HUMAN RENAL SYSTEM PHYSIOLOGY Lecture 9 10 BY
HUMAN RENAL SYSTEM PHYSIOLOGY Lecture 9, 10 BY: LECT. DR. ZAINAB AL-AMILY

objectives • Describe Acid Base balance

Acid-Base Balance • Metabolic activities of the body require precise regulation of acid-base balance, which is reflected by the p. H of ECF. • p. H being regulated within a narrow physiologic range affect: – membrane excitability – enzyme systems – chemical reactions. • Many conditions, pathologic or otherwise, can alter body p. H. • Normally, the concentration of body acids and bases is regulated so that the p. H of ECF is maintained within a very narrow range of 7. 35 to 7. 45.
• This balance is maintained through mechanisms that: generate Buffer acids and bases eliminate. • An acid is a molecule that can release a hydrogen ion (H+) • A base is a molecule that can accept or combine with an H+ ion. • Most of the body’s acids and bases are weak acids and bases. • Most important are carbonic acid (H 2 CO 3), which is a weak acid derived from carbon dioxide (CO 2), and bicarbonate (HCO 3 -), which is a weak base.

• The concentration of H+ ions in body fluids is low compared with other ions. i. e. , the Na+ ion present at a concentration approximately 1 million times that of the H+ ion. • Because of its low concentration in body fluids, the H+ ion concentration is commonly expressed in terms of p. H. • Specifically, p. H represents the negative logarithm (p) of the H+ ion concentration in m. Eq/L. • A p. H value of 7. 0 implies a H+ ion concentration of 10− 7 (0. 0000001 m. Eq/L). • p. H is inversely related to the H+ ion concentration • Low p. H indicates a high concentration of H+ ions and a high p. H, a low concentration.

• Metabolic Acid and HCO 3 - Production • Acids are continuously generated as by-products of metabolic processes. • acids fall into two groups: a. volatile acid (H 2 CO 3) • H 2 CO 3 is in equilibrium with the volatile CO 2, which leaves the body by way of the lungs. • H 2 CO 3 concentration is determined by the lungs and their respiratory capacity. b. nonvolatile or fixed acids. • e. g. , sulfuric, hydrochloric, phosphoric are not eliminated by the lungs. • Instead, they are buffered by body proteins or extracellular buffers, such as HCO 3 - , and then excreted by the kidney.
• CO 2 and HCO 3 - Production • Body metabolism results in the production of approximately 15, 000 mmol of CO 2 each day. • CO 2 is transported in the circulation in three forms: (1) attached to hemoglobin (2) as dissolved CO 2 (i. e. , PCO 2) (3) HCO 3 -
• Dissolved CO 2 and HCO 3 - constitute: – 77% of the CO 2 that is transported in ECF – remaining CO 2 attached to Hb. • Small % of CO 2 combines with water in bloodstream H 2 CO 3 catalyzed by carbonic anhydrase (present in large quantities in RBCs, renal tubular cells, and other tissues in the body). • It is impossible to measure H 2 CO 3 dissolved CO 2 measurements are commonly substituted when calculating p. H.
• Calculation of PH • p. H is calculated with the Henderson-Hasselbalch equation using the dissociation constant for the bicarbonate buffer system (6. 1) and the HCO 3 - to H 2 CO 3 ratio. • p. H = 6. 1 + log HCO 3 -/H 2 CO 3 (CO 2) • The log of 20 is 1. 3. Thus, when the ratio is 20 to 1, the p. H is within the normal range at 7. 4. • The generation of metabolic acids and the availability of bicarbonate to buffer these acids control the HCO 3 - part of the equation.
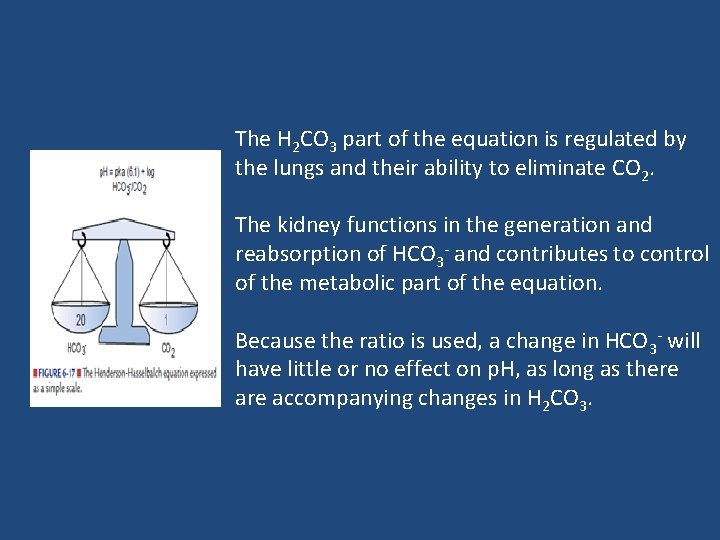
The H 2 CO 3 part of the equation is regulated by the lungs and their ability to eliminate CO 2. The kidney functions in the generation and reabsorption of HCO 3 - and contributes to control of the metabolic part of the equation. Because the ratio is used, a change in HCO 3 - will have little or no effect on p. H, as long as there accompanying changes in H 2 CO 3.

