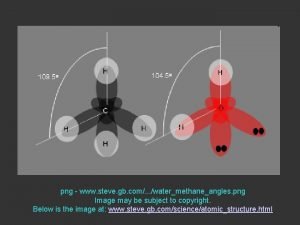
http www 12 6 curve comwpcontentuploads201306WackyWednesday png Density











- Slides: 21

http: //www. 12 -6 curve. com/wp-content/uploads/2013/06/Wacky-Wednesday. png

Density Quiz LET’S REVIEW

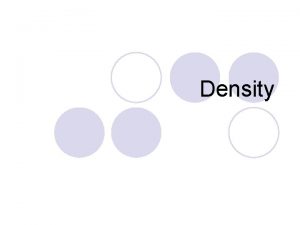
Inquiry Science 1 – Density Quiz Directions: Read all questions carefully. You must show your work to get credit. All calculations must be labeled with proper units. 1. You have gathered the following data in the lab. Use the data to calculate the density of each of the Liquids. Trial 1 Samples Mass g Vol. m. L Trial 2 3 Density Mass Vol. Density g/m. L g/m. L g m. L Liquid 1 4. 45 Liquid 2 3. 95 Liquid 3 6. 06 5 5 5 0. 89 0. 91 9. 05 10 22. 92 25 0. 79 0. 80 0. 81 8 10 20. 16 25 1. 21 1. 24 1. 25 12. 42 10 31. 37 25

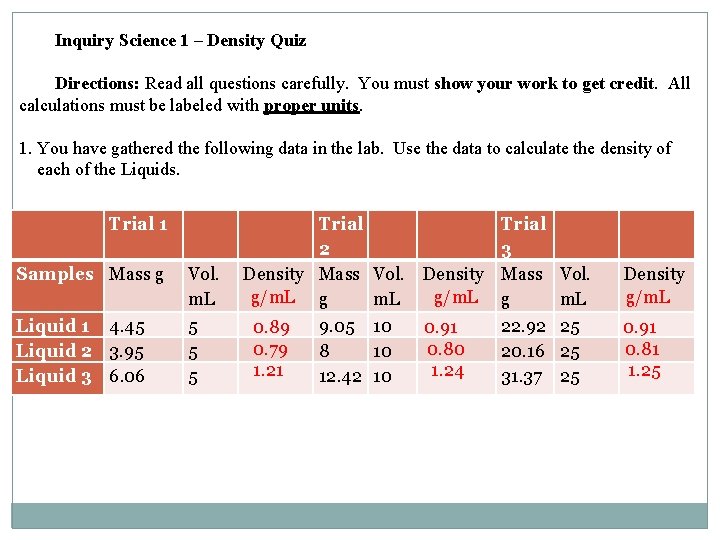
2. On a separate piece of graph paper, graph the mass and volume data for each liquid. Mass (g) 3. Draw a best-fit line for each Liquid. Make sure to label each line. 34 32 30 28 26 24 22 20 18 16 14 12 10 8 6 4 2 Liquid 1 Liquid 2 Liquid 3 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Volume (m. L)

