Human risk assessment perspectives for high risk conditions







- Slides: 42

Human risk assessment perspectives for high risk conditions Jean Lou Dorne Institute of Human Nutrition University of Southampton, UK

Resveratrol Lycopene Isothiocyanates, Sulphorafane Isoflavones Allyl sulphides (Allicin…) Vitamine C, limonene



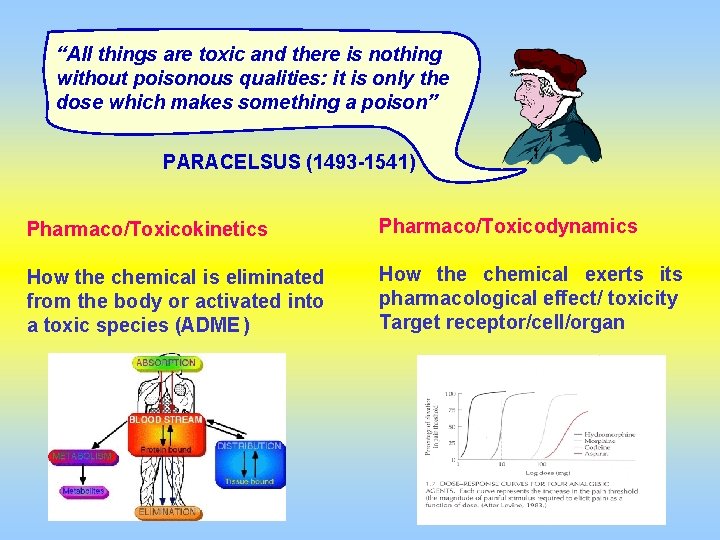
“All things are toxic and there is nothing without poisonous qualities: it is only the dose which makes something a poison” PARACELSUS (1493 -1541) Pharmaco/Toxicokinetics Pharmaco/Toxicodynamics How the chemical is eliminated from the body or activated into a toxic species (ADME ) How the chemical exerts its pharmacological effect/ toxicity Target receptor/cell/organ

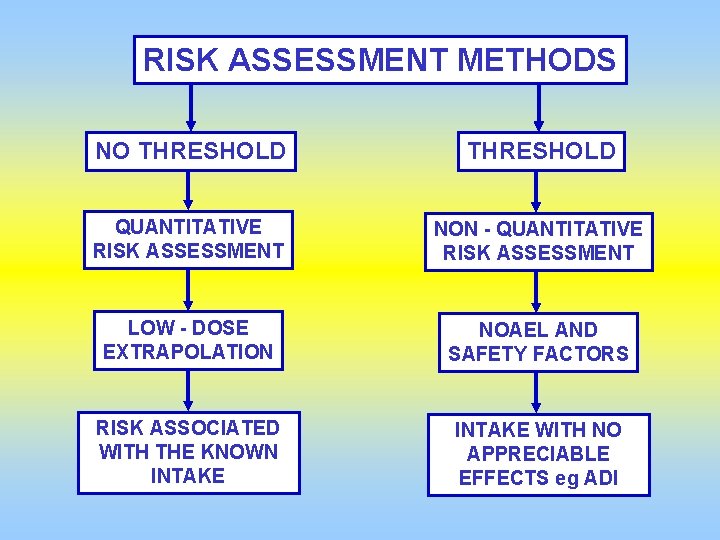
RISK ASSESSMENT METHODS NO THRESHOLD QUANTITATIVE RISK ASSESSMENT NON - QUANTITATIVE RISK ASSESSMENT LOW - DOSE EXTRAPOLATION NOAEL AND SAFETY FACTORS RISK ASSOCIATED WITH THE KNOWN INTAKE WITH NO APPRECIABLE EFFECTS eg ADI

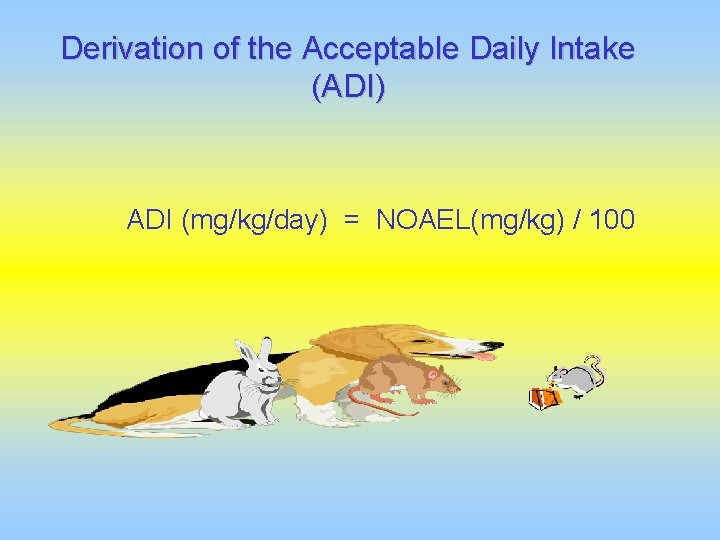
Derivation of the Acceptable Daily Intake (ADI) ADI (mg/kg/day) = NOAEL(mg/kg) / 100