• Acidification of Urine and Bicarbonate Excretion: • Cells of the proximal and distal tubules as well as those of the collecting ducts, like cells of the gastric glands, secrete H+ ions: • The reaction in the proximal tubule is an example of a secondary active transport by the Na+ - H+ antiport (exchanger).
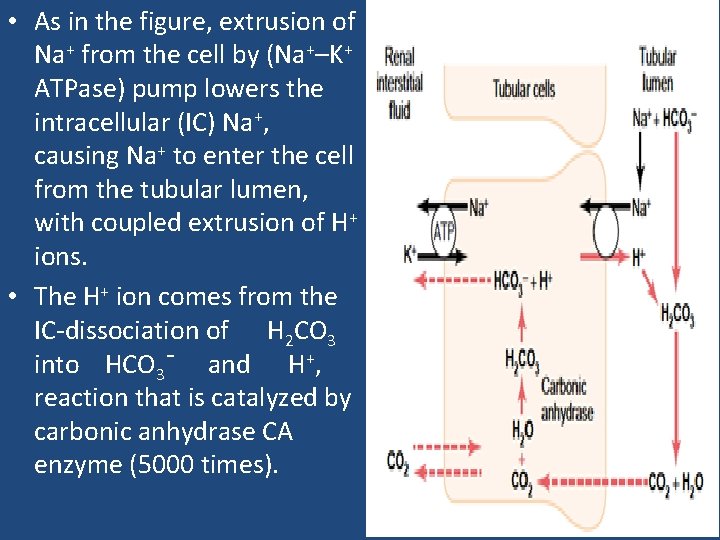
• As in the figure, extrusion of Na+ from the cell by (Na+–K+ ATPase) pump lowers the intracellular (IC) Na+, causing Na+ to enter the cell from the tubular lumen, with coupled extrusion of H+ ions. • The H+ ion comes from the IC-dissociation of H 2 CO 3 into HCO 3¯ and H+, reaction that is catalyzed by carbonic anhydrase CA enzyme (5000 times).

