Human Metaphase Chromosomes Experiment Objectives Preparing staining and

Human Metaphase Chromosomes

Experiment Objectives • Preparing, staining and observing human metaphase chromosomes. Mazen Zaharna Molecular Biology 1/2009

Chromosome Morphology • Chromosomes are not visible under the light microscope in non-dividing cells (interphase cells). • As the cell begins to divide, the threads of chromatin (DNA-protein complex) in the nucleus begin to condense into multiple levels of coiled structures recognizable as chromosomes. • There are two modes of cell division: – mitosis and meiosis. Mitosis is responsible for the proliferation of body (somatic) cells, – whereas meiosis is responsible for the production of gametes. • Because mitotic cells are easy to obtain, morphological studies are generally based on mitotic Mazen Zaharna Molecular Biology 1/2009
Cell division • Cell division can be divided into: q Interphase, q Mitosis q Prophase, q Metaphase, q Anaphase, q Telophase. q Cytokinesis Mazen Zaharna Molecular Biology 1/2009

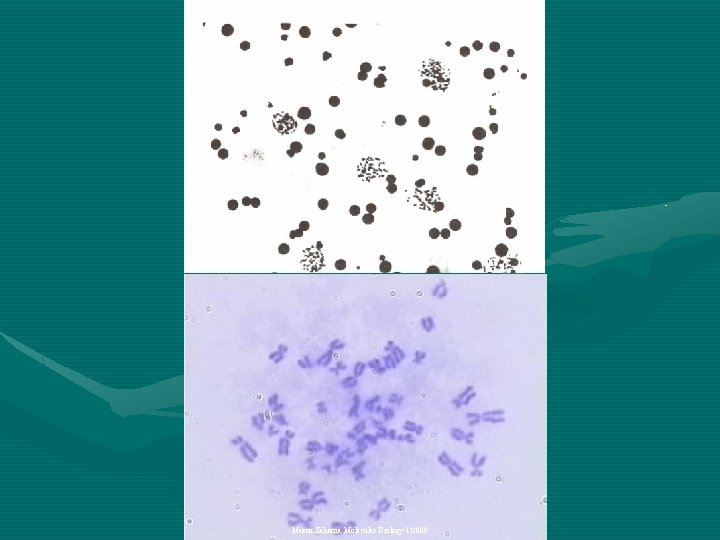
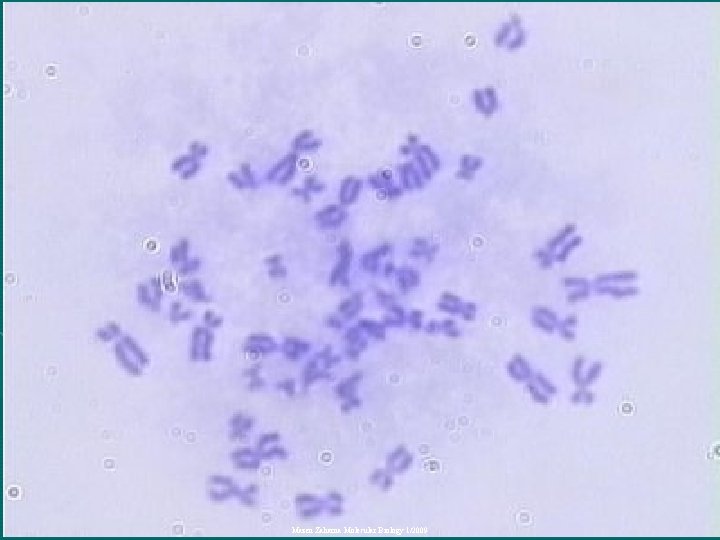
Metaphase • At metaphase the chromosomes are at their most condensed state, • Spindle fibers attaching to the area of the centromere called the kinetochore, forming pole -chromosome fibers. Mazen Zaharna Molecular Biology 1/2009