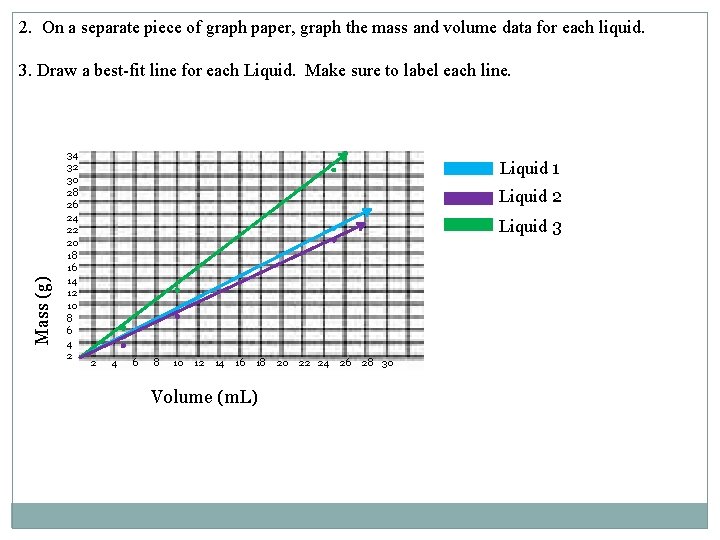
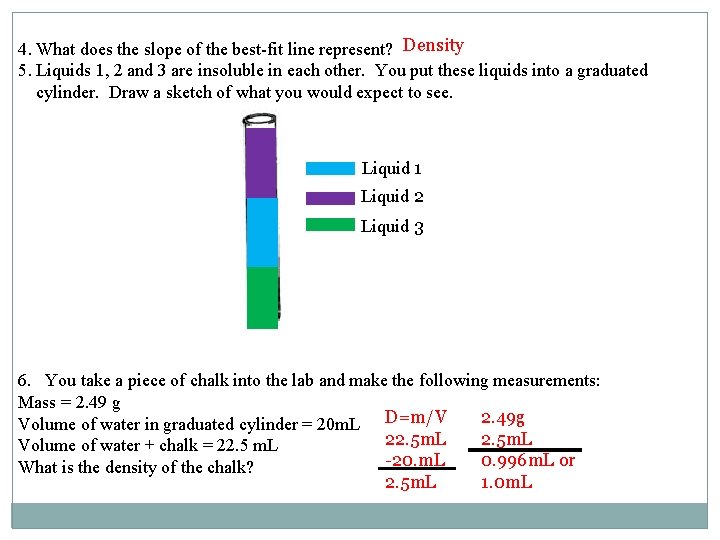
4. What does the slope of the best-fit line represent? Density 5. Liquids 1, 2 and 3 are insoluble in each other. You put these liquids into a graduated cylinder. Draw a sketch of what you would expect to see. Liquid 1 Liquid 2 Liquid 3 6. You take a piece of chalk into the lab and make the following measurements: Mass = 2. 49 g 2. 49 g Volume of water in graduated cylinder = 20 m. L D=m/V 22. 5 m. L Volume of water + chalk = 22. 5 m. L -20. m. L 0. 996 m. L or What is the density of the chalk? 2. 5 m. L 1. 0 m. L

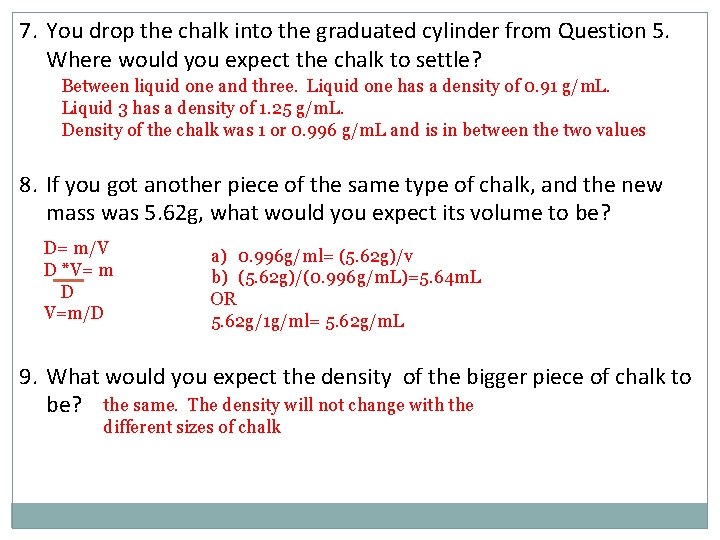
7. You drop the chalk into the graduated cylinder from Question 5. Where would you expect the chalk to settle? Between liquid one and three. Liquid one has a density of 0. 91 g/m. L. Liquid 3 has a density of 1. 25 g/m. L. Density of the chalk was 1 or 0. 996 g/m. L and is in between the two values 8. If you got another piece of the same type of chalk, and the new mass was 5. 62 g, what would you expect its volume to be? D= m/V D *V= m D V=m/D a) 0. 996 g/ml= (5. 62 g)/v b) (5. 62 g)/(0. 996 g/m. L)=5. 64 m. L OR 5. 62 g/1 g/ml= 5. 62 g/m. L 9. What would you expect the density of the bigger piece of chalk to be? the same. The density will not change with the different sizes of chalk