• The bicarbonate ion that is formed diffuses into ISF, along with Na+ ions (through Na+–HCO 3¯ cotransporter) for each H+ ion that is secreted. • In the late distal tubule and the collecting ducts, H+ ion secretion is relatively independent of Na+ ion in the tubular lumen. • aldosterone hormone act on the I- cells that are responsible for secreting acid in this part of the tubule, and will activate an ATP-driven proton pump to increase the distal H+ ion secretion.
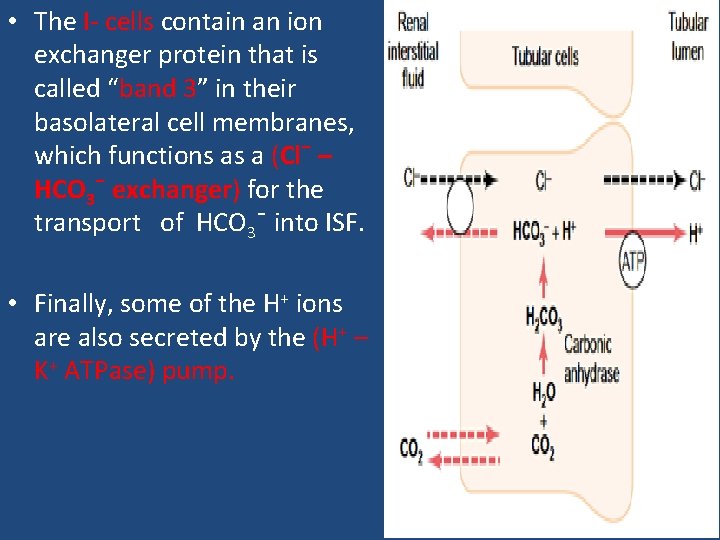
• The I- cells contain an ion exchanger protein that is called “band 3” in their basolateral cell membranes, which functions as a (Cl¯ – HCO 3¯ exchanger) for the transport of HCO 3¯ into ISF. • Finally, some of the H+ ions are also secreted by the (H+ – K+ ATPase) pump.


• When the number of free H+ ions secreted into the tubular fluid threatens to cause the urine to become too acidic, the H+ ions must be carried in some other form. • This is accomplished by combining the H+ ions with intratubular buffers before being excreted in the urine. • There are two important intratubular buffer systems: 1. The phosphate buffer system 2. The ammonia buffer system.
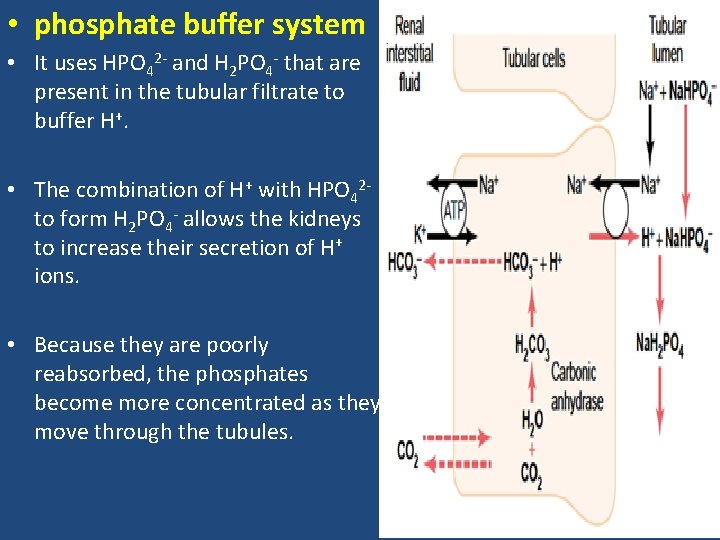
• phosphate buffer system • It uses HPO 42 - and H 2 PO 4 - that are present in the tubular filtrate to buffer H+. • The combination of H+ with HPO 42 - to form H 2 PO 4 - allows the kidneys to increase their secretion of H+ ions. • Because they are poorly reabsorbed, the phosphates become more concentrated as they move through the tubules.
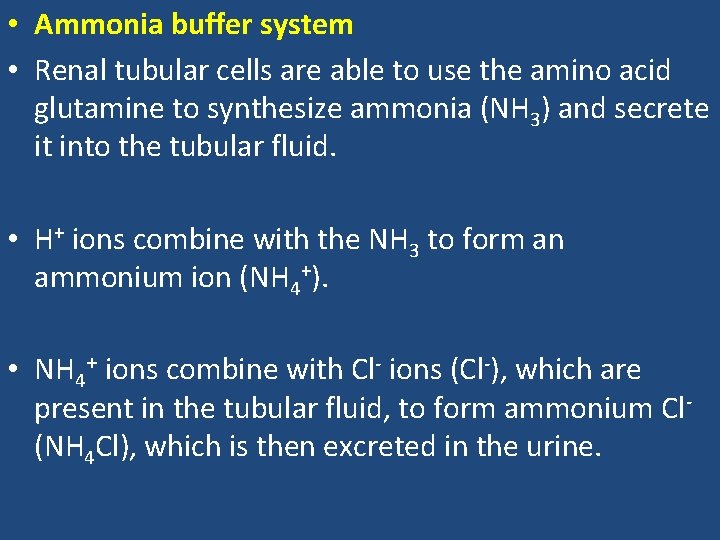
• Ammonia buffer system • Renal tubular cells are able to use the amino acid glutamine to synthesize ammonia (NH 3) and secrete it into the tubular fluid. • H+ ions combine with the NH 3 to form an ammonium ion (NH 4+). • NH 4+ ions combine with Cl- ions (Cl-), which are present in the tubular fluid, to form ammonium Cl- (NH 4 Cl), which is then excreted in the urine.