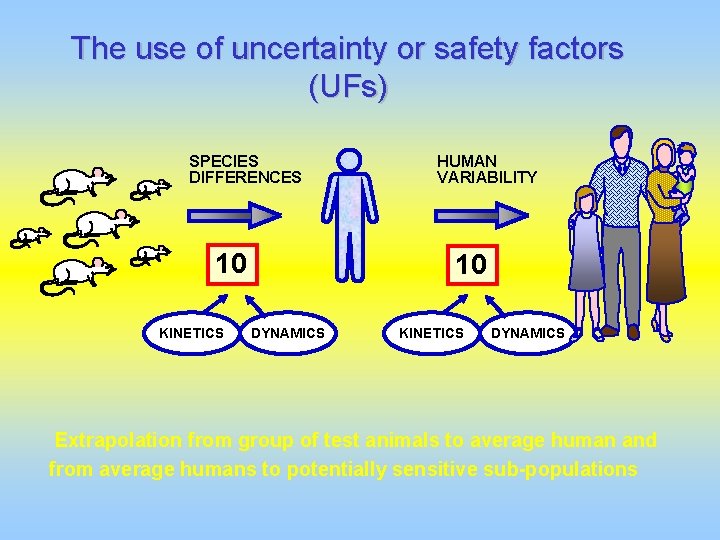
The use of uncertainty or safety factors (UFs) SPECIES DIFFERENCES 10 KINETICS HUMAN VARIABILITY 10 DYNAMICS KINETICS DYNAMICS Extrapolation from group of test animals to average human and from average humans to potentially sensitive sub-populations

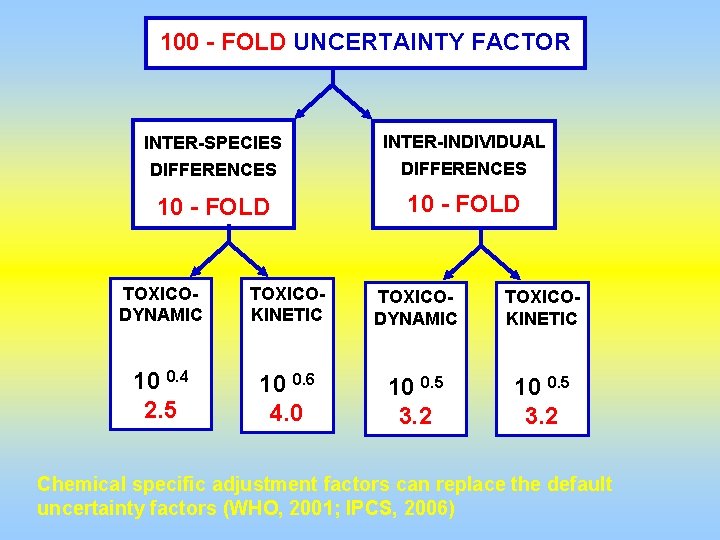
100 - FOLD UNCERTAINTY FACTOR INTER-SPECIES INTER-INDIVIDUAL DIFFERENCES 10 - FOLD TOXICODYNAMIC TOXICOKINETIC 10 0. 4 2. 5 10 0. 6 4. 0 10 0. 5 3. 2 Chemical specific adjustment factors can replace the default uncertainty factors (WHO, 2001; IPCS, 2006)

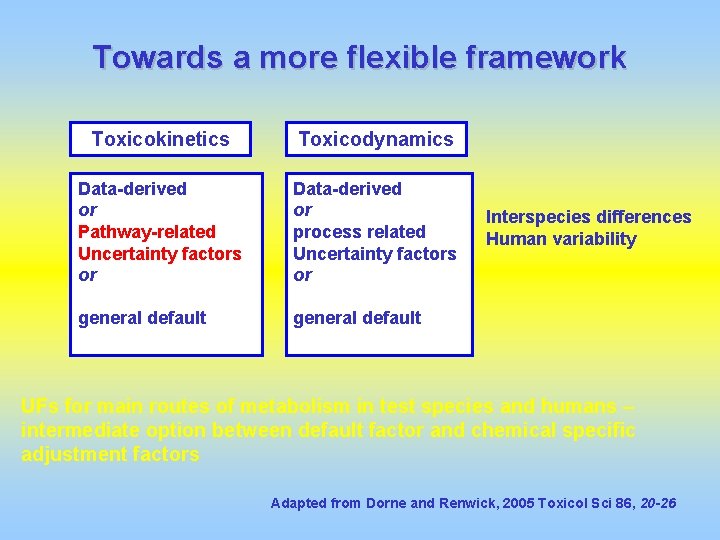
Towards a more flexible framework Toxicokinetics Toxicodynamics Data-derived or Pathway-related Uncertainty factors or Data-derived or process related Uncertainty factors or general default Interspecies differences Human variability UFs for main routes of metabolism in test species and humans – intermediate option between default factor and chemical specific adjustment factors Adapted from Dorne and Renwick, 2005 Toxicol Sci 86, 20 -26

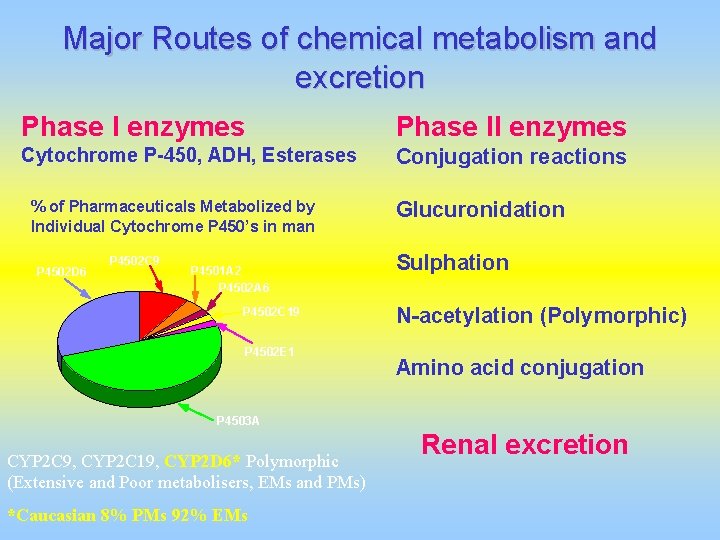
Major Routes of chemical metabolism and excretion Phase I enzymes Phase II enzymes Cytochrome P-450, ADH, Esterases Conjugation reactions % of Pharmaceuticals Metabolized by Individual Cytochrome P 450’s in man P 4502 D 6 P 4502 C 9 P 4501 A 2 P 4502 A 6 P 4502 C 19 P 4502 E 1 Glucuronidation Sulphation N-acetylation (Polymorphic) Amino acid conjugation P 4503 A CYP 2 C 9, CYP 2 C 19, CYP 2 D 6* Polymorphic (Extensive and Poor metabolisers, EMs and PMs) *Caucasian 8% PMs 92% EMs Renal excretion