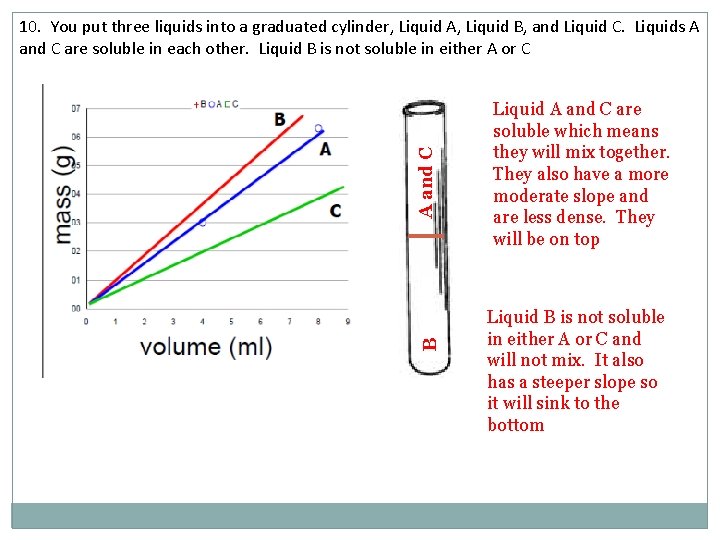
B A and C 10. You put three liquids into a graduated cylinder, Liquid A, Liquid B, and Liquid C. Liquids A and C are soluble in each other. Liquid B is not soluble in either A or C Liquid A and C are soluble which means they will mix together. They also have a more moderate slope and are less dense. They will be on top Liquid B is not soluble in either A or C and will not mix. It also has a steeper slope so it will sink to the bottom

Density Worksheet LET’S REVIEW

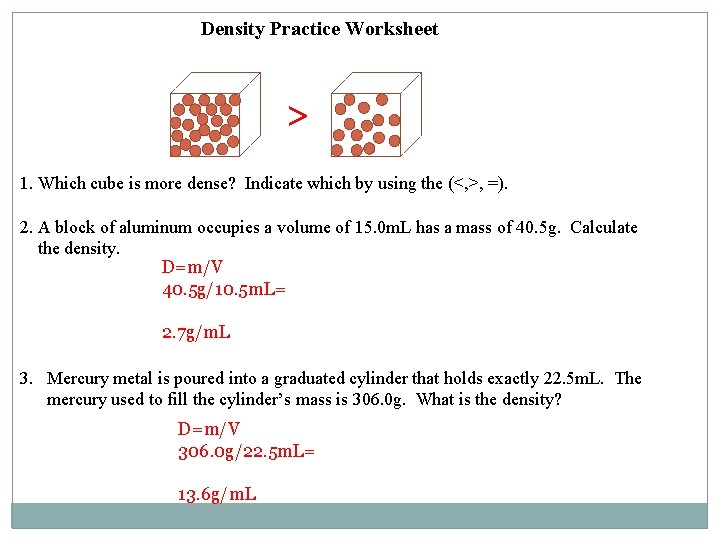
Density Practice Worksheet > 1. Which cube is more dense? Indicate which by using the (<, >, =). 2. A block of aluminum occupies a volume of 15. 0 m. L has a mass of 40. 5 g. Calculate the density. D=m/V 40. 5 g/10. 5 m. L= 2. 7 g/m. L 3. Mercury metal is poured into a graduated cylinder that holds exactly 22. 5 m. L. The mercury used to fill the cylinder’s mass is 306. 0 g. What is the density? D=m/V 306. 0 g/22. 5 m. L= 13. 6 g/m. L

4. What is the mass of isopropyl alcohol with a volume of 200 m. L if the density of the alcohol is 0. 789 g/m. L? D=m/V V*D=m (200 m. L)*(0. 789 g/m. L)= 157. 8 g 5. What is the density of sulfuric acid if the volume is 35. 4 m. L and has a mass of 65. 14 g? D=m/V 65. 14 g/35. 4 m. L= 1. 84 g/m. L

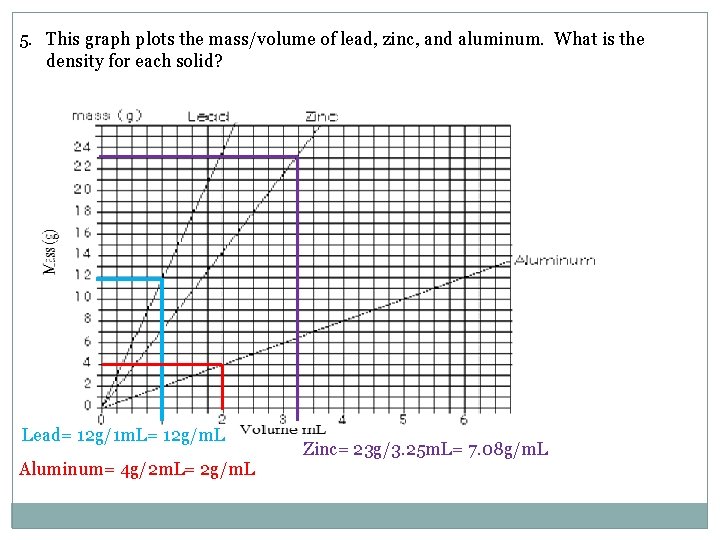
5. This graph plots the mass/volume of lead, zinc, and aluminum. What is the density for each solid? Lead= 12 g/1 m. L= 12 g/m. L Aluminum= 4 g/2 m. L= 2 g/m. L Zinc= 23 g/3. 25 m. L= 7. 08 g/m. L