• Regulation of p. H • The p. H of body fluids is regulated by three major mechanisms: 1. ICF and ECF buffering systems 2. the lungs, which control the elimination of CO 2 3. the kidneys, which eliminate H+ and regulate the elimination of HCO 3 -
• IC and EC Buffer Systems • The moment by-moment regulation of p. H depends on the ICF and ECF buffer systems. • A buffer system consists of a weak acid and the base salt of that acid or of a weak base and its acid salt. • In the process of preventing large changes in p. H, the system swap a strong acid for a weak acid or a strong base for a weak base.
• Major buffer systems protect the p. H of body fluids are: 1. Proteins 2. HCO 3 - buffer system 3. Transcellular H+/K+ exchange system. • These buffer systems are immediately available 4. Bone also represents an important site for the buffering of acids and bases (for long term ).
1. Proteins • Are the largest buffer system in the body. • Albumin and plasma globulins are the major protein buffers in the vascular compartment. • are amphoteric, meaning that they can function as either acids or bases. • They contain many ionizable groups that can release or bind H+. • are largely located within cells, and H+ ions and CO 2 diffuse across cell membranes for buffering by intracellular proteins

2. bicarbonate buffer system • uses H 2 CO 3 as its weak acid and HCO 3 - as its weak base. • It substitutes the weak H 2 CO 3 for a strong acid such as HCl or the weak HCO 3 - base for a strong base such as sodium hydroxide. • HCO 3 -/H 2 CO 3 buffer system is an efficient system because the buffer components can be readily added or removed from the body. • Metabolism provides an sufficient supply of CO 2 as body requirement • The kidney can form new HCO 3 - when excess acid is added, and it can excrete HCO 3 - when excess base is added.
3. Transcellular H+/K+ exchange system • Both ions are positively charged, and both ions move freely between the ICF and ECF compartments. • When excess H+ is present in the ECF, it moves into the body cells in exchange for K+. • When excess K+ is present in the ECF, it moves into the cell in exchange for H+. • On the average, serum K+ rises by approximately 0. 6 m. Eq/L for every 0. 1 unit fall in p. H. • Thus, alterations in K+ levels affect acid-base balance, and changes in acid-base balance influence K+ levels.

• In acidosis, increased levels of serum K+ cause the resting membrane potential to become less negative and • in alkalosis, decreased levels cause the resting membrane potential to become more negative. • Changes in neural excitability are further influenced by alterations in ionized Ca 2+. • In acidosis: – ionized portion of the extracellular Ca 2+ is increased making neurons less excitable • In alkalosis: – amount of ionized Ca 2+ is reduced making neurons more excitable.


1. Secretion of K+ by the distal tubule will be decreased by (A) metabolic acidosis (B) a high-K+ diet (C) hyperaldosteronism (D) spironolactone administration (E) thiazide diuretic administration
• Respiratory Control Mechanisms • provides for the elimination of CO 2 into the air and plays a major role in acid-base regulation. • The respiratory control of p. H is rapid, occurring within minutes, and is maximal within 12 to 24 hours. • it does not return the p. H to normal.

