HUKUM KEDUA TERMODINAMIKA Pengantar Catatan Umum In Chapter


















- Slides: 47

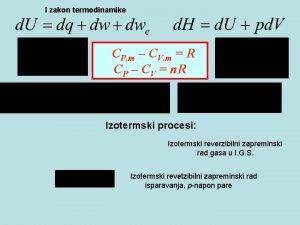
HUKUM KEDUA TERMODINAMIKA Pengantar

Catatan Umum • In Chapter 6 we mentioned the fact that all real changes have a direction which we consider natural. • The transformation in the opposite sense would be unnatural ; it would be unreal. In nature, rivers run from the mountains to the sea, never in the opposite way • Yet the first law of thermodynamics tells us nothing of this preference of one direction over the opposite one. • The first law requires only that the energy of the universe remain the same before and after the change takes place. In the changes described above, the energy of the universe is not one whit altered ; the transformation may go in either direction and satisfy the first law

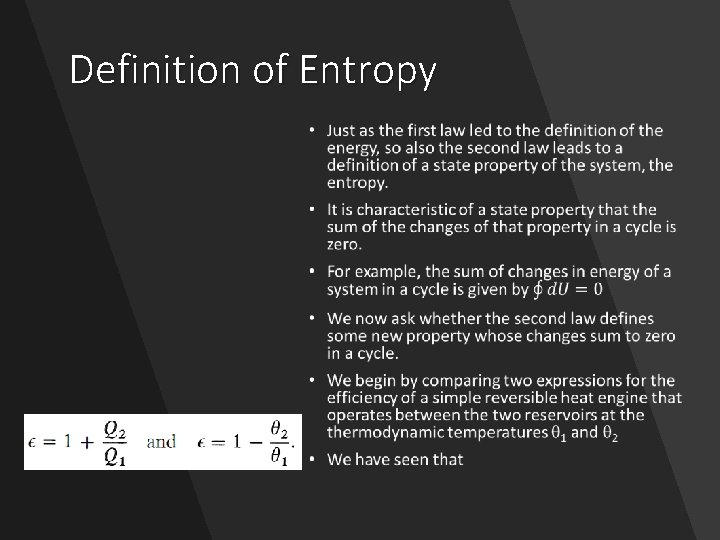
• It would be helpful if a system possessed one or more properties that always change in one direction when the system undergoes a natural change, and change in the opposite direction if we imagine the system to undergo an " unnatural change. " • Fortunately, there exists such a property of a system, the entropy, as well as several others derived from it. • To prepare a foundation for the mathematical definition of the entropy, we must divert our attention briefly to the characteristics of cyclic transformations. Having done that, we will return to chemical systems and the chemical implications of the second law

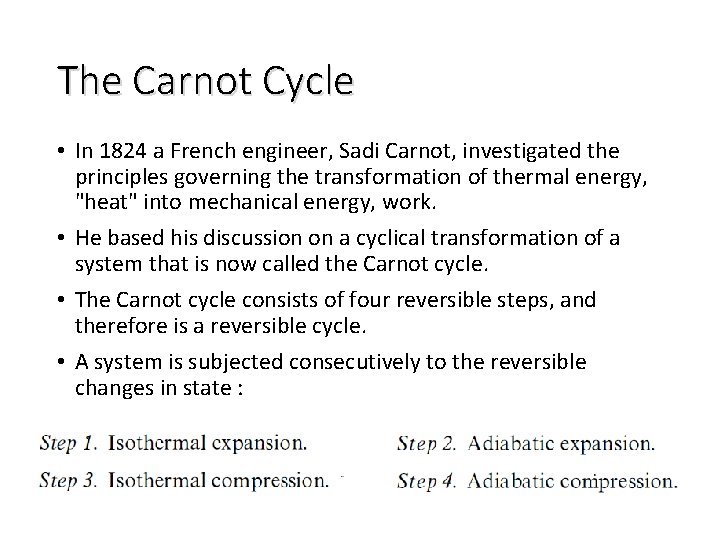
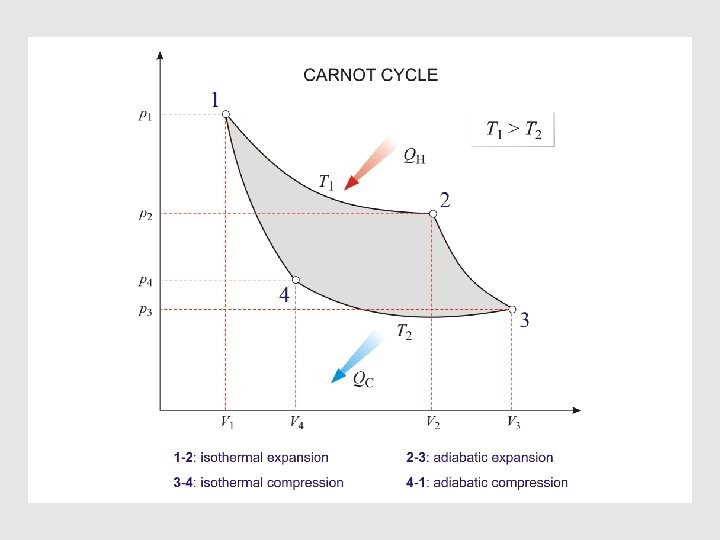
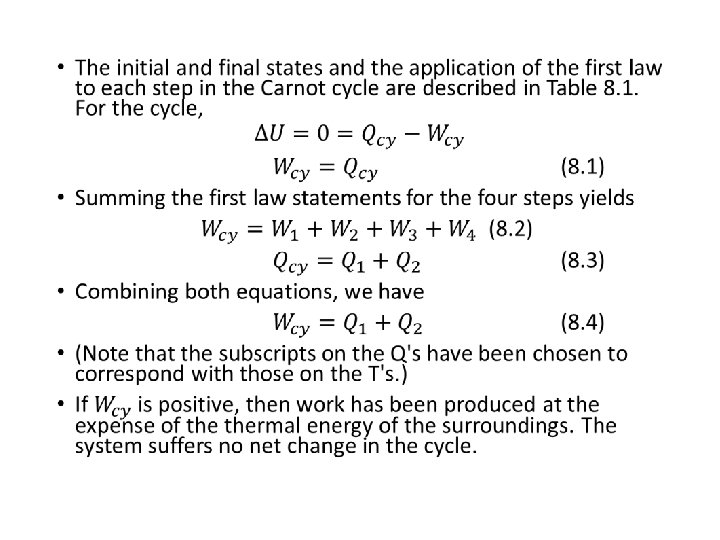
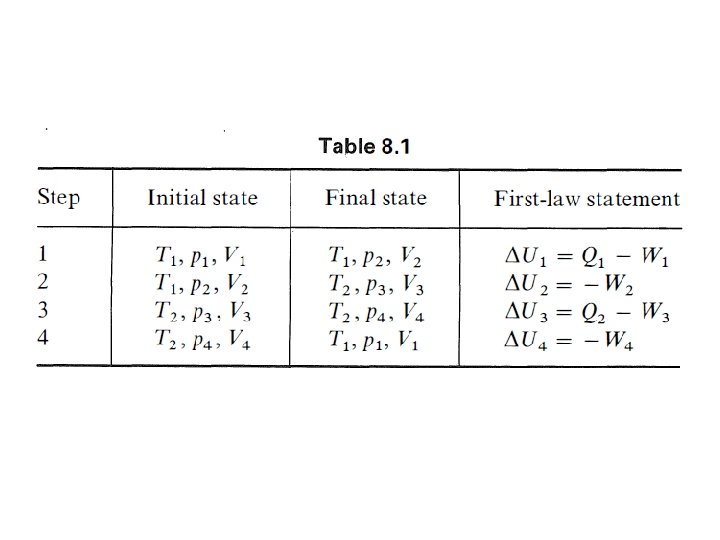
The Carnot Cycle • In 1824 a French engineer, Sadi Carnot, investigated the principles governing the transformation of thermal energy, "heat" into mechanical energy, work. • He based his discussion on a cyclical transformation of a system that is now called the Carnot cycle. • The Carnot cycle consists of four reversible steps, and therefore is a reversible cycle. • A system is subjected consecutively to the reversible changes in state :