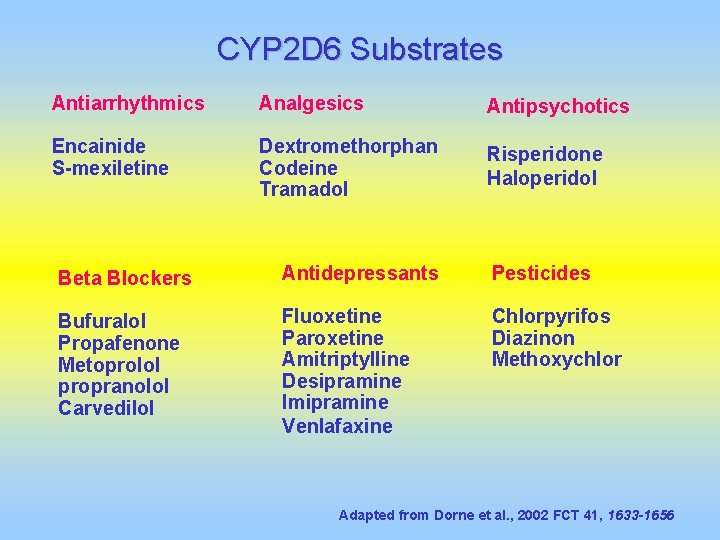
CYP 2 D 6 Substrates Antiarrhythmics Analgesics Antipsychotics Encainide S-mexiletine Dextromethorphan Codeine Tramadol Risperidone Haloperidol Beta Blockers Antidepressants Pesticides Bufuralol Propafenone Metoprolol propranolol Carvedilol Fluoxetine Paroxetine Amitriptylline Desipramine Imipramine Venlafaxine Chlorpyrifos Diazinon Methoxychlor Adapted from Dorne et al. , 2002 FCT 41, 1633 -1656

Introducing metabolic and toxicokinetic data into risk assessment

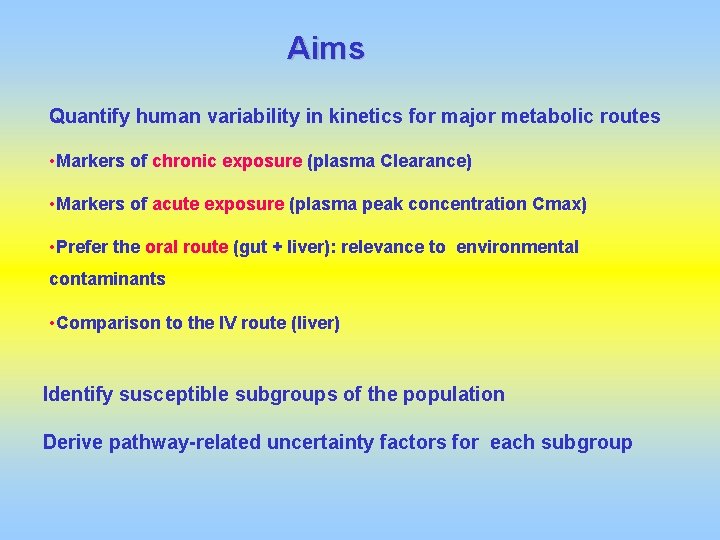
Aims Quantify human variability in kinetics for major metabolic routes • Markers of chronic exposure (plasma Clearance) • Markers of acute exposure (plasma peak concentration Cmax) • Prefer the oral route (gut + liver): relevance to environmental contaminants • Comparison to the IV route (liver) Identify susceptible subgroups of the population Derive pathway-related uncertainty factors for each subgroup

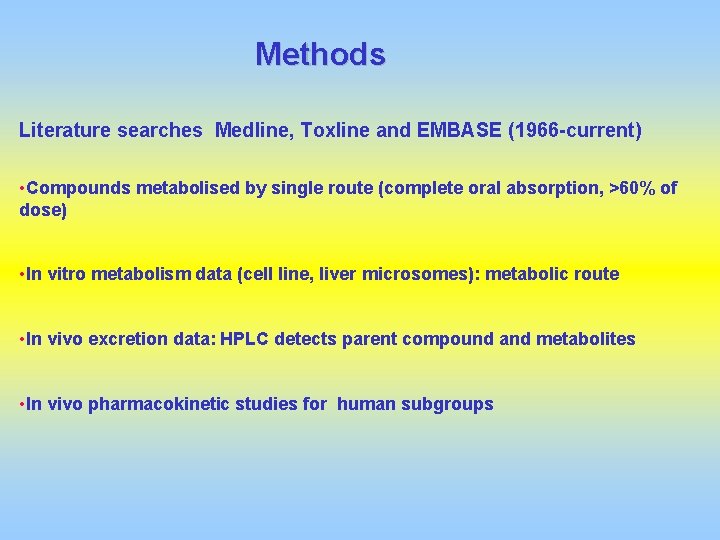
Methods Literature searches Medline, Toxline and EMBASE (1966 -current) • Compounds metabolised by single route (complete oral absorption, >60% of dose) • In vitro metabolism data (cell line, liver microsomes): metabolic route • In vivo excretion data: HPLC detects parent compound and metabolites • In vivo pharmacokinetic studies for human subgroups