• Renal Control Mechanisms • The kidneys regulate acid-base balance by excreting acidic or alkaline urine. • The renal mechanisms CANNOT adjust the p. H within minutes, as respiratory mechanisms can, but they continue to function for days until the p. H has returned to normal or near-normal range. • The kidneys filter HCO 3 - in the glomerulus and then reabsorb it in the tubules as a means of maintaining ECF levels.

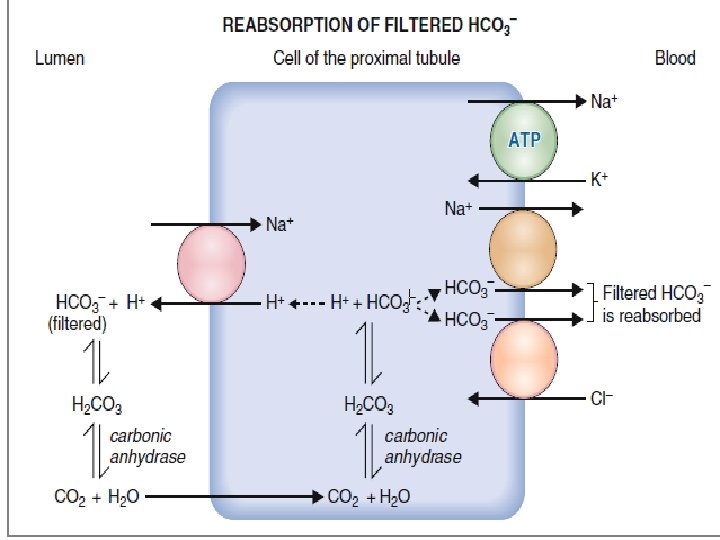
• Almost 99. 9% of the filtered HCO 3 is reabsorbed • The reabsorption rate can be calculated by comparing the filtered load of HCO 3 with the excretion rate of HCO 3 • If the glomerular filtration rate (GFR) is 180 L/day and the plasma HCO 3 concentration is 24 m. Eq/L, then the filtered load is 4320 m. Eq/day (180 L/day × 24 m. Eq/L). • The measured excretion rate of HCO 3 is merely 2 m. Eq/day. Therefore, the reabsorption rate of HCO 3 is 4318 m. Eq/day, which is 99. 9% of the filtered load. • Most filtered HCO 3 reabsorption occurs in the proximal tubule and only small quantities are reabsorbed in the loop of Henle, distal tubule, and collecting duct.

• HCO 3 - do not readily cross the membranes of renal tubular cells; HCO 3 - that has been filtered in the glomerulus cannot be directly reabsorbed. • Instead, HCO 3 - ions first combine with H+ ions that have been secreted into the tubular fluid to form H 2 CO 3, which is converted to CO 2 and water. • The resulting CO 2 can readily cross the tubular membrane and enter the tubular cell, where it combines with water, under the influence of carbonic anhydrase, to generate a new H 2 CO 3 molecule. • The newly formed H 2 CO 3, in turn, dissociates to form a HCO 3 - and a H+ ion.

• The HCO 3 - that is formed moves out of the tubular cell into the bloodstream and the H+ is secreted into the tubular fluid.


• Aldosterone also influences H+ ion elimination by the kidney. • It acts in the collecting duct to indirectly stimulate H+ ion secretion, while increasing Na+ ion reabsorption and K+ secretion. • Hyperaldosteronism tends to lead to a decrease in serum K+ levels and increased p. H and alkalosis caused by increased H+ ion secretion. • Hypoaldosteronism leads to increased K+ levels, decreased H+ ion secretion, and acidosis.
• One of the mechanisms that the kidneys use in regulating the p. H of the ECF is the conservation or elimination of HCO 3 -ions. • Cl- is the most abundant anion in the ECF and can substitute for HCO 3 - when an anion shift is needed. • i. e. , serum HCO 3 - levels normally increase as (HCl is secreted into the stomach after a heavy meal, causing what is called the postprandial alkaline tide. • Later, as the Cl- ion is reabsorbed in the small intestine, the p. H returns to normal.

- Slides: 39