Human Research Protection Program 101 March 19 20







































- Slides: 42

Human Research Protection Program 101 March 19 -20 Cincinnati, OH

Structure of Oversight of Human Subjects Research Presented by: Marisue Cody, Ph. D, RN

Objectives • • • Describe the oversight structure of human subjects research in the VA Describe the responsibilities of the institution Describe the responsibilities of the investigator

HRPPP (Human Research Participant Protection Programs) • Institute of Medicine report 2001 § Preserving the Public Trust • Broader system with multiple functional elements • Advocated accreditation of the HRPPP

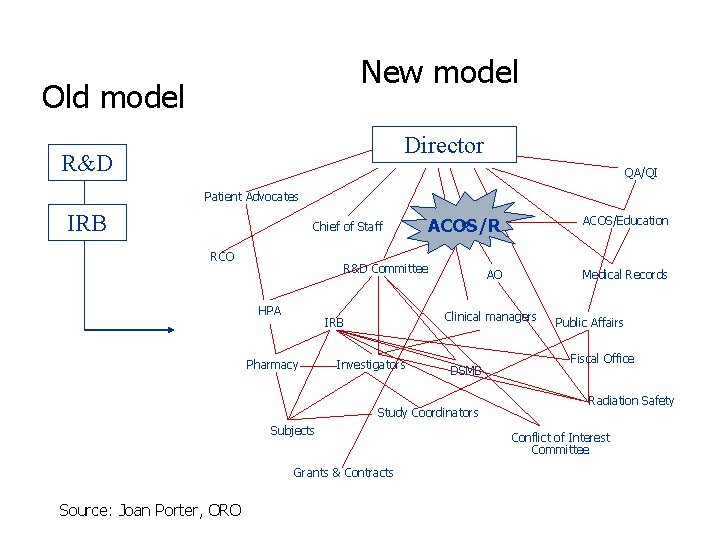
New model Old model Director R&D QA/QI Patient Advocates IRB Chief of Staff RCO R&D Committee HPA AO Investigators DSMB Study Coordinators Subjects Grants & Contracts Source: Joan Porter, ORO Medical Records Clinical managers IRB Pharmacy ACOS/Education ACOS/R Public Affairs Fiscal Office Radiation Safety Conflict of Interest Committee

Authority & Responsibilities • • United States Code (U. S. C. ) Code of Federal Regulations (CFR) Directives Handbooks Manuals Memorandums Professional standards

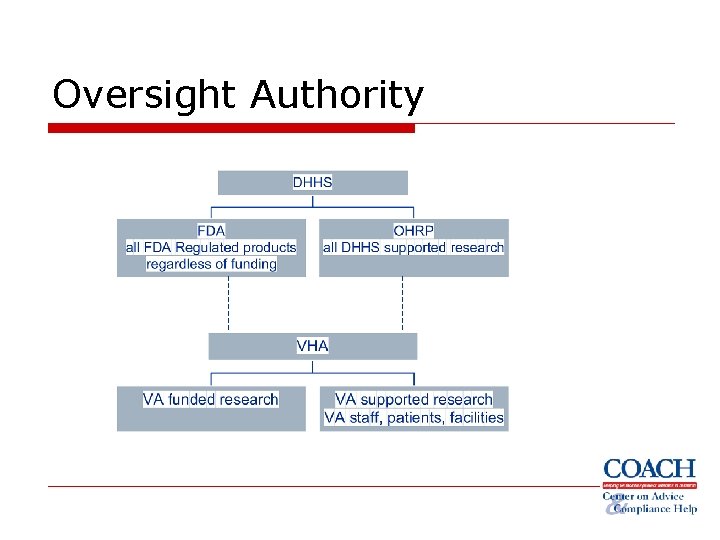
Oversight Authority

• • • All research covered by the Common Rule FWA option (all research that is not exempt) Who? § § Institution IRB