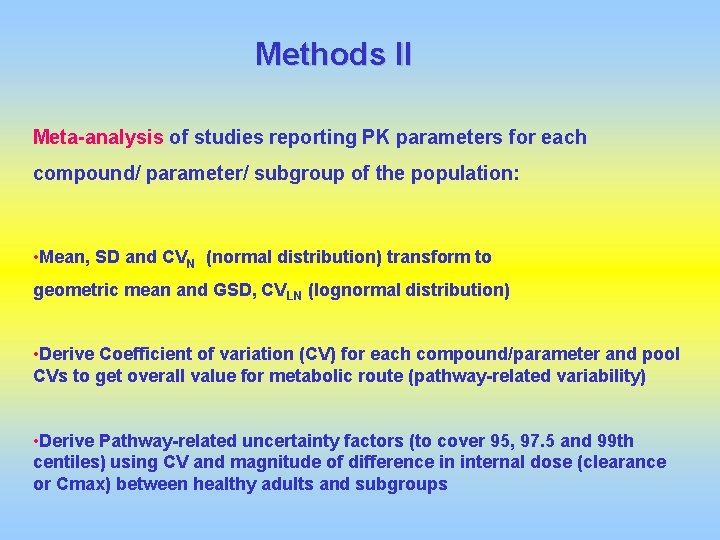
Methods II Meta-analysis of studies reporting PK parameters for each compound/ parameter/ subgroup of the population: • Mean, SD and CVN (normal distribution) transform to geometric mean and GSD, CVLN (lognormal distribution) • Derive Coefficient of variation (CV) for each compound/parameter and pool CVs to get overall value for metabolic route (pathway-related variability) • Derive Pathway-related uncertainty factors (to cover 95, 97. 5 and 99 th centiles) using CV and magnitude of difference in internal dose (clearance or Cmax) between healthy adults and subgroups

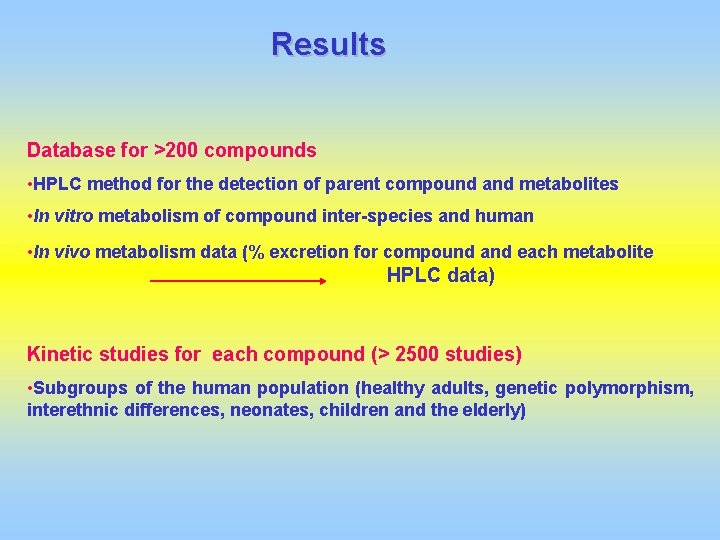
Results Database for >200 compounds • HPLC method for the detection of parent compound and metabolites • In vitro metabolism of compound inter-species and human • In vivo metabolism data (% excretion for compound and each metabolite HPLC data) Kinetic studies for each compound (> 2500 studies) • Subgroups of the human population (healthy adults, genetic polymorphism, interethnic differences, neonates, children and the elderly)

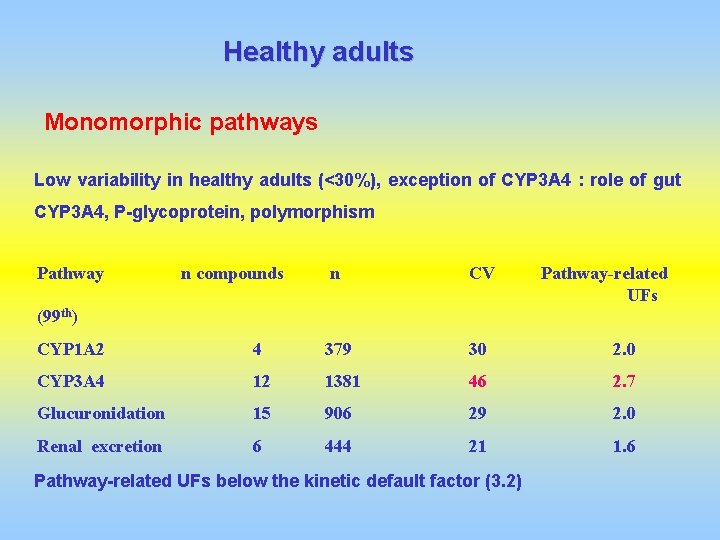
Healthy adults Monomorphic pathways Low variability in healthy adults (<30%), exception of CYP 3 A 4 : role of gut CYP 3 A 4, P-glycoprotein, polymorphism Pathway n compounds n CV Pathway-related UFs (99 th) CYP 1 A 2 4 379 30 2. 0 CYP 3 A 4 12 1381 46 2. 7 Glucuronidation 15 906 29 2. 0 Renal excretion 6 444 21 1. 6 Pathway-related UFs below the kinetic default factor (3. 2)

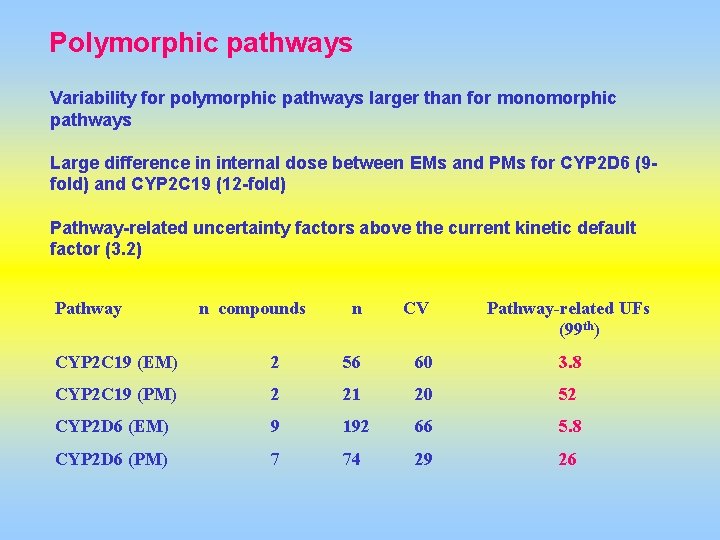
Polymorphic pathways Variability for polymorphic pathways larger than for monomorphic pathways Large difference in internal dose between EMs and PMs for CYP 2 D 6 (9 fold) and CYP 2 C 19 (12 -fold) Pathway-related uncertainty factors above the current kinetic default factor (3. 2) Pathway n compounds n CV Pathway-related UFs (99 th) CYP 2 C 19 (EM) 2 56 60 3. 8 CYP 2 C 19 (PM) 2 21 20 52 CYP 2 D 6 (EM) 9 192 66 5. 8 CYP 2 D 6 (PM) 7 74 29 26