Hukum Kedua Termodinamika •


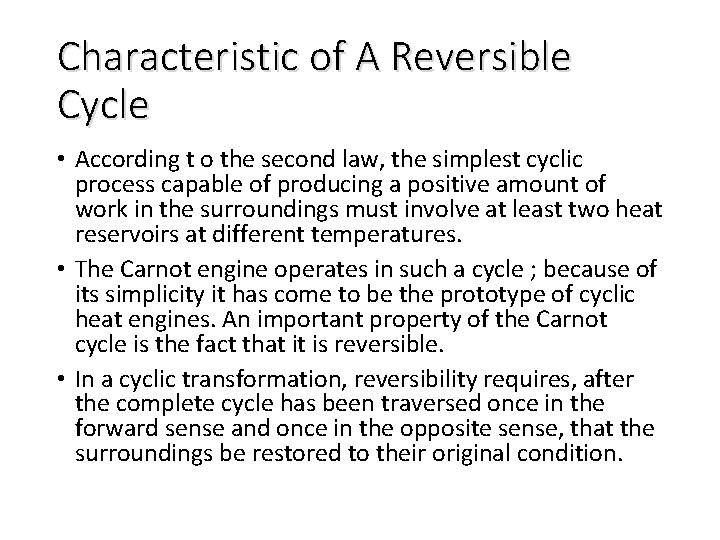
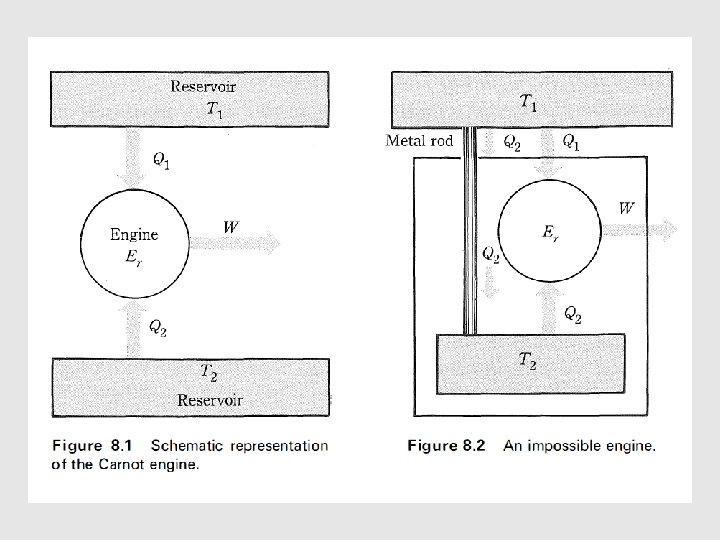
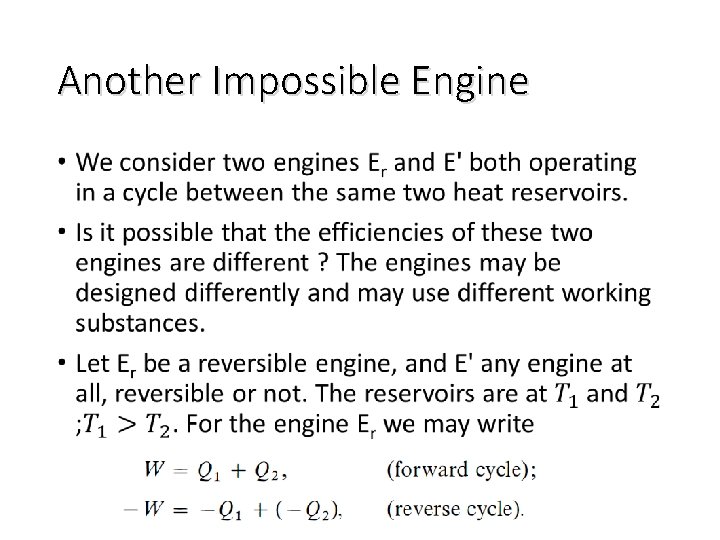
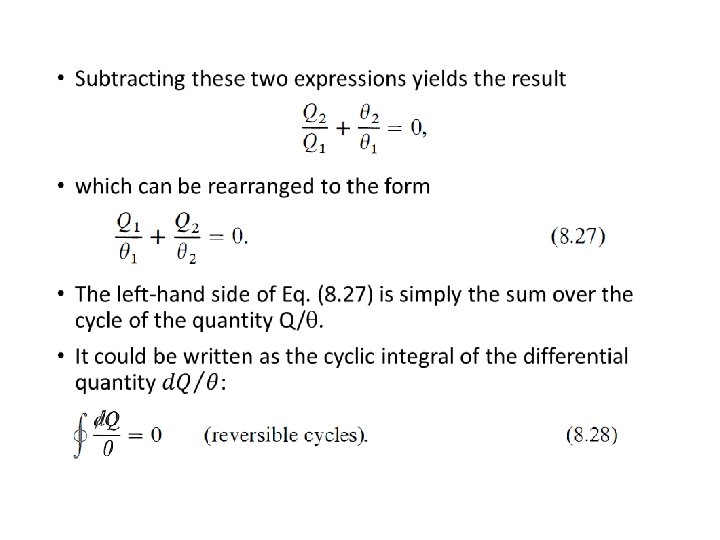
Characteristic of A Reversible Cycle • According t o the second law, the simplest cyclic process capable of producing a positive amount of work in the surroundings must involve at least two heat reservoirs at different temperatures. • The Carnot engine operates in such a cycle ; because of its simplicity it has come to be the prototype of cyclic heat engines. An important property of the Carnot cycle is the fact that it is reversible. • In a cyclic transformation, reversibility requires, after the complete cycle has been traversed once in the forward sense and once in the opposite sense, that the surroundings be restored to their original condition.