• • FDA regulated drugs, biologics and devices Who? § § Investigators IRBs

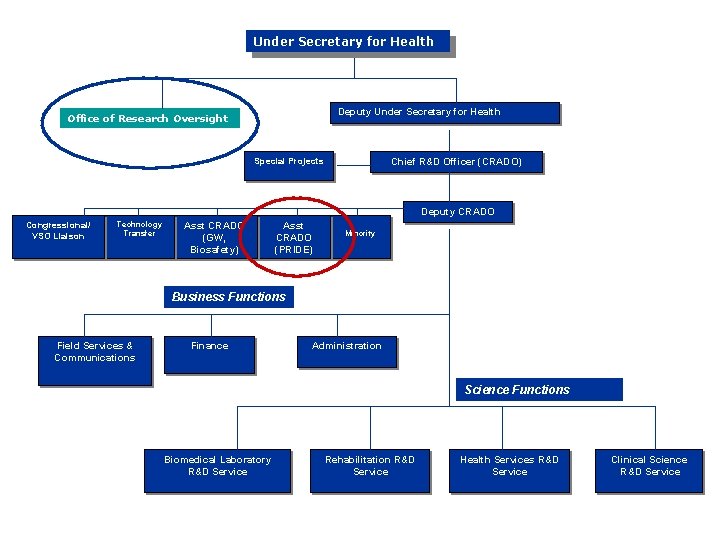
Under Secretary for Health Deputy Under Secretary for Health Office of Research Oversight Special Projects Chief R&D Officer (CRADO) Deputy CRADO Congressional/ VSO Liaison Technology Transfer Asst CRADO (GW, Biosafety) Asst CRADO (PRIDE) Minority Business Functions Field Services & Communications Finance Administration Science Functions Biomedical Laboratory R&D Service Rehabilitation R&D Service Health Services R&D Service Clinical Science R&D Service

Regulations vs. guidance • When does “guidance” not have to be followed? § § • Suggested practice? Must practice? Must vs. should?

Institutional Responsibilities

Regulatory Guidelines for the Institution • • • DHHS – FWA FDA – none VA § § VHA Directive 2003 -031 (funding of the facility HRP) VHA Directive 1200 (Facility R&D program) M-3 Part I, Chapter 2 (Organizational Structure of R&D Committee) M-3 Part I, Chapter 3 (Functions)

Assurance of Compliance (. 103) • • • Statement of principles governing the institution Designation of one or more IRBs List of IRB members Written procedures the IRB will follow Written procedures for reporting unanticipated problems involving risk, and any suspension or termination of IRB approval Executed by individual authorized to act for the institution (in the VA this is the Medical Center Director)

Federalwide Assurance (FWA) • This documents your institutional commitment to comply with the Common Rule. • It is required from each institution “engaged” in covered research: http: //www. hhs. gov/ohrp/policy/index. html#engagement • Turn to Tab 8 in your notebook.

Review by institution (. 112) • Research approved by an IRB may be subject to further review by official of the institution • Those officials MAY NOT approve the research if it is not approved by the IRB

Medical Center Director Responsibilities (VHA Directive 1200) • • Responsible for R&D program, advised and assisted by R&D Committee R&D funds used for research Research reimburses medical care appropriation for research participation Ensure ethical conduct of research

R&D Responsibilities

Scope of R&D Committee • • Responsible through the COS to the Medical Center Director, for oversight of the research program Responsible for maintaining high standards throughout the R&D program Assuring scientific & ethical quality of research ACOS/R&D & AO/R&D assist the Committee with its duties

Scope (Cont. ) • • • R&D Committee approval must be obtained prior to initiating any research Applicable “subcommittee” approvals must be obtained prior to final R&D Committee approval A R&D Committee may serve as the R&D Committee of record for another VA only § § MOU required Local accountability is a key point

Specific Areas • • • Plan & develop broad objectives Determine extent to which R&D committee has met its objectives Review budgetary & other resource needs Oversee all R&D activities Review certain written agreements Review & evaluate all “subcommittees”

“Subcommittees” • Required “subcommittees”: § § § • IRB IACUC Biosafety Option for other “subcommittees” § § § Scientific review Continuing review Others as needed