7. What volume of silver metal will have a mass of exactly 2500. 0 g? The density of silver is 10. 5 g/m. L a) D=m/V: 2500 g/10. 5 g/m. L= b) V=m/D 238. 1 m. L 8. Water has a density of 1 g/ml. What is the mass of the water if it fills a 10 ml container? a) D=m/V: b) V*D=m (1 g/m. L) * (10. om. L)= 10 g 9. A certain gas expands to fill a 3 L container. Its mass is measured to be 0. 6 kg. What is its density? 0. 6 kg *1000= 600 g 3. 0 L * 1000 = 3000 m. L a) 1 L= 1000 m. L b) 1 kg= 1000 g = 0. 2 g/m. L 10. Will an object with a density of 0. 97 g/ml float or sink in water? Explain An object with a density of 0. 97 g/m. L will float in water. Water has a density of 1 g/m. L and the object is less dense.

Learning Target Review Name: Period: Date: I can manipulate the density formula. 1. 1. 2. Rate yourself (scale 1 -5): Explain 2. I can graph density 1. 2. Rate yourself (scale 1 -5): Explain 3. I understand how Reacting to Density Part I helps us to understand the Law of Conservation of Matter 1. 2. Rate yourself (scale 1 -5): Explain

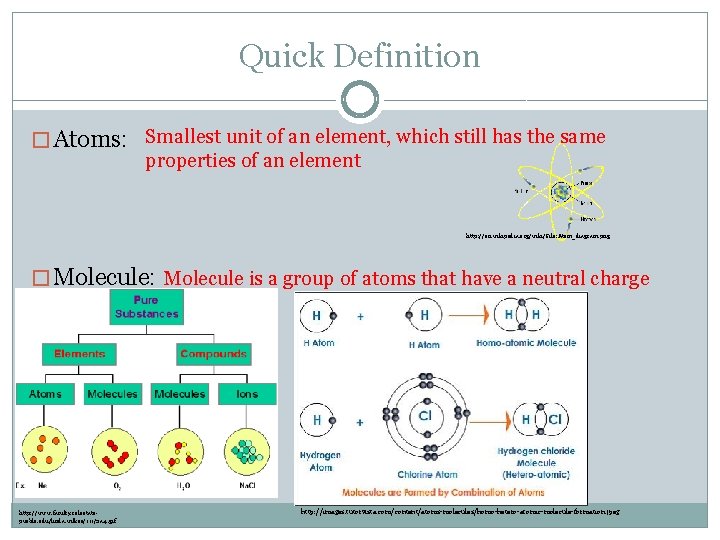
Quick Definition � Atoms: Smallest unit of an element, which still has the same properties of an element http: //en. wikipedia. org/wiki/File: Atom_diagram. png � Molecule: Molecule is a group of atoms that have a neutral charge http: //www. faculty. colostatepueblo. edu/linda. wilkes/111/2 a. 4. gif http: //images. tutorvista. com/content/atoms-molecules/homo-hetero-atomic-molecule-formation. jpeg

Stop and Think pg 84 1 -4 � 1. The gas produced was CO 2 the same gas we exhale during breathing. Suppose both the cap and the balloon methods captured exactly 50 particles (molecules) of CO 2 A) sketch these gas particles for both methods (balloon and cap) include highlight comments and captions for each sketch. What I see: Gas particles are farther apart in the balloon method What it means: Gas density is greater in the capped method; increasing the bottles pressure water Modified by” Tiffany adamdshttp : //www. allproducts. com/plastic/youcheng/plastic_soda_bottles-l. jpg http: //2. imimg. com/data 2/PX/HN/MY-224366/double-lip-closures 250 x 250. jpg

Stop and Think pg 84 � 1 b) Why should there be the same number of gas particles in each bottle, provided the same amount of water and the size tablet? Matter can neither be created nor destroyed (Law of Conservation), so the amount of gas should have been the same no matter how you conducted the experiment. As long as the same amounts of water and tablet were used.

