Human Anatomy and Physiology Respiration Gas exchange Gas




- Slides: 23

Human Anatomy and Physiology Respiration: Gas exchange

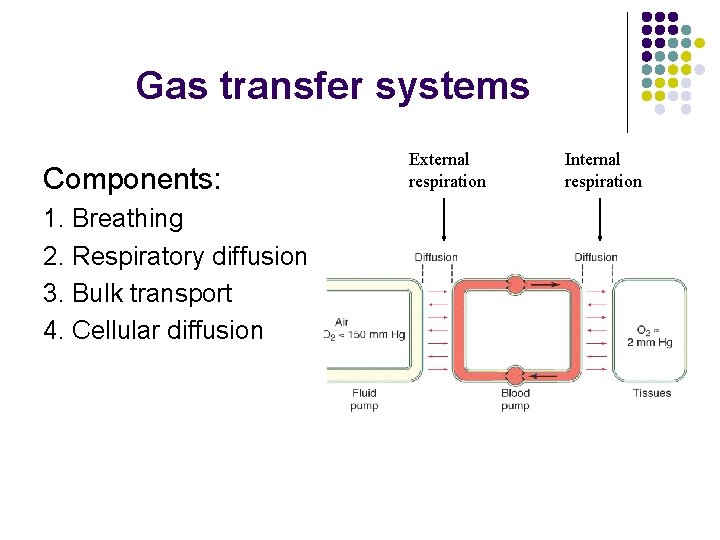
Gas transfer systems Components: 1. Breathing 2. Respiratory diffusion 3. Bulk transport 4. Cellular diffusion External respiration Internal respiration

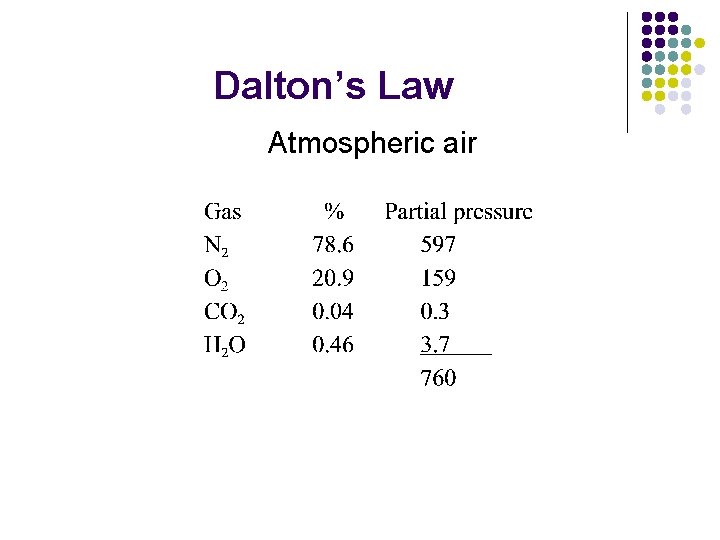
Dalton’s Law l l l PT = P 1 + P 2 + P 3 etc. Therefore each gas has a partial pressure (pgas) Pgas = % of total mixture

Dalton’s Law Atmospheric air

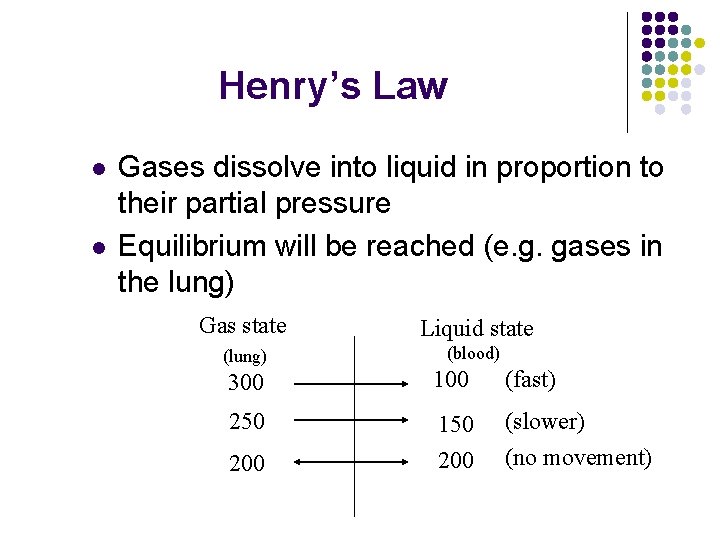
Henry’s Law l l Gases dissolve into liquid in proportion to their partial pressure Equilibrium will be reached (e. g. gases in the lung) Gas state Liquid state (lung) (blood) 300 100 (fast) 250 150 200 (slower) (no movement) 200