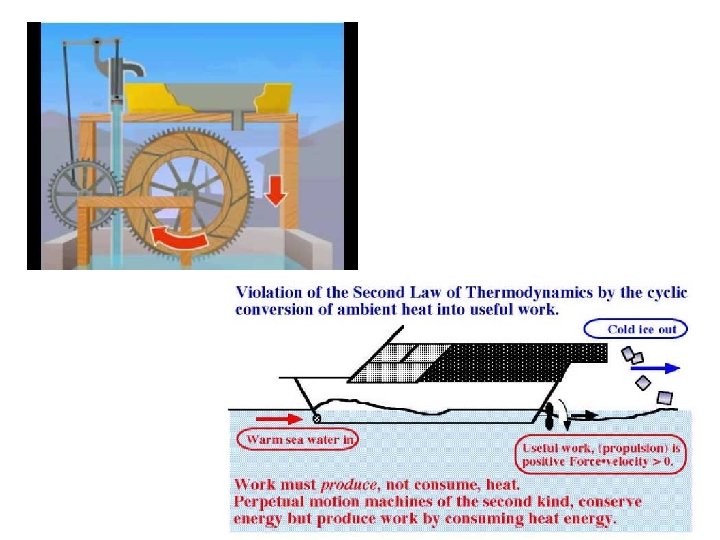
A Perpetual-Motion Machine of The Second Kind •




• Suppose that we install the impossible engine shown in Fig. 8. 2 in the living room. The room itself can serve as the heat reservoir. We set the machine in motion. (Note that we do not have to plug it in !) • The machine is now extracting heat from the room and producing mechanical work. Anyone with an ounce of frugality would use this work to run an electrical generator. • As we gaily pen a note to the local power company stating that we no longer need their services, we note that the room is getting chilly. Air conditioning ! Very nice in the summer, but definitely a nuisance in the winter. • In winter we can put the machine outdoors. The heat is then extracted from the atmosphere ; that engine will run for a long time before the atmosphere cools by so much as one degree ; meanwhile we aren't paying any light bills. The delightful thing about this engine is that of course the atmosphere will never go permanently cold on us. As we use the stored electrical energy, it is returned to the universe mainly in the form of " heat. " • Unfortunately, this wonderful machine is not available on the market. • The truth of the matter is that experience has shown that it is not possible to build such a machine. It is a perpetual-motion machine of the second kind.

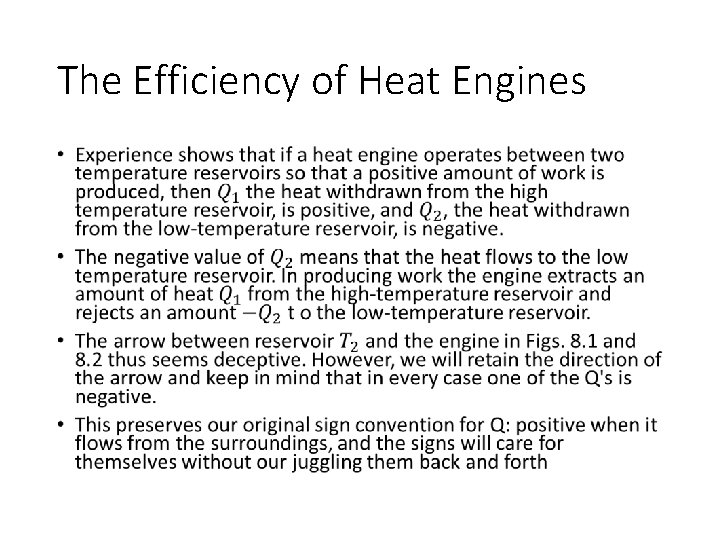
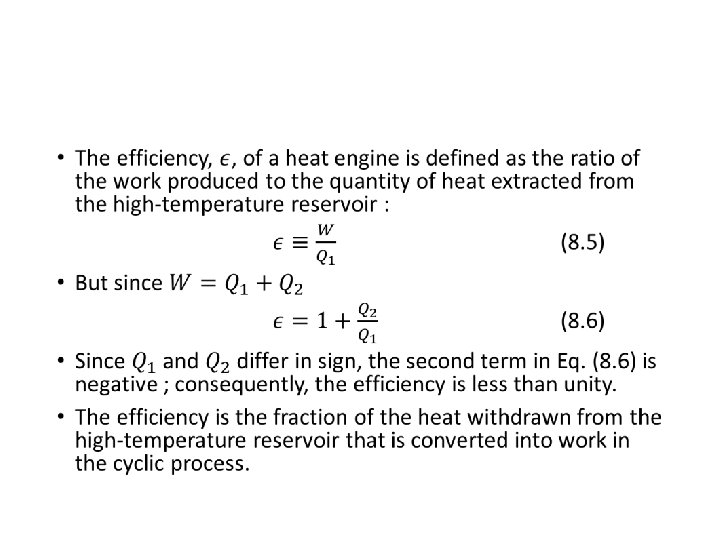
The Efficiency of Heat Engines •


