Human Parvoviruses Acknowledgment Addis Ababa University Jimma University

Human Parvoviruses

Acknowledgment • • Addis Ababa University Jimma University Hawassa University Haramaya University of Gonder American Society of Clinical Pathology(ASCP) Center for Disease Control and Prevention(CDC)

Objectives • Explain the properties of Parvovirus • Describe the pathogenesis and clinical features Parvovirus infection • Illustrate epidemiology parvovirus infection • Describe the diagnosis parvovirus infection

Outline • • • Classification and structure Properties Pathogenesis and clinical features Diagnosis summary

Human Parvoviruses • Parvoviruses are the smallest viruses ►In Latin, parvum meaning small • Posses ss. DNA genome • One known human pathogen (parvovirus B 19) • The family Parvoviridae consists of two subfamilies: § Densovirinae ……. are all viruses of insects § Parvovirinae……. . . contains viruses of vertebrates
Human parvovirus B 19 (B 19 V) Structure • In electron micrographs of negatively stained preparations B 19 V appears as : § Non-enveloped § Icoashedral with a diameter varying from 18 to 25 nm • The virus do not contain lipids or carbohydrates • As with all parvovirus particles, B 19 V:

§ Stable over a wide range of p. H § Resistant to lipid solvents § Not quite resistant to heat as other parvoviruses § Inactivated by formalin, β-propiolactone, oxidizing agents & γ-irradiation (Blumel et al. , 2002)

Parvovirus structure Fig. From Medical Microbiology, 4 th ed. , Murray, Rosenthal, Kobayashi & Pfaller, Mosby Inc. , 2002.
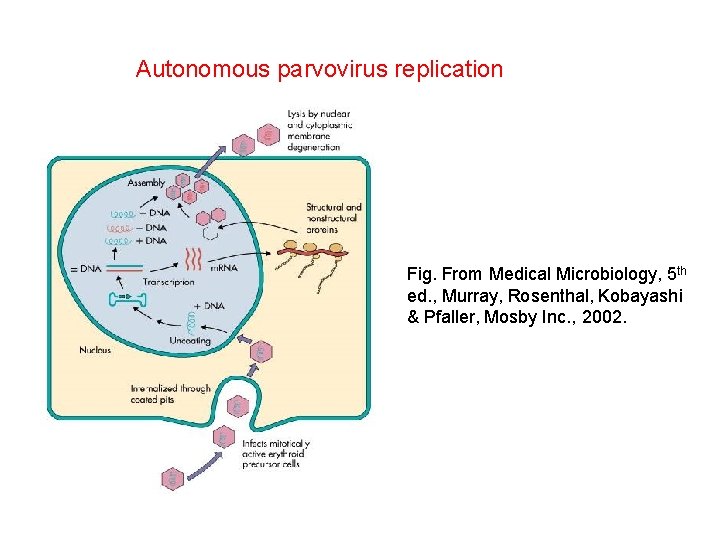
Autonomous parvovirus replication Fig. From Medical Microbiology, 5 th ed. , Murray, Rosenthal, Kobayashi & Pfaller, Mosby Inc. , 2002.
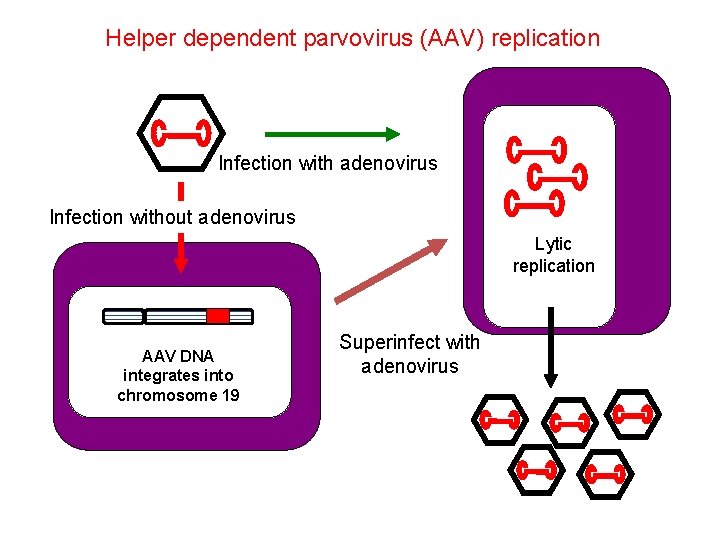
Helper dependent parvovirus (AAV) replication Infection with adenovirus Infection without adenovirus Lytic replication AAV DNA integrates into chromosome 19 Superinfect with adenovirus
Pathogenesis Two studies of adult volunteers have provided a basis for understanding the pathogenesis of B 19 infection, which has two phases: First phase of the illness Characterized by viremia that develops approximately 6 days after intranasal inoculation of B 19 into susceptible individuals who lack serum antibodies to the virus