Responsibilities Related to the Facility’s Research Program • • • Have oversight responsibilities, not responsibilities for day to day management Need to receive sufficient information (annual, quarterly reports or other information) to fulfill areas of responsibility Information on compliance, QA, requests for WOC appointments, & special programs such as Biosafety, Animal Welfare, & HRPP

Review of Research • Initial review of research: § • • • Final approval only after receive approval from applicable “subcommittees” Continuing review to occur each year Review during a convened meeting Quorum required to approve research: § Majority of voting members present for discussion & vote

Review of Research (Cont. ) • Review includes: § § § § Budget Personnel Equipment needs Supply needs Relevance to VA PI’s qualifications Scientific merit Conflicts of Interest

“Just-In-Time” • Concurrence from R&D Committee to submit after a preliminary review § § • Appropriateness of scientific methodology Relevance of the research to VA’s mission Investigator’s qualification Adequacy of resources Review by full board or expedited procedure

“Just-In-Time” Continued • • Develop SOPs on how to conduct preliminary review Concurrence ≠ approval to initiate research § Prior to initiating research PI must: ü Submit to required subcommittees (IRB, IACUC etc, ) ü Submit to R&D Committee ü Obtain approvals from all

Institutional Review Board

Institutional Review Board (IRB) • Fulfill regulatory requirements set forth in Common Rule (38 CFR 16) • Authority and responsibilities detailed in VHA Handbook 1200. 5 • FDA (21 CFR 56)

Common Rule and VHA 1200. 5 Common Rule § § Establish IRBs Ensure Informed Consent of Subjects VHA Handbook 1200. 5 § § Medical Center Responsibilities IRB Composition & Responsibilities Investigator Responsibilities Investigational Drugs & Devices in VA

What is covered? (. 101) • All research involving human subjects conducted, supported or otherwise subject to regulation by any federal department or agency which takes action to make this policy application. § In the VA, that is all research involving human subjects conducted completely or partially in VA facilities, approved off-site locations, facilities, and/or by VA researchers while on official VA duty time (VHA Handbook 1200. 5, p. 6).

Research … a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to “generalizable” knowledge. (45 CFR 46. 102 d) ion… t a rm Wid es info d a pre

Human subject …means a living individual about whom an investigator (whether professional or student) conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information

Investigator Responsibilities

Investigator’s Responsibilities Common Rule • § Obtain legally effective informed consent VHA 1200. 5 Paragraph 10 • § § § § Training and credentialing Research plan Consent process Reporting of SAEs and/or AEs Amendments IRB Review Record retention HIPAA

Study Staff • Delegated authority • Training and credentialing

FDA and GCP (ICH E 6) • Investigator qualifications and agreements § § • • • Permit auditing and inspection Maintain list of appropriately qualified personnel to whom delegated significant trial-related duties Adequate resources (patients, time, staff) Medical care of trial subjects Communicate with the IRB Compliance with the protocol Investigational product accountability Informed consent of trial subjects

FDA and GCP (ICH E 6) • • • Accurate, complete, legible & timely reports Written progress reports Safety reporting Appropriate follow-up for trial subjects Submit final report to institution

Responsible Conduct of Research • Nine core components 1. 2. 3. 4. 5. 6. 7. 8. 9. data acquisition, management, sharing, and ownership mentor/trainee responsibilities publication practices and responsible authorship peer review collaborative science human subjects research involving animals research misconduct conflicts of interest and commitment

IOM Recommendations for PI Responsibilities • • Appropriate training and credentials Training in ethics and regulatory requirements Scientifically and ethically sound protocol Submit for scientific and ethical review Disclosure of potential conflicts of interest Voluntary and effective informed consent Conduct study according to approved protocol Submit all amendments Institute of Medicine. 2003. Responsible Research: A Systems Approach to Protecting Research Participants. Washington DC: The National Academies Press.

IOM Recommendations (continued) • • Ensure appropriate safety monitoring and continuing review activities Acknowledge and report violations, errors, problems Report results in a responsible manner When appropriate, communicate results to participants or participant communities Institute of Medicine. 2003. Responsible Research: A Systems Approach to Protecting Research Participants. Washington DC: The National Academies Press.