Another Impossible Engine •

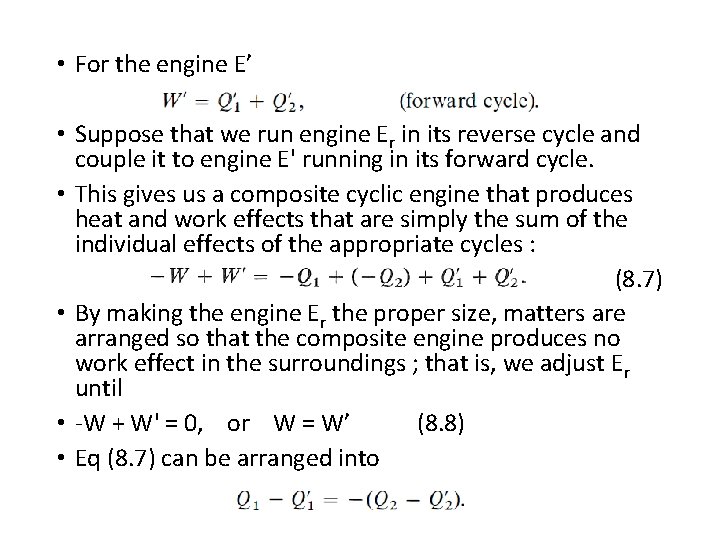
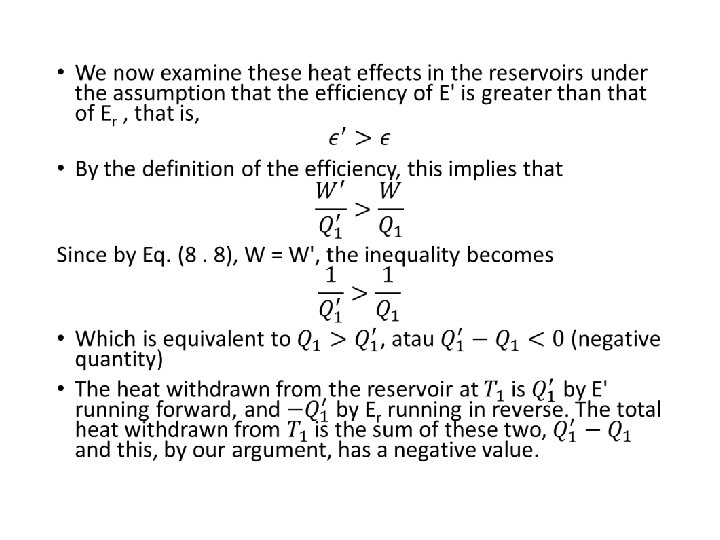
• For the engine E’ • Suppose that we run engine Er in its reverse cycle and couple it to engine E' running in its forward cycle. • This gives us a composite cyclic engine that produces heat and work effects that are simply the sum of the individual effects of the appropriate cycles : (8. 7) • By making the engine Er the proper size, matters are arranged so that the composite engine produces no work effect in the surroundings ; that is, we adjust Er until • -W + W' = 0, or W = W’ (8. 8) • Eq (8. 7) can be arranged into



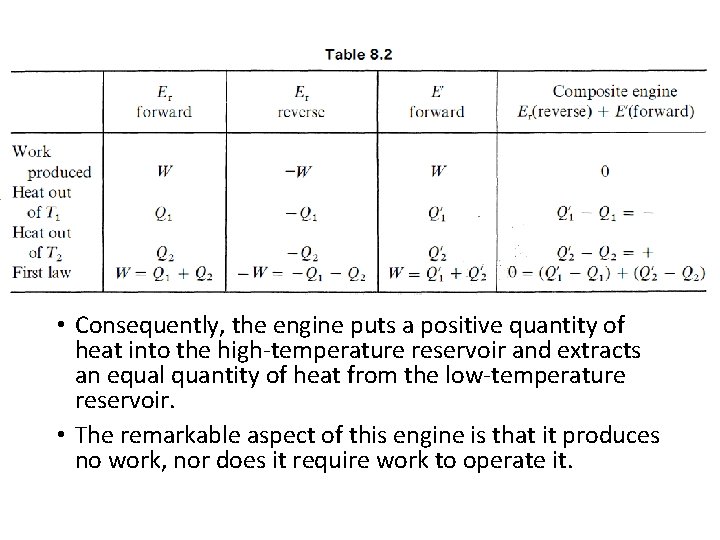
• Consequently, the engine puts a positive quantity of heat into the high-temperature reservoir and extracts an equal quantity of heat from the low-temperature reservoir. • The remarkable aspect of this engine is that it produces no work, nor does it require work to operate it.

