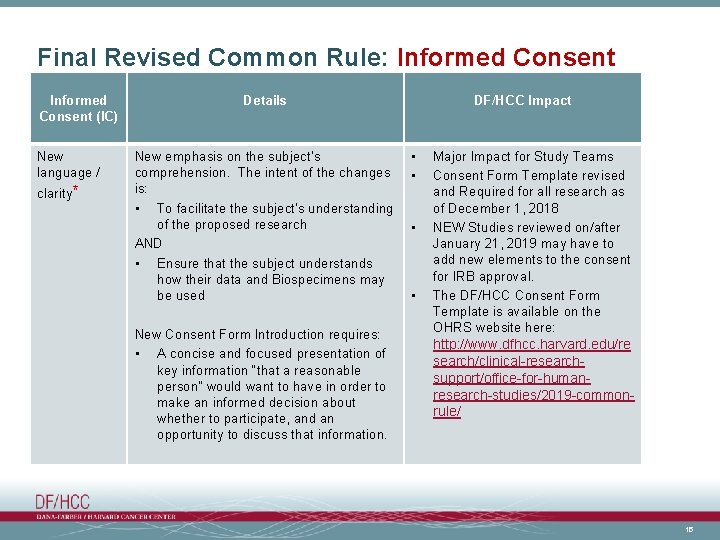
Final Revised Common Rule: Informed Consent (IC) New language / clarity* Details New emphasis on the subject’s comprehension. The intent of the changes is: • To facilitate the subject’s understanding of the proposed research AND • Ensure that the subject understands how their data and Biospecimens may be used New Consent Form Introduction requires: • A concise and focused presentation of key information “that a reasonable person” would want to have in order to make an informed decision about whether to participate, and an opportunity to discuss that information. DF/HCC Impact • • Major Impact for Study Teams Consent Form Template revised and Required for all research as of December 1, 2018 NEW Studies reviewed on/after January 21, 2019 may have to add new elements to the consent for IRB approval. The DF/HCC Consent Form Template is available on the OHRS website here: http: //www. dfhcc. harvard. edu/re search/clinical-researchsupport/office-for-humanresearch-studies/2019 -commonrule/ 15
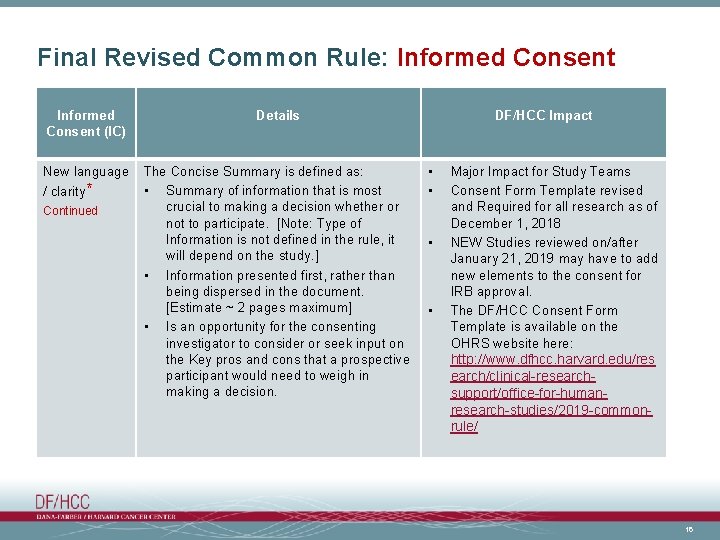
Final Revised Common Rule: Informed Consent (IC) Details New language The Concise Summary is defined as: • Summary of information that is most crucial to making a decision whether or not to participate. [Note: Type of Information is not defined in the rule, it will depend on the study. ] • Information presented first, rather than being dispersed in the document. [Estimate ~ 2 pages maximum] • Is an opportunity for the consenting investigator to consider or seek input on the Key pros and cons that a prospective participant would need to weigh in making a decision. / clarity* Continued DF/HCC Impact • • Major Impact for Study Teams Consent Form Template revised and Required for all research as of December 1, 2018 NEW Studies reviewed on/after January 21, 2019 may have to add new elements to the consent for IRB approval. The DF/HCC Consent Form Template is available on the OHRS website here: http: //www. dfhcc. harvard. edu/res earch/clinical-researchsupport/office-for-humanresearch-studies/2019 -commonrule/ 16
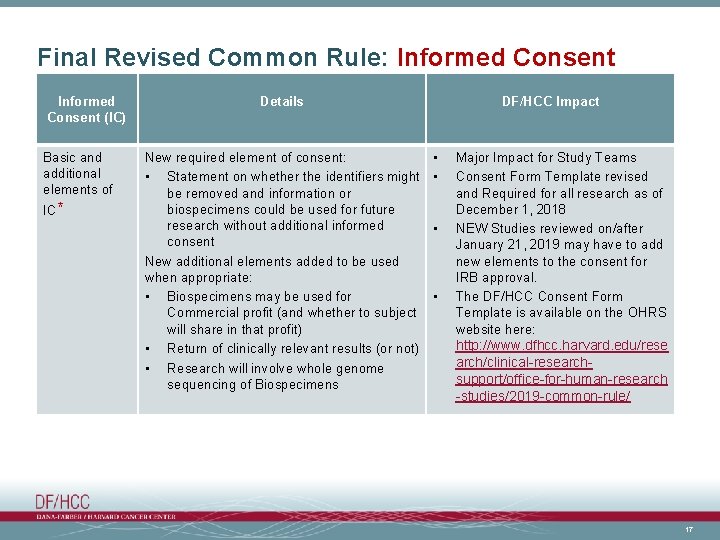