Chromosome Analysis • The best mitotic stage for chromosome analysis is prometaphase or metaphase. • A typical metaphase chromosome consists of two arms separated by a primary constriction or centromere. • Each of the two sister-chromatids contains a highly coiled double helix of DNA. Mazen Zaharna Molecular Biology 1/2009
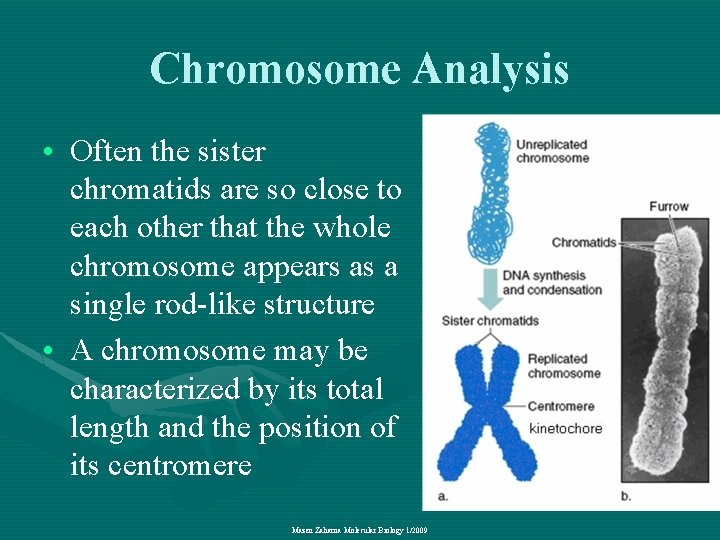
Chromosome Analysis • Often the sister chromatids are so close to each other that the whole chromosome appears as a single rod-like structure • A chromosome may be characterized by its total length and the position of its centromere Mazen Zaharna Molecular Biology 1/2009
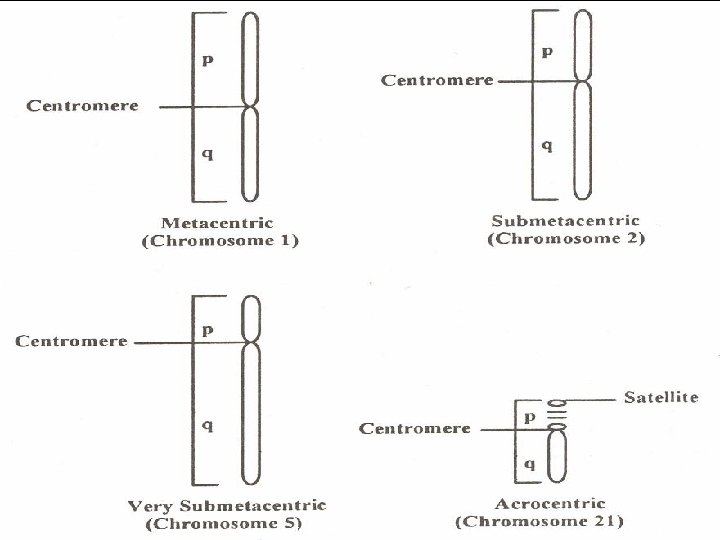
Mazen Zaharna Molecular Biology 1/2009

Types of Tissue • A variety of tissue types can be used to obtain chromosome preparations. • Some examples include peripheral blood, bone marrow, amniotic fluid and products of conception. • In the case of blood cell culture only cells that are actively dividing can be used for cytogenetic studies. • Normally only white blood cells are used for cytogenetic analysis. • Specific techniques differ according to the type of tissue used. Mazen Zaharna Molecular Biology 1/2009

Overview of Procedure 1. 2. 3. 4. 5. 6. 7. Collection of blood Cell culture Stopping the cell division at Metaphase Hypotonic treatment of red & white blood cells Fixation Slide preparation Staining Mazen Zaharna Molecular Biology 1/2009
1 - Collection of blood • Draw 5 ml of venous blood into a sterile heparinized tube containing 0. 1 ml of sodium heparin (500 units/ml). Mazen Zaharna Molecular Biology 1/2009