• Again in our imagination we install this engine in the living room. We pour a bucket of hot water into one end a bucket of cold water into the other end, then set the machine in motion. • It commences to pump heat from the cold end to the hot end. Before long the water in the hot end is boiling while that in the cold end is freezing. • If the designer has been provident enough to make the cold end in the shape of an insulated box, we can keep the beer in that end and boil the coffee on the hot end. • Any thrifty (hemat) homemaker would be delighted with this gadget. What a kitchen appliance : a combination stoverefrigerator! • And again, no bill from the power company. • Experience shows that it is not possible to build this engine ; this is another example of perpetual motion of the second kind.



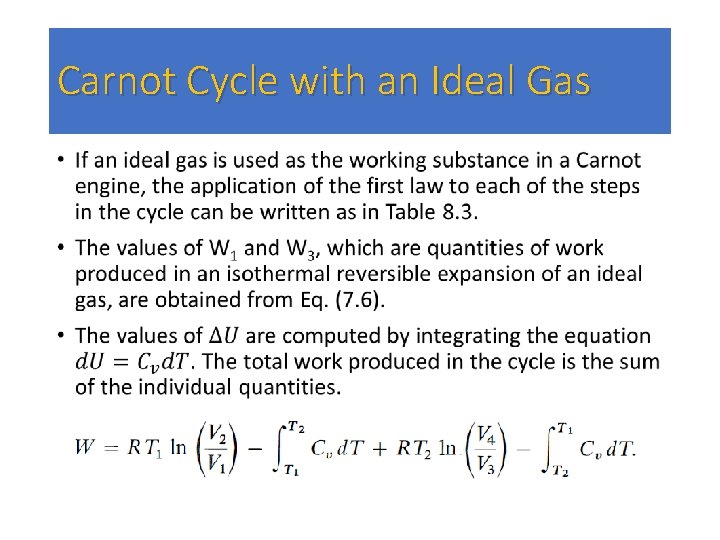
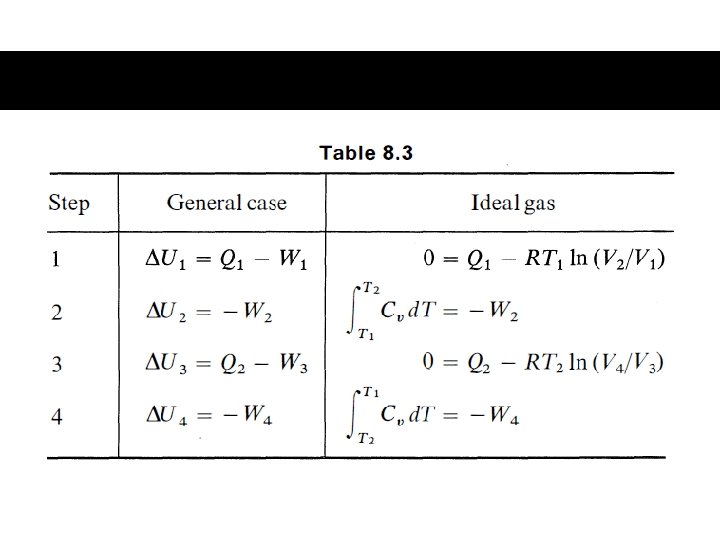
Carnot Cycle with an Ideal Gas •


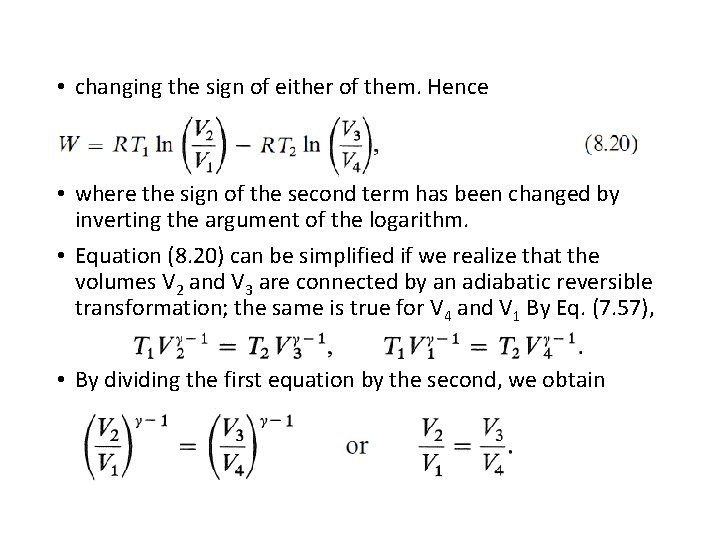
• changing the sign of either of them. Hence • where the sign of the second term has been changed by inverting the argument of the logarithm. • Equation (8. 20) can be simplified if we realize that the volumes V 2 and V 3 are connected by an adiabatic reversible transformation; the same is true for V 4 and V 1 By Eq. (7. 57), • By dividing the first equation by the second, we obtain

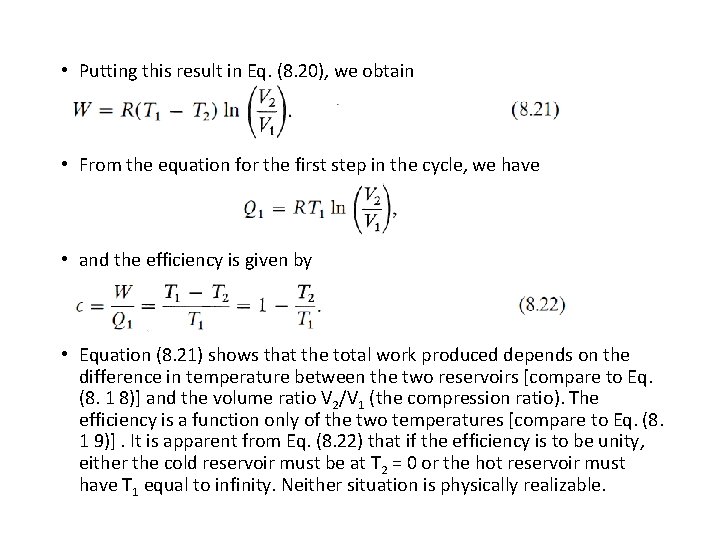
• Putting this result in Eq. (8. 20), we obtain • From the equation for the first step in the cycle, we have • and the efficiency is given by • Equation (8. 21) shows that the total work produced depends on the difference in temperature between the two reservoirs [compare to Eq. (8. 1 8)] and the volume ratio V 2/V 1 (the compression ratio). The efficiency is a function only of the two temperatures [compare to Eq. (8. 1 9)]. It is apparent from Eq. (8. 22) that if the efficiency is to be unity, either the cold reservoir must be at T 2 = 0 or the hot reservoir must have T 1 equal to infinity. Neither situation is physically realizable.