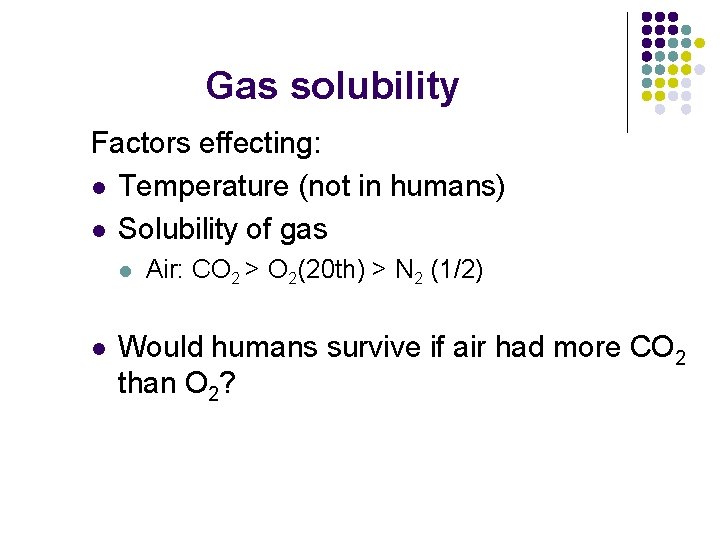
Gas solubility Factors effecting: l Temperature (not in humans) l Solubility of gas l l Air: CO 2 > O 2(20 th) > N 2 (1/2) Would humans survive if air had more CO 2 than O 2?

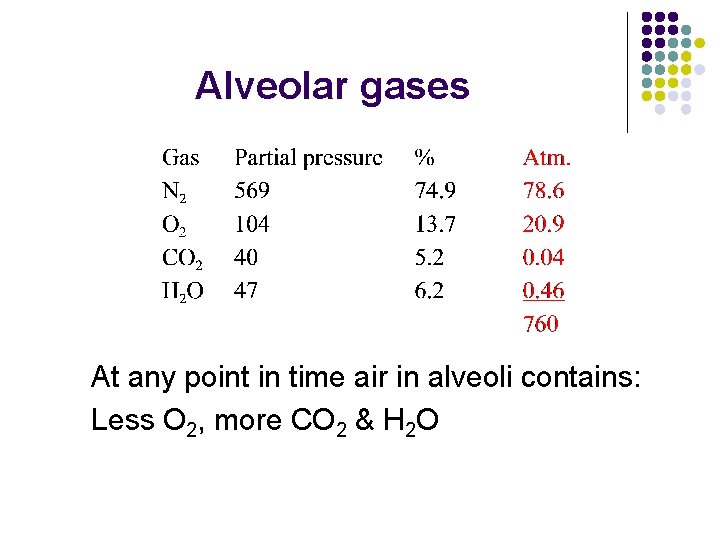
Alveolar gases At any point in time air in alveoli contains: Less O 2, more CO 2 & H 2 O

Why is gas composition different? l l O 2 diffuses into blood, CO 2 in opposite direction Humid air in conductive pathway Air in alveoli a mixture of air from more than one breath How can humans alter gas composition? l Increase rate and depth of breathing