Final Revised Common Rule: Informed Consent (IC) Basic and additional elements of IC* Details New required element of consent: • Statement on whether the identifiers might be removed and information or biospecimens could be used for future research without additional informed consent New additional elements added to be used when appropriate: • Biospecimens may be used for Commercial profit (and whether to subject will share in that profit) • Return of clinically relevant results (or not) • Research will involve whole genome sequencing of Biospecimens DF/HCC Impact • • Major Impact for Study Teams Consent Form Template revised and Required for all research as of December 1, 2018 NEW Studies reviewed on/after January 21, 2019 may have to add new elements to the consent for IRB approval. The DF/HCC Consent Form Template is available on the OHRS website here: http: //www. dfhcc. harvard. edu/rese arch/clinical-researchsupport/office-for-human-research -studies/2019 -common-rule/ 17
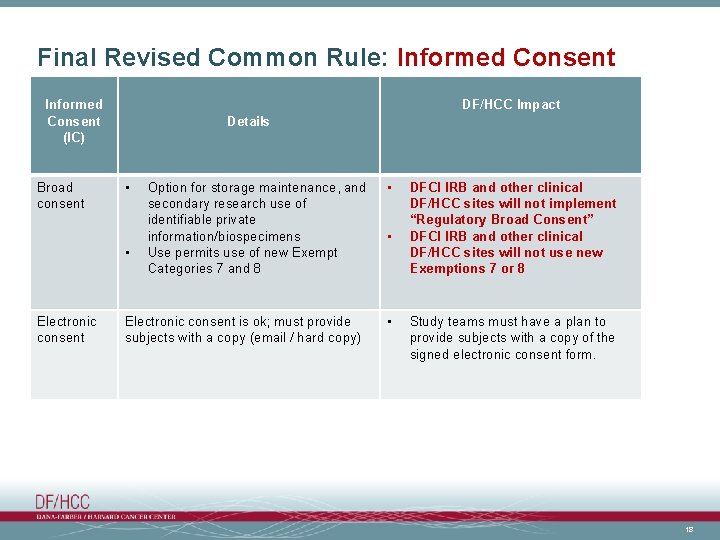
Final Revised Common Rule: Informed Consent (IC) Broad consent DF/HCC Impact Details • • Electronic consent Option for storage maintenance, and secondary research use of identifiable private information/biospecimens Use permits use of new Exempt Categories 7 and 8 Electronic consent is ok; must provide subjects with a copy (email / hard copy) • • • DFCI IRB and other clinical DF/HCC sites will not implement “Regulatory Broad Consent” DFCI IRB and other clinical DF/HCC sites will not use new Exemptions 7 or 8 Study teams must have a plan to provide subjects with a copy of the signed electronic consent form. 18
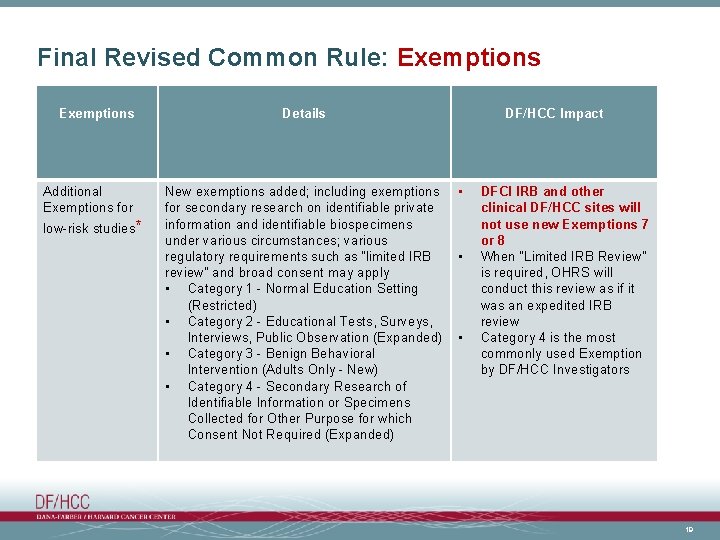
Final Revised Common Rule: Exemptions Additional Exemptions for low-risk studies* Details New exemptions added; including exemptions for secondary research on identifiable private information and identifiable biospecimens under various circumstances; various regulatory requirements such as “limited IRB review” and broad consent may apply • Category 1 - Normal Education Setting (Restricted) • Category 2 - Educational Tests, Surveys, Interviews, Public Observation (Expanded) • Category 3 - Benign Behavioral Intervention (Adults Only - New) • Category 4 - Secondary Research of Identifiable Information or Specimens Collected for Other Purpose for which Consent Not Required (Expanded) DF/HCC Impact • • • DFCI IRB and other clinical DF/HCC sites will not use new Exemptions 7 or 8 When “Limited IRB Review” is required, OHRS will conduct this review as if it was an expedited IRB review Category 4 is the most commonly used Exemption by DF/HCC Investigators 19