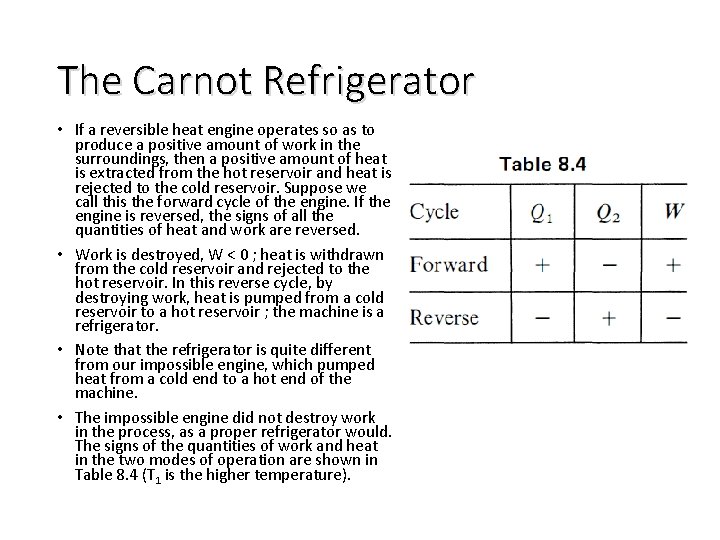
The Carnot Refrigerator • If a reversible heat engine operates so as to produce a positive amount of work in the surroundings, then a positive amount of heat is extracted from the hot reservoir and heat is rejected to the cold reservoir. Suppose we call this the forward cycle of the engine. If the engine is reversed, the signs of all the quantities of heat and work are reversed. • Work is destroyed, W < 0 ; heat is withdrawn from the cold reservoir and rejected to the hot reservoir. In this reverse cycle, by destroying work, heat is pumped from a cold reservoir to a hot reservoir ; the machine is a refrigerator. • Note that the refrigerator is quite different from our impossible engine, which pumped heat from a cold end to a hot end of the machine. • The impossible engine did not destroy work in the process, as a proper refrigerator would. The signs of the quantities of work and heat in the two modes of operation are shown in Table 8. 4 (T 1 is the higher temperature).

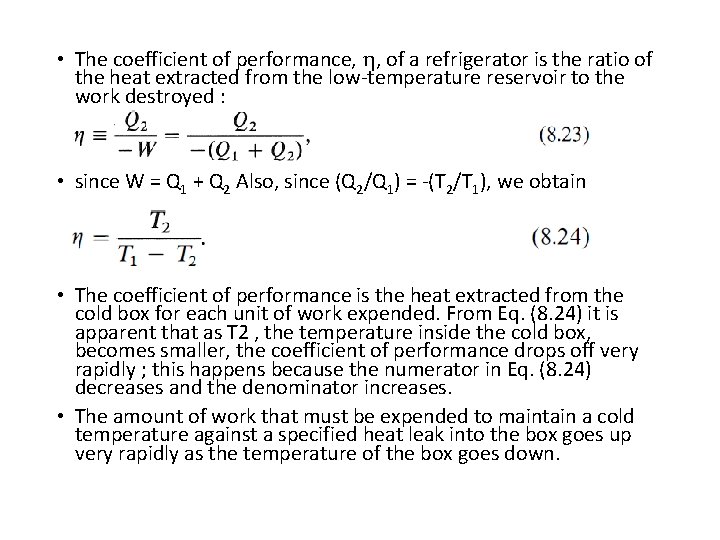
• The coefficient of performance, , of a refrigerator is the ratio of the heat extracted from the low-temperature reservoir to the work destroyed : • since W = Q 1 + Q 2 Also, since (Q 2/Q 1) = -(T 2/T 1), we obtain • The coefficient of performance is the heat extracted from the cold box for each unit of work expended. From Eq. (8. 24) it is apparent that as T 2 , the temperature inside the cold box, becomes smaller, the coefficient of performance drops off very rapidly ; this happens because the numerator in Eq. (8. 24) decreases and the denominator increases. • The amount of work that must be expended to maintain a cold temperature against a specified heat leak into the box goes up very rapidly as the temperature of the box goes down.

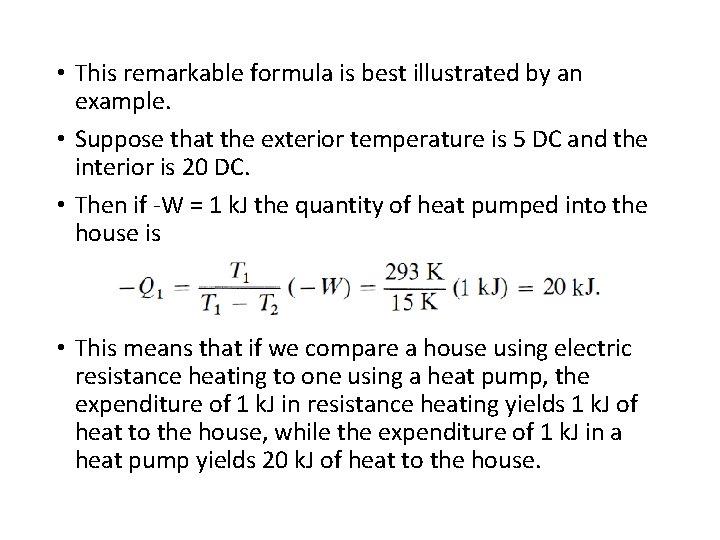
• This remarkable formula is best illustrated by an example. • Suppose that the exterior temperature is 5 DC and the interior is 20 DC. • Then if -W = 1 k. J the quantity of heat pumped into the house is • This means that if we compare a house using electric resistance heating to one using a heat pump, the expenditure of 1 k. J in resistance heating yields 1 k. J of heat to the house, while the expenditure of 1 k. J in a heat pump yields 20 k. J of heat to the house.