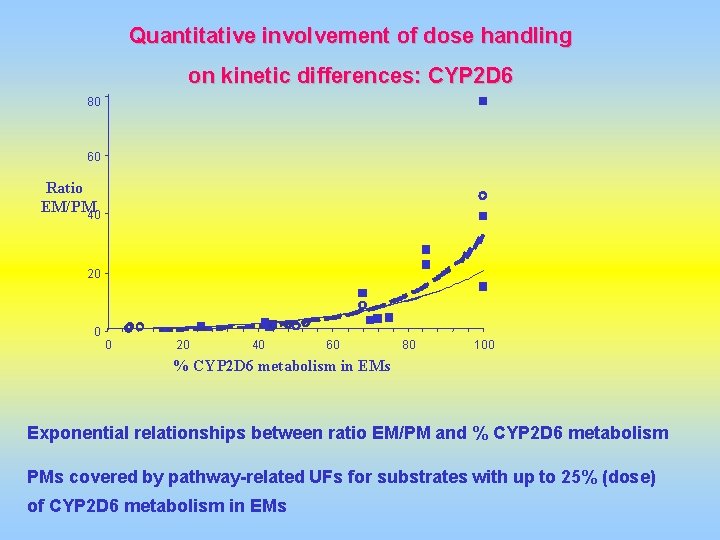
Quantitative involvement of dose handling on kinetic differences: CYP 2 D 6 80 60 Ratio EM/PM 40 20 0 0 20 40 60 80 100 % CYP 2 D 6 metabolism in EMs Exponential relationships between ratio EM/PM and % CYP 2 D 6 metabolism PMs covered by pathway-related UFs for substrates with up to 25% (dose) of CYP 2 D 6 metabolism in EMs

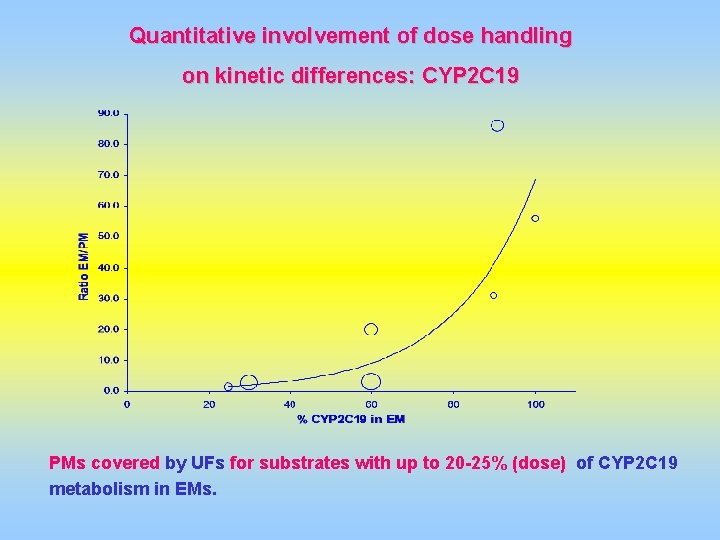
Quantitative involvement of dose handling on kinetic differences: CYP 2 C 19 PMs covered by UFs for substrates with up to 20 -25% (dose) of CYP 2 C 19 metabolism in EMs.

Results: Subgroups of the population Interethnic differences Historically smaller database for non-Caucasian subjects: Modern man : mixture of ethnic groups and more so in the future ! Less variability in Asian vs Caucasian for CYP 2 D 6 and CYP 2 C 19 (+ different frequencies of phenotypes) Pathway-related uncertainty factors above kinetic default for CYP 2 C 19 and NAT metabolism Ex relationship for CYP 2 C 19 and ratio EMs/PMs in Asian healthy adults (R 2=0. 87) : Slope 100% metabolism via CYP 2 C 19 gives a ratio of 30 (80 in Caucasian !)

Children and neonates Potential susceptible subgroups of the population: -Immaturity of phase I, phase II and renal excretion (particularly for neonates) -Quantify differences in internal dose from in vivo PK database -Provide pathway-related UFs for these subgroups -Identify datagaps