• Viremia lasts about 1 week; its clearance is correlated with the development of Ig. M antibodies to B 19, which remain detectable for up to a few months • Ig. G antibodies develop several days later and persist indefinitely • Non-specific systemic symptoms lasting 2 or 3 days occur early during the viremic phase, include: § Headache, malaise, myalgia, fever, chills, and pruritus § Accompanied by reticulocytopenia and excretion of the virus from the respiratory tract
• Several days after the onset of symptoms: § Decline in hemoglobin conc. (maintained for 7 to 10 days) § Examination of bone marrow samples reveals a marked depletion of erythroid precursor cells § Transient mild lymphopenia, neutropenia, & throbocytopenia

A second phase • Begins around 17 or 18 days after virus inoculation & after: § Clearance of viremia § Cessation of viral shedding in throat secretions § Resolution of reticulocytopenia • This phase occurs in the presence of rising serum titers of antibody to B 19

Parvovirus pathogenesis Fig. From Medical Microbiology, 5 th ed. , Murray, Rosenthal & Pfaller, Mosby Inc. , 2005.
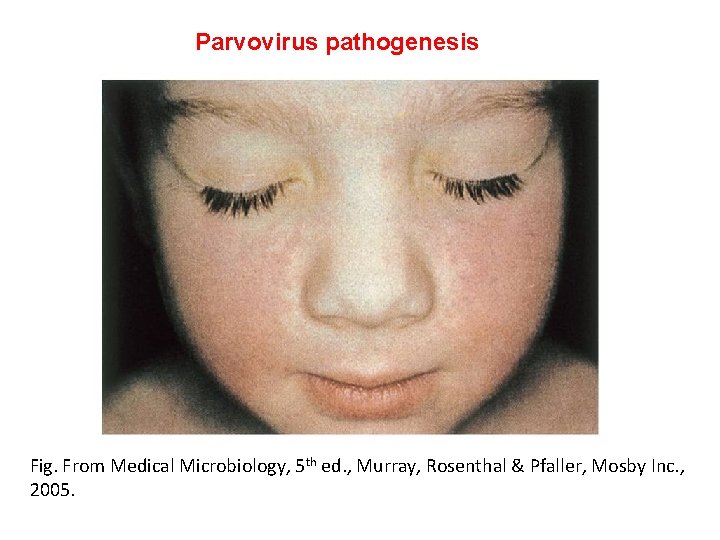
Parvovirus pathogenesis Fig. From Medical Microbiology, 5 th ed. , Murray, Rosenthal & Pfaller, Mosby Inc. , 2005.

Epidemiology of parvovirus B 19 • Serological studies indicate that infection is worldwide • Infections occurring in all populations • In temperate climates, infection occurs throughout the year • Outbreaks are more common in late winter, spring and the early summer months
Transmission • The usual route of viral transmission under natural conditions is unknown but may be respiratory or through direct contact • B 19 can be transmitted during therapy with clotting factor • concentrate & other plasma derivatives. It • Is now known that infusion of plasma pools containing high titre virus (>107 IU ml− 1) can transmit infection

B 19 V Infection in Pregnancy • Maternal B 19 infections usually do not adversely affect the fetus • More often, the fetus remains uninfected. • Couples in which the pregnant woman is infected should be counselled as to the relatively low risk of fetal infection • It is estimated that fewer than 10% of maternal B 19 infections in the first 20 weeks of pregnancy lead to fetal death
Laboratory diagnosis Specimens: § Serum (principal specimen) § Tissue biopsy A. Virus Detection Culture of B 19 in erythroid progenitor cells derived from: § Human bone marrow, umbilical cord, peripheral blood, or fetal liver sources § Failed to grow in conventional cell culture lines & animal model
B. Serologic tests • ELISA (detection of B 19 -specific Ig. M & Ig. G antibodies) • Haemagglutination-based assays C. Molecular technique • Detection of viral DNA by quantitative PCR is the mainstay of detection of B 19 V • Low levels of viral DNA (<104 IU (genome copies) ml− 1) can be detected for months, or even years after acute infection

Treatment No specific treatment for B 19 V infection • Except intravenous administration of human Ig in cases of persistent infection in immuno-compromised patient • No vaccine for B 19 is currently available Prevention and control • Isolating of susceptible individuals …. If possible • Vaccination of animals to prevent animal B 19 V
Summary • Structure Small (5 kb) linear ss. DNA genome, naked capsid • Pathogenesis § respiratory transmission § replication in nucleus, very host dependent, needs S phase cells or helper virus § viremia § antibody important in immunity

• Diagnosis serology, viral nucleic acid • Treatment/prevention No specific treatment

References • Medical Microbiology, 5 th ed. , Murray, Rosenthal & Pfaller, Mosby Inc. , 2005 • Basic Virology. Edward K. Wagner, 3 rd ed. , 2006 • Medical Microbiology, Jawtz, Melnick, & Adelberg’s, 24 th Edition, vishal • Medical Microbiology, An Introduction to Infectious Disease, Sherris, 4 th Edition, 2004 25
- Slides: 25