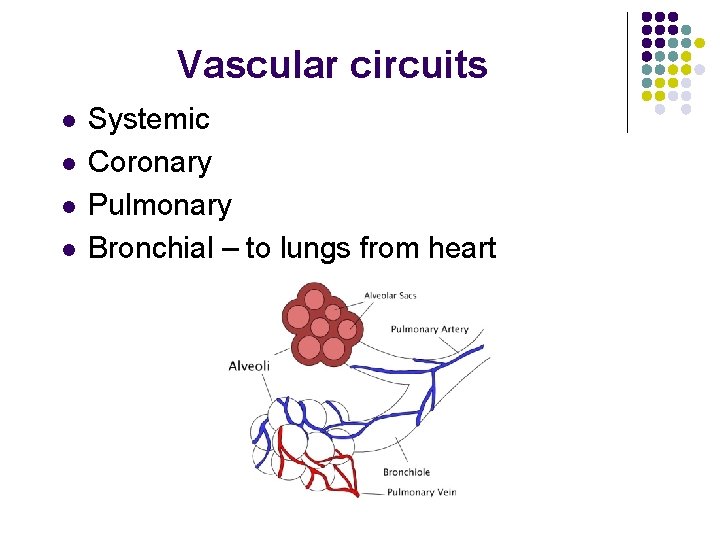
Vascular circuits l l Systemic Coronary Pulmonary Bronchial – to lungs from heart

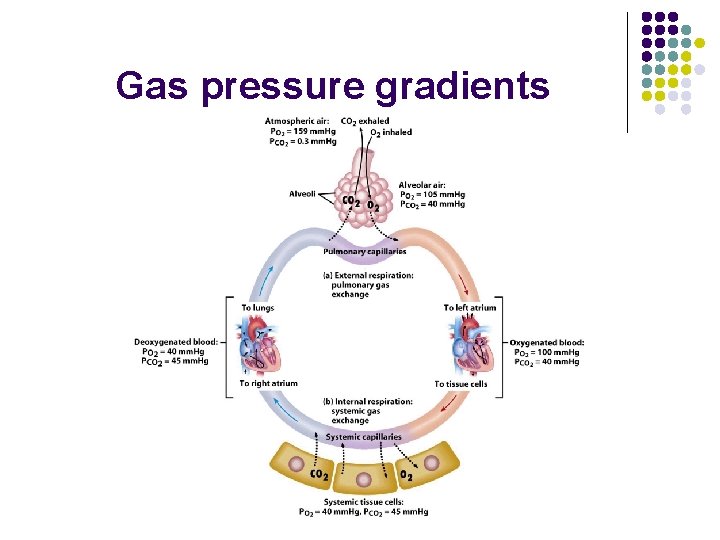
Gas pressure gradients

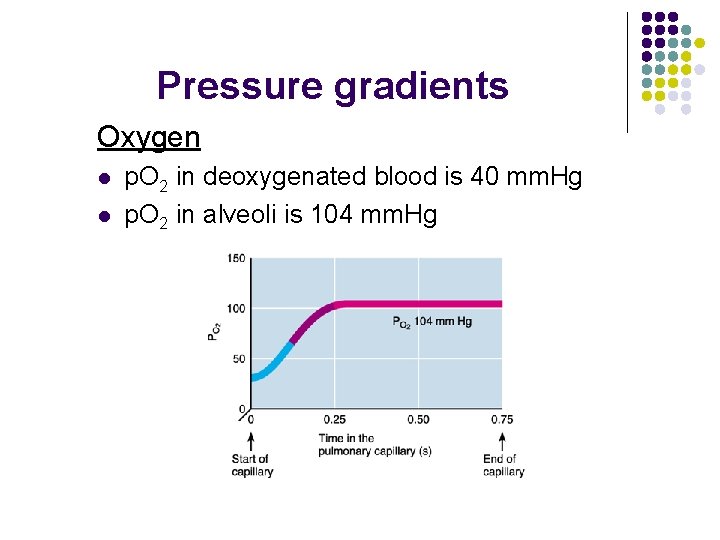
Pressure gradients Oxygen l l p. O 2 in deoxygenated blood is 40 mm. Hg p. O 2 in alveoli is 104 mm. Hg