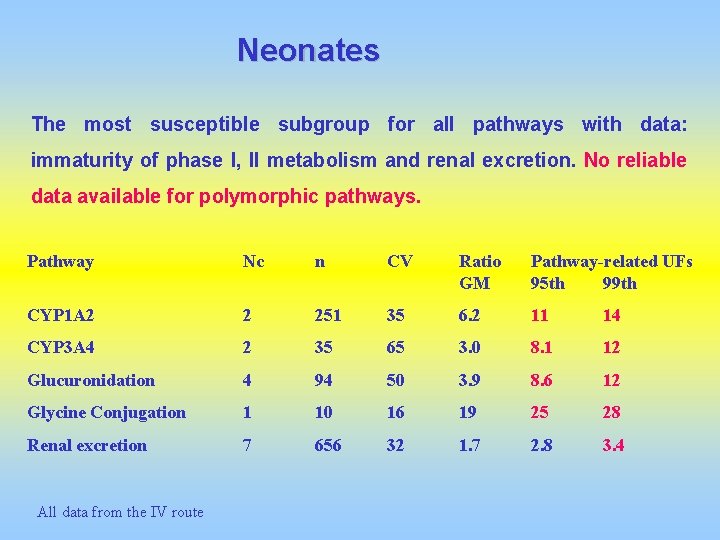
Neonates The most susceptible subgroup for all pathways with data: immaturity of phase I, II metabolism and renal excretion. No reliable data available for polymorphic pathways. Pathway Nc n CV Ratio GM Pathway-related UFs 95 th 99 th CYP 1 A 2 2 251 35 6. 2 11 14 CYP 3 A 4 2 35 65 3. 0 8. 1 12 Glucuronidation 4 94 50 3. 9 8. 6 12 Glycine Conjugation 1 10 16 19 25 28 Renal excretion 7 656 32 1. 7 2. 8 3. 4 All data from the IV route

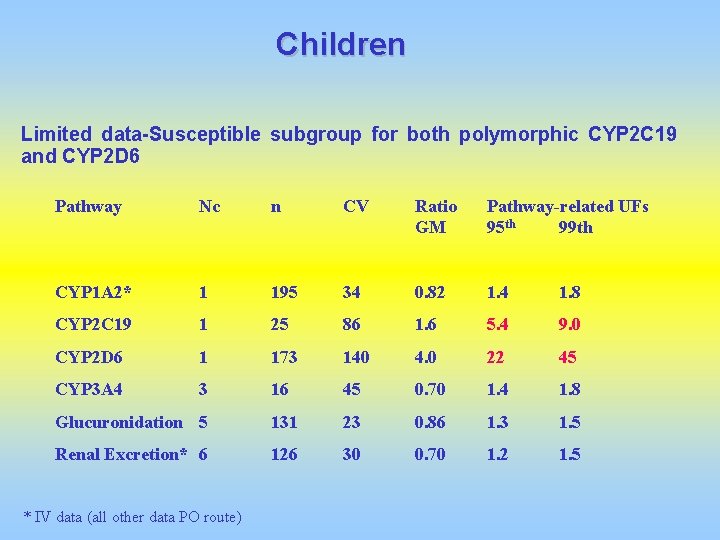
Children Limited data-Susceptible subgroup for both polymorphic CYP 2 C 19 and CYP 2 D 6 Pathway Nc n CV Ratio GM Pathway-related UFs 95 th 99 th CYP 1 A 2* 1 195 34 0. 82 1. 4 1. 8 CYP 2 C 19 1 25 86 1. 6 5. 4 9. 0 CYP 2 D 6 1 173 140 4. 0 22 45 CYP 3 A 4 3 16 45 0. 70 1. 4 1. 8 Glucuronidation 5 131 23 0. 86 1. 3 1. 5 Renal Excretion* 6 126 30 0. 70 1. 2 1. 5 * IV data (all other data PO route)

Polymorphism in metabolism and Children and neonates: Examples Fluoxetine and paroxetine metabolised largely via CYP 2 D 6 and other CYP isoforms (CYP 2 C 9, CYP 3 A 4 and CYP 2 C 19) Large inter-individual differences in kinetics in healthy adults and Holden, C. Prozac Treatment of Newborn Mice Raises children: up to 10 -18 -fold variation in clearance in healthy adults Anxiety. Science. 2004 Oct 29; 306(5697): 792. PMs (including 2 PM children) Ibuprofen and indomethacin in preterm neonates : up to 10 -fold difference decrease in clearance : immature CYP 2 C 9, glucuronidation and renal excretion. Lansoprazole (CYP 2 C 19 -CYP 3 A 4): 1 neonate and 1 infant PM (3 and 7 -fold decrease in clearance)

Predicting human variability in toxicokinetics using Monte Carlo modelling

Latin hypercube sampling: variant of Monte Carlo (random), stratified sampling throughout the distribution. Compounds handled by multiple pathways : predict variability and uncertainty factors for healthy adults, children and neonates. Combine distributions describing pathway –related variability and quantitative metabolism data. Compare Simulated data and published kinetic data.

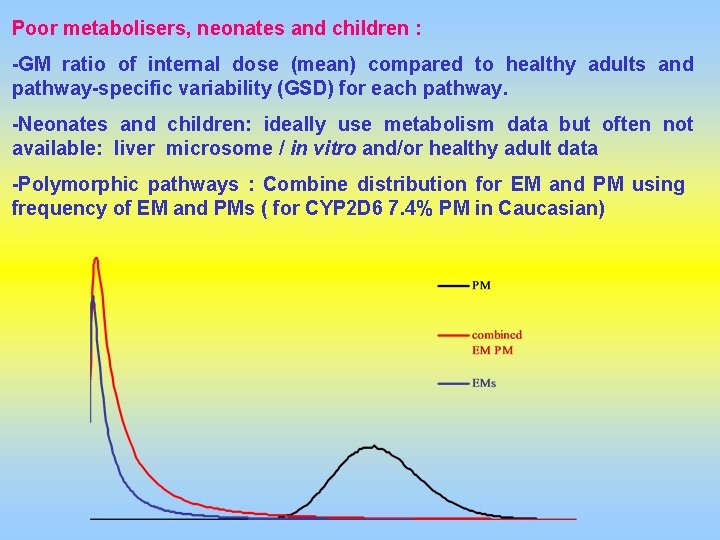
Poor metabolisers, neonates and children : -GM ratio of internal dose (mean) compared to healthy adults and pathway-specific variability (GSD) for each pathway. -Neonates and children: ideally use metabolism data but often not available: liver microsome / in vitro and/or healthy adult data -Polymorphic pathways : Combine distribution for EM and PM using frequency of EM and PMs ( for CYP 2 D 6 7. 4% PM in Caucasian)