• The advantage of the heat pump over resistance heating is apparent even though the coefficients of performance of real machines are substantially below theoretical maximum given by the second law. With the given temperatures, the coefficients of performance of real machines range from 2 to 3 (still good multiplication factors). • However, when the exterior temperature drops below 5 DC the heat pump runs into trouble. Under the usual heating demand, it is difficult to supply cold air at a sufficient rate to keep the cold coil at the ambient temperature. • The coil temperature drops and the performance ratio decreases, as shown by Eq. (8. 26). If we try to assess the relative economy of a heat pump versus burning fossil fuel directly, we must bear in mind that, if the electrical energy to run the heat pump comes from fossil fuel, the power plant is subject to the Carnot limitation. • The overall efficiency of a modern steam power plant is about 35 percent. Thus, just to break even on fossil fuel consumption, the heat pump coefficient of performance must be at least 1/0. 35 = 2. 9

Definition of Entropy •



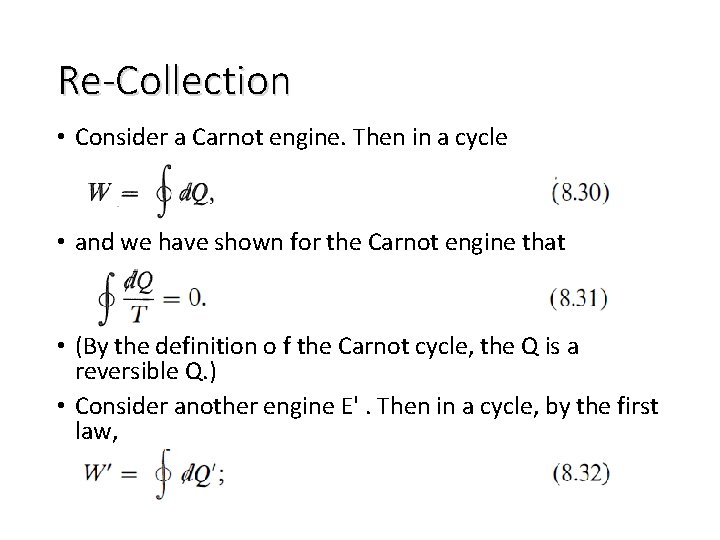
Re-Collection • Consider a Carnot engine. Then in a cycle • and we have shown for the Carnot engine that • (By the definition o f the Carnot cycle, the Q is a reversible Q. ) • Consider another engine E'. Then in a cycle, by the first law,

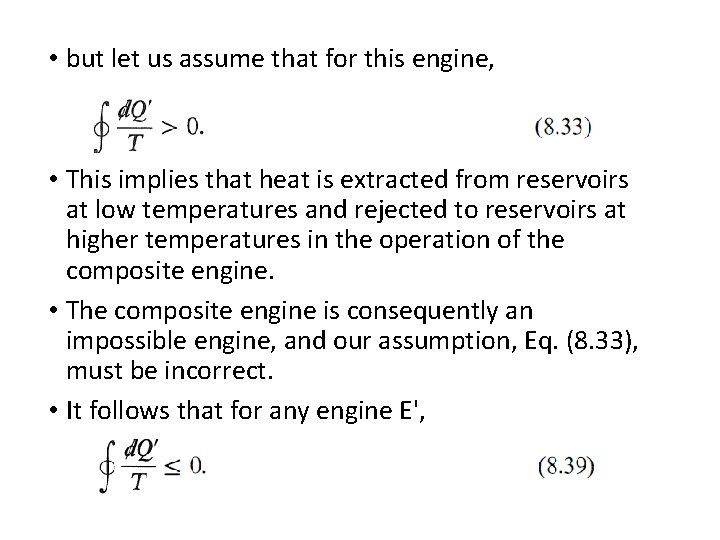
• but let us assume that for this engine, • This implies that heat is extracted from reservoirs at low temperatures and rejected to reservoirs at higher temperatures in the operation of the composite engine. • The composite engine is consequently an impossible engine, and our assumption, Eq. (8. 33), must be incorrect. • It follows that for any engine E',

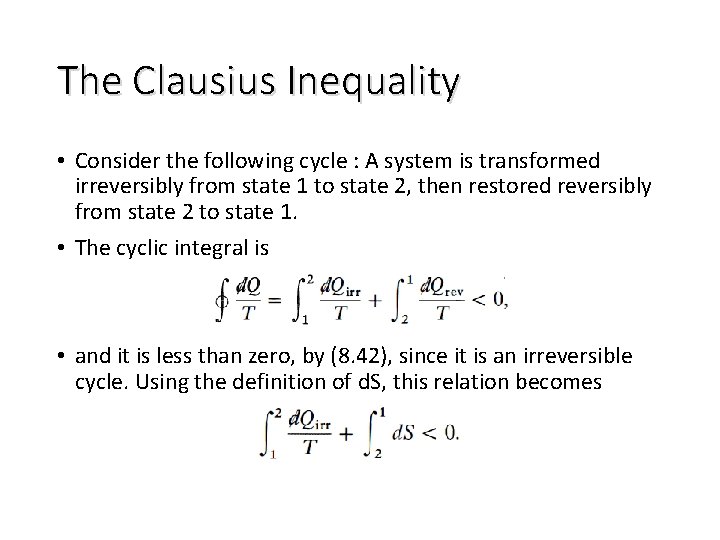
The Clausius Inequality • Consider the following cycle : A system is transformed irreversibly from state 1 to state 2, then restored reversibly from state 2 to state 1. • The cyclic integral is • and it is less than zero, by (8. 42), since it is an irreversible cycle. Using the definition of d. S, this relation becomes