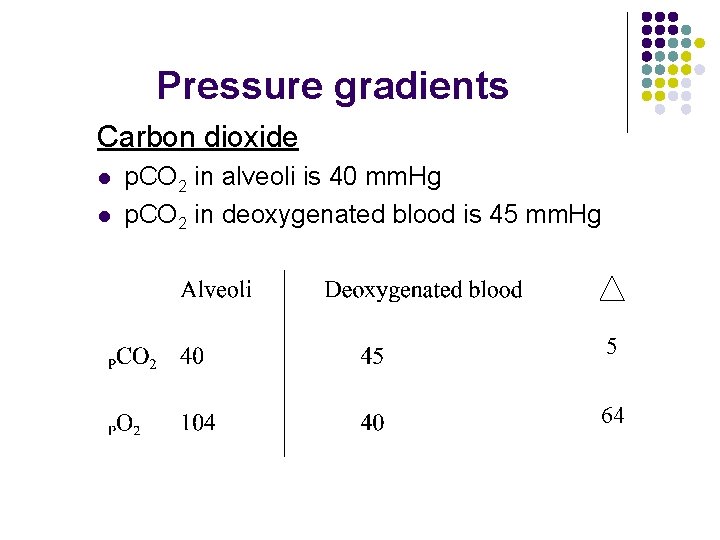
Pressure gradients Carbon dioxide l l p. CO 2 in alveoli is 40 mm. Hg p. CO 2 in deoxygenated blood is 45 mm. Hg 5 64

Pressure gradients l Relatively the same amount of O 2 and CO 2 are exchanged. Why? Answer: Solubility

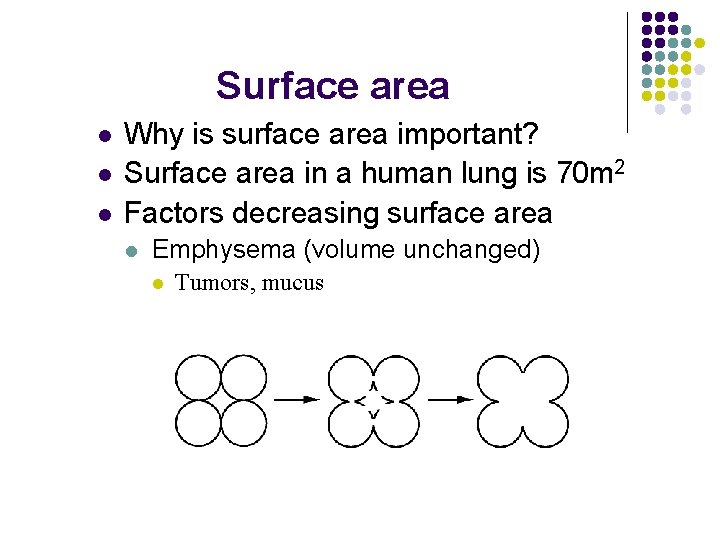
Surface area l l l Why is surface area important? Surface area in a human lung is 70 m 2 Factors decreasing surface area l Emphysema (volume unchanged) l Tumors, mucus

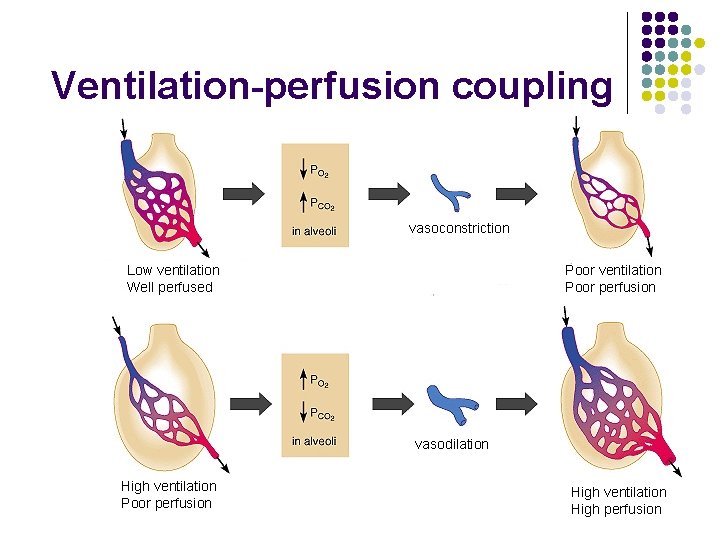
Ventilation-perfusion coupling vasoconstriction Low ventilation Well perfused Poor ventilation Poor perfusion vasodilation High ventilation Poor perfusion High ventilation High perfusion