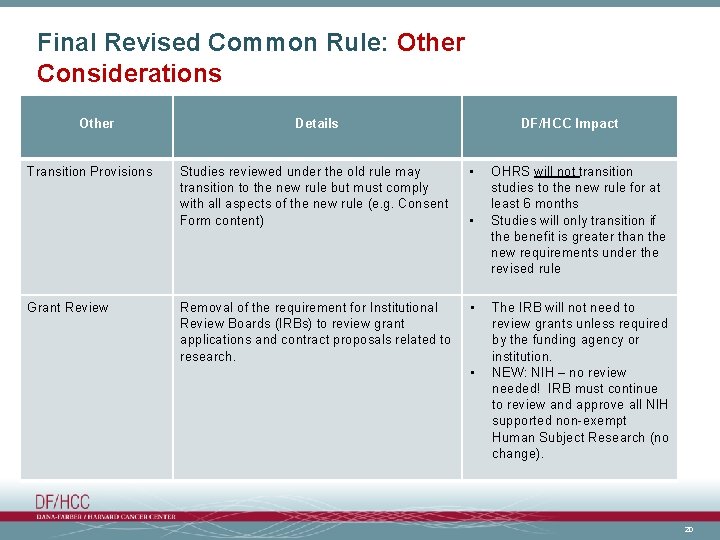
Final Revised Common Rule: Other Considerations Other Transition Provisions Grant Review Details DF/HCC Impact Studies reviewed under the old rule may transition to the new rule but must comply with all aspects of the new rule (e. g. Consent Form content) • Removal of the requirement for Institutional Review Boards (IRBs) to review grant applications and contract proposals related to research. • • • OHRS will not transition studies to the new rule for at least 6 months Studies will only transition if the benefit is greater than the new requirements under the revised rule The IRB will not need to review grants unless required by the funding agency or institution. NEW: NIH – no review needed! IRB must continue to review and approve all NIH supported non-exempt Human Subject Research (no change). 20
Other Requirements: 21 st Century Cures Act Aims to minimize administrative burden on researchers Highlights include: • HHS Secretary to Harmonize FDA regulations and the revised Common Rule (Note: They will not be aligned when the Final Rule is implemented) • Requires Drug Manufacturers to publicly post their expanded access policies and provide points of contacts for requests. • Removes Local IRB oversight requirement for device (IDE) studies • Requires NIH to ensure that centers and institutes collaborate on projects collecting similar data • Requests Guidance from HHS on HIPAA Privacy Rule and future uses of specimens and data • Consent alteration or waivers for minimal risk FDA-governed research 21

What’s needed to prepare and implement? 22
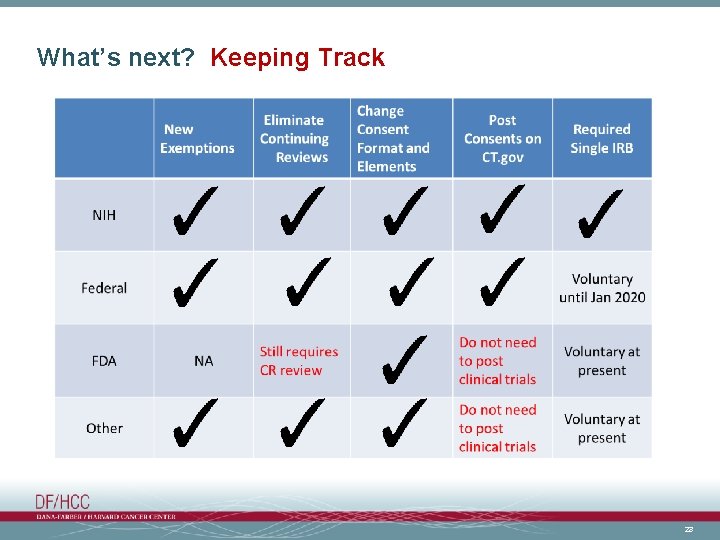
What’s next? Keeping Track 23

What’s needed to prepare and implement? 1. Update and Require use of Revised Consent Form Template (done) http: //www. dfhcc. harvard. edu/research/clinical-research-support/office-forhuman-research-studies/2019 -common-rule/ 2. Revise and Finalize DFCI IRB Policies and Procedures and Guidance Documents (ongoing) 3. Update Systems and IRB Documentation including Forms to accommodate Rule Change (ongoing) 4. Train Research Community (ongoing): • Revised Consent Form Training: November 9 th and 28 th 2018 • IRB Member Consent Form Training: December 10 th – December 20 th 2018 • OHRS Staff Rule Change Training: December 19 th 2018 • IRB Chair Rule Change Training: December 20 th 2018 • Rule Change Training: January 4 h and 10 th 2019 24