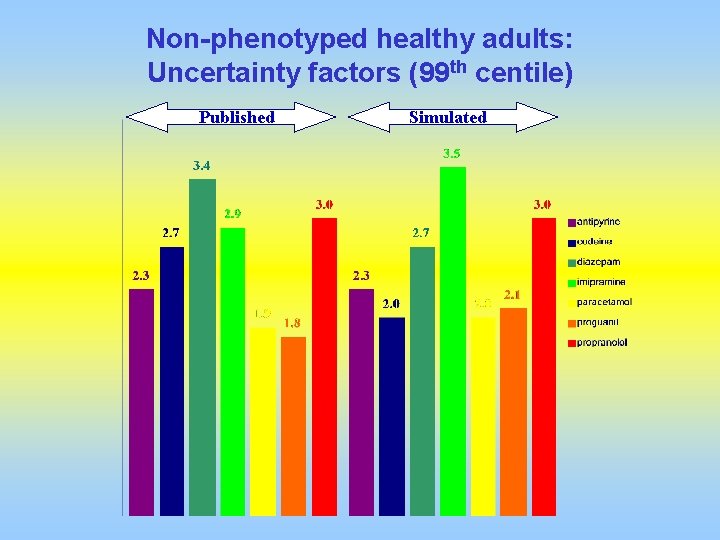
Non-phenotyped healthy adults: Uncertainty factors (99 th centile) Published Simulated

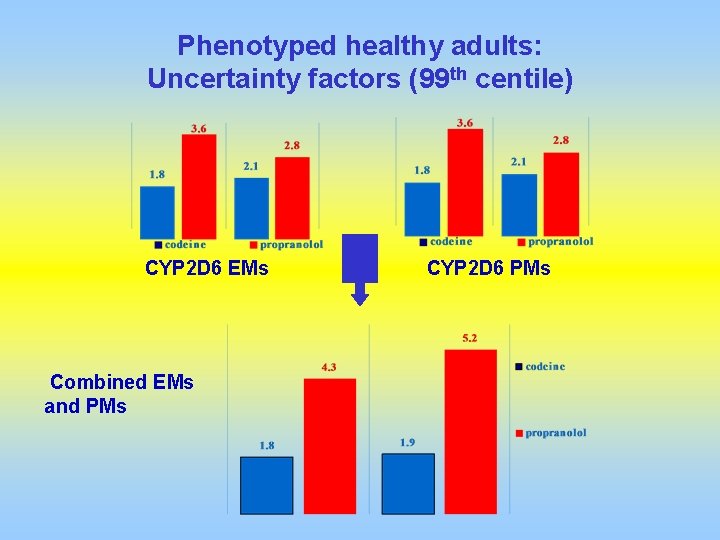
Phenotyped healthy adults: Uncertainty factors (99 th centile) CYP 2 D 6 EMs Combined EMs and PMs CYP 2 D 6 PMs

Pharmacokinetic interaction between probe substrates of polymorphic CYPs • Literature searches for interaction studies between major probe substrates (> 70% of the dose metabolised by each CYP) of CYP 2 D 6 and CYP 2 C 19, inhibitors and inducers of each enzyme. • UFs to cover percentiles for subgroup of population Relevance: a number of pesticides are substrates and inhibit polymorphic CYPs (chlorpyrifos, diazinon). . Extensive metabolisers (EMs) are at risk if the metabolite produced the toxicant. Poor metabolisers (PMs) would be at risk if the parent compound is the toxicant.

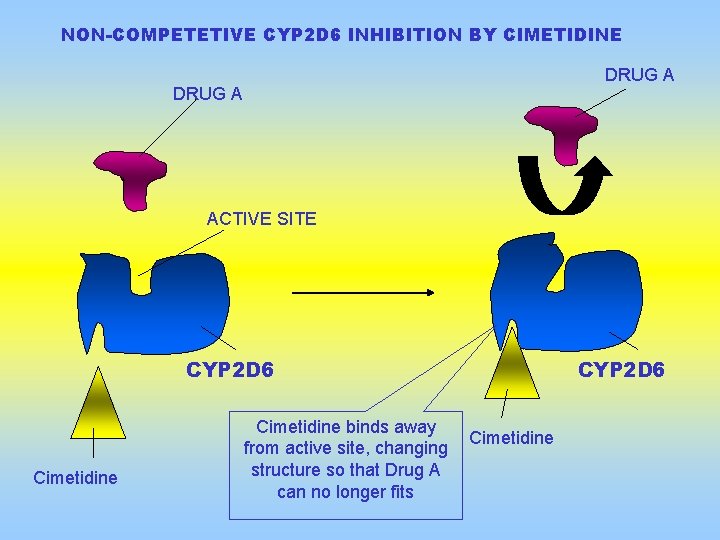
NON-COMPETETIVE CYP 2 D 6 INHIBITION BY CIMETIDINE DRUG A ACTIVE SITE CYP 2 D 6 Cimetidine binds away from active site, changing structure so that Drug A can no longer fits CYP 2 D 6 Cimetidine

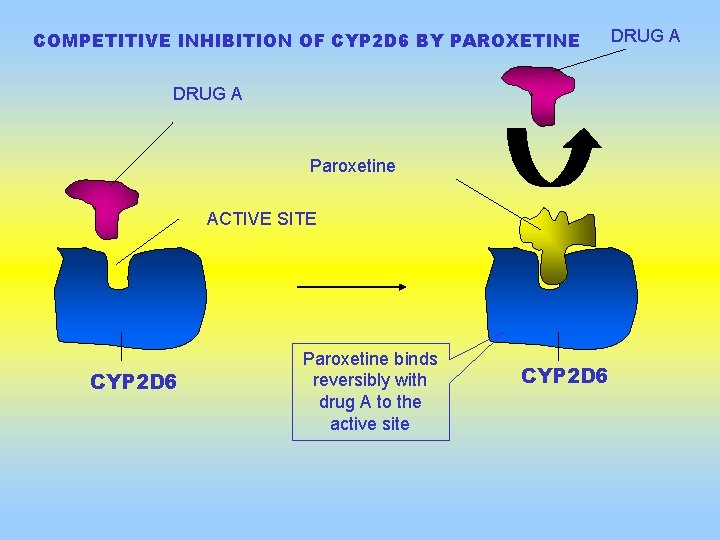
COMPETITIVE INHIBITION OF CYP 2 D 6 BY PAROXETINE DRUG A Paroxetine ACTIVE SITE CYP 2 D 6 Paroxetine binds reversibly with drug A to the active site CYP 2 D 6 DRUG A