Gas transport in blood l Methods of transport l l l Dissolved in plasma (3 ml per liter) Problem: C. O. would need to be 80 l/min Bound to a respiratory pigment (Hb) (200 ml per liter) Solution: Hb carries both O 2 and CO 2 simultaneously

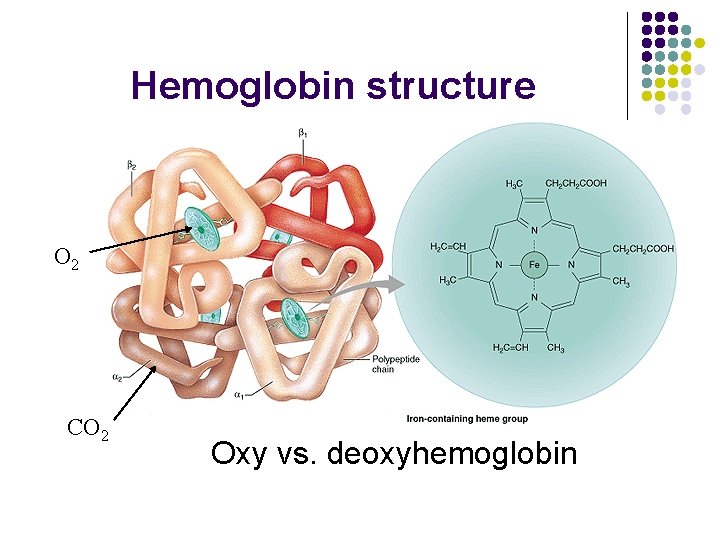
Hemoglobin structure O 2 CO 2 Oxy vs. deoxyhemoglobin

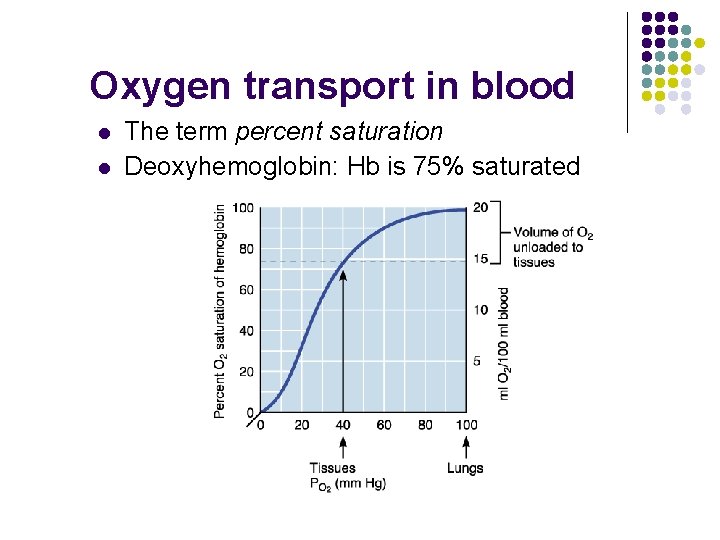
Oxygen transport in blood l l The term percent saturation Deoxyhemoglobin: Hb is 75% saturated