What’s still needed to prepare and implement? • • Guidance from OHRP • Read the text of the revised Common Rule Read about the revised Common Rule Revised Common Rule Q&As • Activities Deemed Not to be Research: Public Health Surveillance, 2018 Requirements • Scholarly and Journalistic Activities Deemed Not to be Research: 2018 Requirements • When Continuing Review Is Not Required During the 6 -Month Delay Period • Elimination of Institutional Review Board (IRB) Review of Research Applications and Proposals: 2018 Requirements Guidance from SACHRP • • Guidance from FDA • • https: //www. hhs. gov/ohrp/sachrp-committee/recommendations/sachrp-recommendations/index. html Impact of Certain Provisions of the Revised Common Rule on FDA-Regulated Clinical Investigations (10/2018) https: //www. fda. gov/downloads/Regulatory. Information/Guidances/UCM 623211. pdf Guidance from NIH • NIH Implementation of the Burden-Reducing Provisions of the 2018 Common Rule: https: //grants. nih. gov/grants/guide/notice-files/NOT-OD-18211. html • NIH Implementation of the Final Rule on the Federal Policy for the Protection of Human Subjects (Common Rule): https: //grants. nih. gov/grants/guide/notice-files/NOT-OD-19 -050. html 25

Additional Recommended Training on the Revised Common Rule: The CITI program has a new optional training module on the Common Rule changes. Please keep in mind CITI will not describe specific plans for implementing this new rule at DF/HCC. Additional information on implementation plans will be communicated separately. • CITI program: http: //www. dfhcc. harvard. edu/research/clinicalresearch-support/office-of-data-quality/servicessupport/education-citi/
More Info to Come! • 2019 Common Rule Webpage: OHRS has created a new page on the OHRS website where updates concerning the revised common rule will be placed.

Thank you! OHRS Common Rule Website: http: //www. dfhcc. harvard. edu/research/clinical-researchsupport/office-for-human-research-studies/2019 common-rule/ Office for Human Research Studies (OHRS) Dana-Farber Cancer Institute 450 Brookline Avenue, OS 229 Boston, MA 02215 Phone: 617 632 -3029 Fax: 617 632 -2686 http: //www. dfhcc. harvard. edu/research/clinical-researchsupport/ 28
- Slides: 28