2 - Cell Culture • Sterile technique must be used throughout the cell culture preparation, because it is possible to cause major contamination during this procedure • 70% of the problems are due to a lack of good sterile technique • Antibiotics do not eliminate problems of gross contamination which result from poor sterile technique or antibiotic-resistant mutants • Autoclaving renders pipettes, glassware, and solutions sterile Mazen Zaharna Molecular Biology 1/2009
2 - Cell Culture Medium • Pipette 10 ml RPMI 1640 medium with L-Glutamine into a 15 ml labeled sterile culture tube • Supplement the medium with the following: Penicillin-Streptomycin Stock solution 10 µl (100000 u penicillin/ml – 100 mg/ml Streptomycin Phytohemagglutinin 0. 3 ml 20 µg/ml Fetal bovine Serum 20% 2 ml Mazen Zaharna Molecular Biology 1/2009
2 - Cell Culture Incubation • Add 1 ml of whole heparinized blood into the tube containing the supplemented medium • Mix contents of tube with gentle inversion • Incubate in 5% CO 2 incubator at 37 o. C for 72 hours Mazen Zaharna Molecular Biology 1/2009

3 - Stopping cell division at Metaphase • Pre-warm the Colchine (0. 04 mg/ml) in incubator at 37 o. C • Add 25 µl of pre-warmed Colchine to the culture o • Mix gently and incubate at 37 C for 30 -60 minutes Mazen Zaharna Molecular Biology 1/2009
4 - Hypotonic treatment of red & white blood cells • Centrifuge for 10 minutes at 2000 rpm • Discard supernatant without disturbing the cells leaving 0. 5 ml of fluid • Add 1 ml of pre-warmed hypotonic solution (0. 075 M KCl) at 37 o. C • Mix and then add 9 ml of hypotonic solution • Mix well by Pasteur pipette • Incubate at 37 o. C incubator for 17 minutes • hypotonic solution should not be in contact with cells more than 27 minutes (may cause rupture of WBCs) Mazen Zaharna Molecular Biology 1/2009

5 - Fixation • Fixative must be prepared fresh • Add 3 parts of chilled absolute methanol: 1 part glacial acetic acid Mazen Zaharna Molecular Biology 1/2009

5 - Fixation • Centrifuge for 10 minutes at 1000 – 1500 rpm • Remove supernatant leaving about 0. 5 ml of fluid on top of cells • At this time there is probably a small whitish or reddish film at the bottom of the tube • The film contain red blood cell debris and enlarged WBCs Mazen Zaharna Molecular Biology 1/2009

5 - Fixation • • • Add 5 ml of fixative to the tube Mix with a Pasteur pipette 3 -4 times Place in refrigerator for 30 minutes Centrifuge the tube for 10 minutes at 1000 -1500 rpm Remove supernatant and add another 6 ml of cold fixative, & mix well Centrifuge the tube for 10 minutes at 1000 -1500 rpm Repeat the last two steps Remove the supernatant leaving 1 ml of fluid at the bottom The remaining material will be used to make the slides Mazen Zaharna Molecular Biology 1/2009

6 - Slides Preparation • The slide must be exceptionally clean • Lay slides on a paper towel • Withdraw a few drops of cell suspension into a pipette • From a height of 20 cm, drop 2 or 3 drops of fluid on each slide • Allow the slides to dry Mazen Zaharna Molecular Biology 1/2009

7 - Staining • Stain the slides by immersion in fresh Giemsa stain for 7 -10 minutes • Remove slides from stain & rinse in distilled water • Observe under microscope X 40 then under oil immersion Mazen Zaharna Molecular Biology 1/2009
Mazen Zaharna Molecular Biology 1/2009
Mazen Zaharna Molecular Biology 1/2009

• http: //www. biology. arizona. edu/human_bio/ac tivities/karyotyping/patient_a. html • http: //www. youtube. com/watch? v=E 0 Wk. Zr 81 9 UU Mazen Zaharna Molecular Biology 1/2009
- Slides: 24