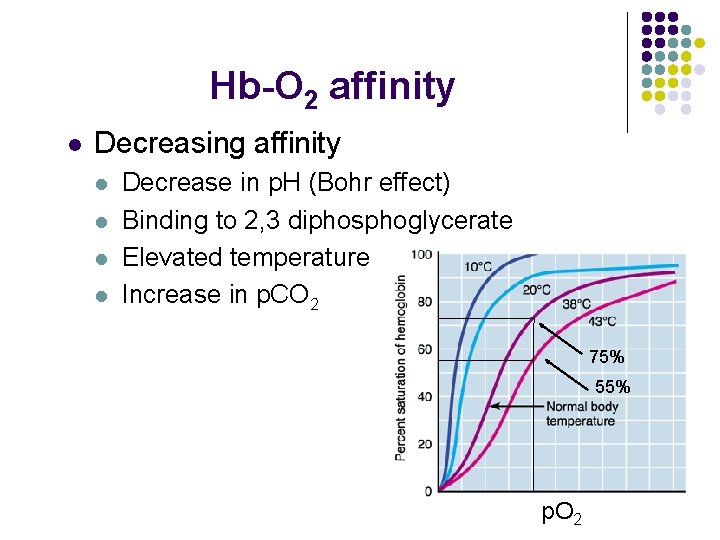
Hb-O 2 affinity l Decreasing affinity l l Decrease in p. H (Bohr effect) Binding to 2, 3 diphosphoglycerate Elevated temperature Increase in p. CO 2 75% 55% p. O 2

Oxygen transport l Hypoxia: inadequate O 2 to tissues l l Anemic: few RBC’s Ischemic: impaired or blocked blood circulation Histotoxic: body cells unable to use O 2 even though enough delivered (cyanide) Hypoxemic: reduced arterial p. O 2 (CO 2 poisoning)

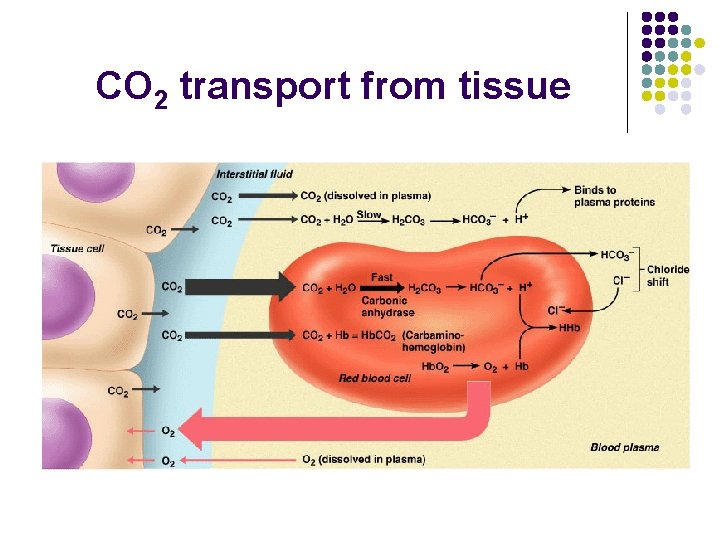
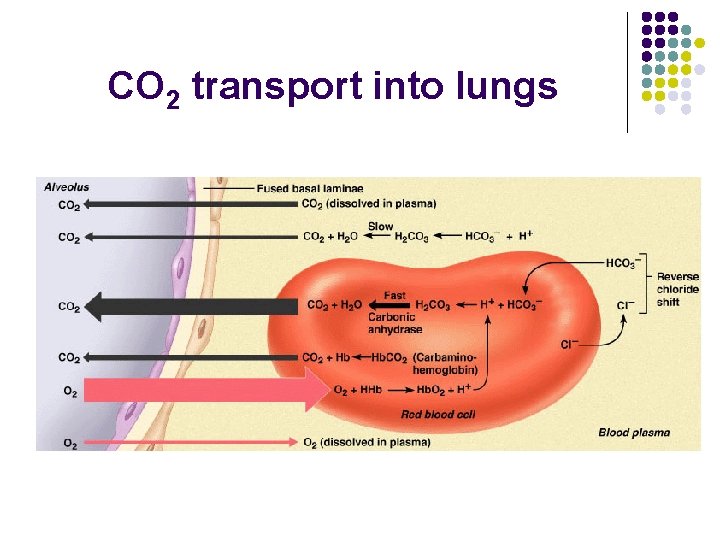
CO 2 transport l Ways to transport l l l Dissolved in plasma (7 - 10%) Bound to Hb (20 - 30%) Bicarbonate ion (60 - 70%)

CO 2 transport from tissue

CO 2 transport into lungs