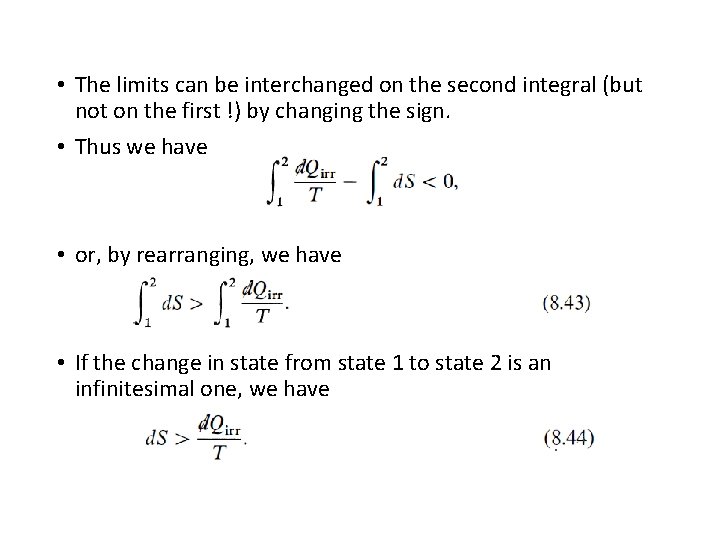
• The limits can be interchanged on the second integral (but not on the first !) by changing the sign. • Thus we have • or, by rearranging, we have • If the change in state from state 1 to state 2 is an infinitesimal one, we have


• Any natural change occurring within an isolated system is attended by an increase in entropy of the system. • The entropy of an isolated system continues to increase so long as changes occur within it. When the changes cease, the system is in equilibrium and the entropy has reached a maximum value. • Therefore the condition of equilibrium in an isolated system is that the entropy have a maximum value. • These, then, are also fundamental properties of the entropy : (1) the entropy of an isolated system is increased by any natural change which occurs within it ; and (2) the entropy of an isolated system has a maximum value at equilibrium.

• Changes in a non-isolated system produce effects in the system and in the immediate surroundings. • The system and its immediate surroundings constitute a composite isolated system in which the entropy increases as natural changes occur within it. Thus in the universe the entropy increases continually as natural changes occur within it. • Clausius expressed the two laws of thermodynamics in the famous aphorism : “The energy of the universe is constant ; the entropy strives to reach a maximum“.
 Hukum kedua termodinamika entropi
Hukum kedua termodinamika entropi Termodinamika 2
Termodinamika 2 Hukum 2 termodinamika
Hukum 2 termodinamika Catatan dalam buku jurnal merupakan catatan akuntansi yang
Catatan dalam buku jurnal merupakan catatan akuntansi yang Lapisan hukum
Lapisan hukum Hukum pertama termodinamika
Hukum pertama termodinamika Pengertian hukum ke nol termodinamika
Pengertian hukum ke nol termodinamika Prinsip carnot
Prinsip carnot Hukum pertama termodinamika
Hukum pertama termodinamika Hukum termodinamika disebut juga sebagai
Hukum termodinamika disebut juga sebagai Hukum 2 termodinamika
Hukum 2 termodinamika Aplikasi hukum termodinamika 2
Aplikasi hukum termodinamika 2 Hukum termodinamika 1
Hukum termodinamika 1 Gas ideal
Gas ideal Silabus pengantar ilmu hukum
Silabus pengantar ilmu hukum Rps pengantar ilmu hukum
Rps pengantar ilmu hukum Silabus pengantar ilmu hukum
Silabus pengantar ilmu hukum Hukum gerakan newton
Hukum gerakan newton Gambar model sistem umum perusahaan
Gambar model sistem umum perusahaan Prinsip hukum umum
Prinsip hukum umum Fungsi hukum taklifi
Fungsi hukum taklifi Hukum hukum perkembangan peserta didik
Hukum hukum perkembangan peserta didik Materi himpunan matematika diskrit
Materi himpunan matematika diskrit Hukum dasar listrik
Hukum dasar listrik Peta konsep hukum dasar kimia kelas 10
Peta konsep hukum dasar kimia kelas 10 Letak filsafat hukum dalam ilmu hukum
Letak filsafat hukum dalam ilmu hukum Hukum-hukum himpunan matematika diskrit
Hukum-hukum himpunan matematika diskrit Teori himpunan matematika diskrit
Teori himpunan matematika diskrit Hukum hukum logika proposisi
Hukum hukum logika proposisi Konsep hukum pascal
Konsep hukum pascal Gerak lurus dan hukum newton
Gerak lurus dan hukum newton Peta konsep hukum newton tentang gravitasi dan hukum kepler
Peta konsep hukum newton tentang gravitasi dan hukum kepler Jelaskan bunyi hukum ohm dan hukum 1 kirchoff
Jelaskan bunyi hukum ohm dan hukum 1 kirchoff Macam-macam hukum taklifi
Macam-macam hukum taklifi Tijdsgebied
Tijdsgebied Ppqpq
Ppqpq Hukum aljabar vektor
Hukum aljabar vektor Kedudukan hukum islam dalam kurikulum fakultas hukum
Kedudukan hukum islam dalam kurikulum fakultas hukum Hukum minimum liebig menyatakan bahwa
Hukum minimum liebig menyatakan bahwa Pengertian teorema de morgan
Pengertian teorema de morgan Strojarski računalni tehničar oroslavje
Strojarski računalni tehničar oroslavje Termokimia dan termodinamika
Termokimia dan termodinamika Thermodinamika
Thermodinamika Termodinamik sistema nima
Termodinamik sistema nima Cp-cv=r/m
Cp-cv=r/m Termodinamika teknik kimia
Termodinamika teknik kimia Cv termodinamika
Cv termodinamika Reaksi spontan termodinamika
Reaksi spontan termodinamika