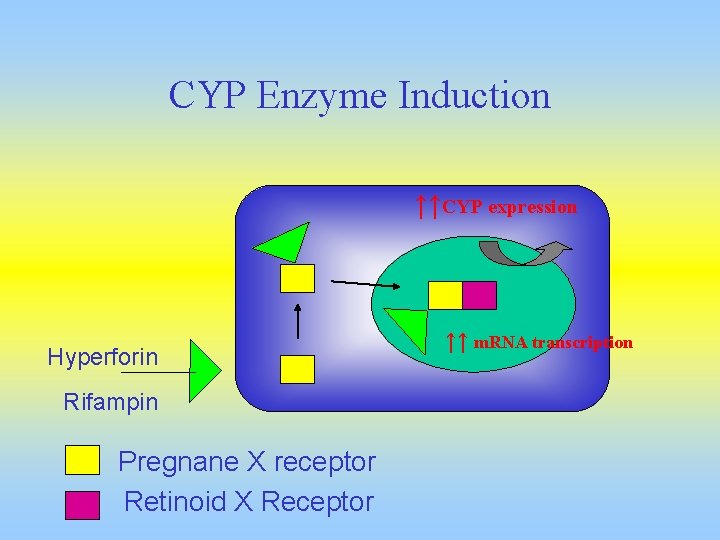
CYP Enzyme Induction ↑↑CYP expression Hyperforin Rifampin Pregnane X receptor Retinoid X Receptor ↑↑ m. RNA transcription

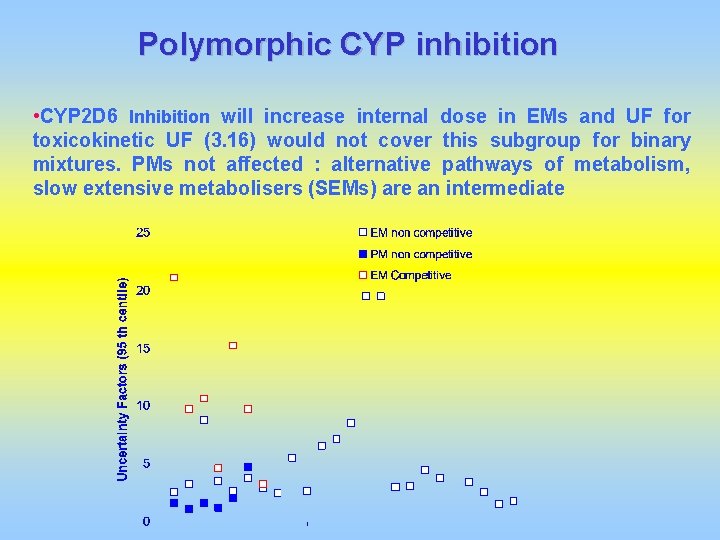
Polymorphic CYP inhibition • CYP 2 D 6 Inhibition will increase internal dose in EMs and UF for toxicokinetic UF (3. 16) would not cover this subgroup for binary mixtures. PMs not affected : alternative pathways of metabolism, slow extensive metabolisers (SEMs) are an intermediate

INHIBITION/ INDUCTION • Inhibition/induction of polymorphic CYP increase/decrease exposure to therapeutic drugs in EMs (and PMs for induction). Current UF for human variability in toxicokinetics (3. 16) would not cater for these interactions • Results variable ; detailed analysis to classify interaction according to constant of inhibition (Ki) • In vivo database on therapeutic doses much higher than pesticide levels but only in vivo data quantifying human variability in toxicokinetic interactions.

RELEVANCE TO HUMAN RISK ASSESSMENT • Current levels of exposure of organophosphates (< 10 u. M) : shown to inhibit imipramine metabolism in human recombinant enzymes and liver microsomes (Di Consiglio et al. , 2005). • Many pesticides known to either inhibit or induce cytochrome P-450 isoforms in animals and man • More work to characterise their potential in vivo effects at the current level of exposure using recombinant technology and toxicokinetic assays (Hodgson and Rose, 2005).

CONCLUSIONS Human data are essential To replace default uncertainty factors with chemical-specific data To identify high risk subgroups regarding susceptibility to chemical toxicity Most susceptible subgroups Poor metabolisers (Healthy adults), neonates, children for polymorphic enzymes but very little data Most suceptible subgroups (mixtures) Extensive metabolisers for polymorphic enzymes with inhibitors if metabolite toxic Need for well characterised metabolism before compound on the market Use of in vitro techniques Many pesticides metabolised via polymorphic CYPs

CONCLUSIONS II Advanced statistical techniques Uncertainty analysis, Probabilistic and Bayesian approaches Analysis of toxicodynamics (mechanisms of toxicity) Very little data, use of pharmacodynamic data In vitro, in silico data and OMICS Regulatory bodies, Risk managers ? Integrate data (including susceptible subgroups…) in the risk assessment process Industry Integrate relevant data (compound specific metabolism PK, PD, TK, TD…) and relevant modelling techniques for risk assessment of compounds before market

Many thanks to Professor Emeritus Andrew Renwick OBE and -The Department of Health (UK), -Health and Safety Executive (UK), -Food Standard Agency (UK), -European Commission within NO MIRACLE for funding this work