Stop and Think pg 84 1 -4 � 1 c) Are the carbon dioxide molecules moving or stationary? How do you know? • The molecules have to be moving because otherwise the balloon/bottle would collapse. • There has to be something in the balloon after the reaction because it began to inflate • Something extinguished the flame and we could see it wasn’t a solid or a liquid • We can physically see that there were no solids or liquids in the balloon • The only way pressure could have built up in the balloon/bottle would be for those molecules to collide with each other and move around the container

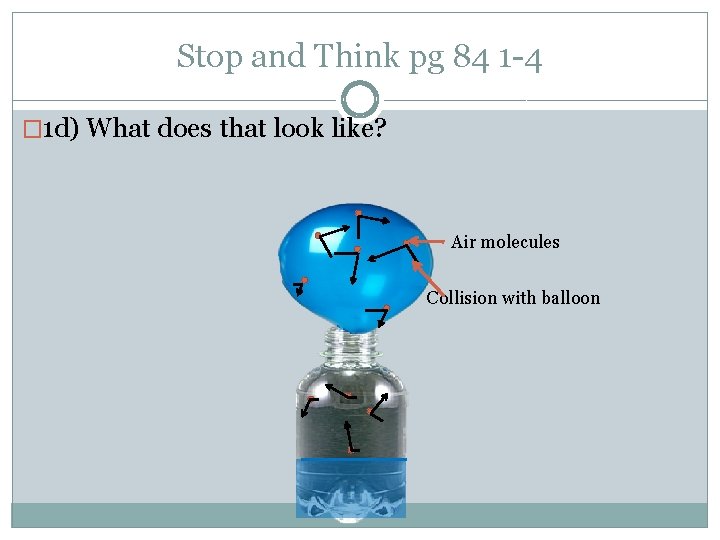
Stop and Think pg 84 1 -4 � 1 d) What does that look like? Air molecules Collision with balloon

Stop and Think pg 84 1 -4 � 2. In which method is the pressure inside the bottle the greatest? Give evidence. • The capped bottle has more pressure inside. • Since the capped bottle was started at room pressure and remained the same size (volume), the carbon dioxide gas had to produce extra pressure above room pressure • All the gas that was created had to go somewhere. In the balloon, there was more volume so less pressure in that bottle • In the capped bottle? Can’t go anywhere so there is a higher pressure

Stop and Think pg 84 1 -4 � 3. In which method is the density of the carbon dioxide gas the greatest? What evidence supports your answer? • The capped bottle has the higher gas density. • You know this because the same amount of molecules are produced for both methods (provided the tablet was the same size), so mass was the same. • The volume was less because the bottle was capped and therefore there was not the extra room for the molecules to go as in the balloon. • Since the volume was less (mass the same), then the density is greater. water

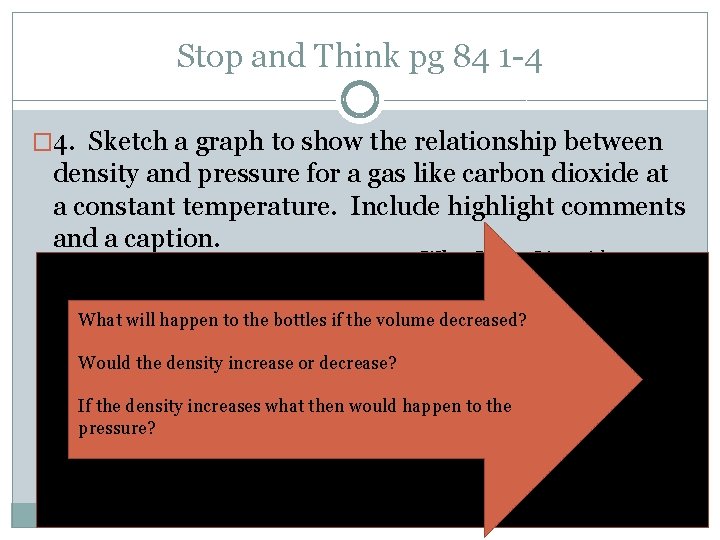
Stop and Think pg 84 1 -4 � 4. Sketch a graph to show the relationship between density and pressure for a gas like carbon dioxide at a constant temperature. Include highlight comments and a caption. What I See: Line with a constant positive slope Gas density What will happen to the bottles if the volume decreased? What it Means: Gas density is proportional to pressure Would the density increase or decrease? If the density increases what then would happen to the This graph shows how density of a gas increases as pressure? increases. This is due to molecules begin pushed together. With more molecules in the same place the density increases Would it increase or decrease? Pressure
 Commensalism emoji
Commensalism emoji Linear density of fcc 110
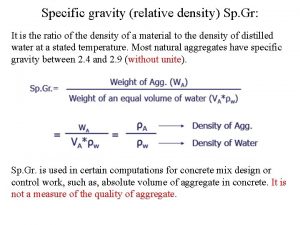
Linear density of fcc 110 Units of specific gravity
Units of specific gravity Nda full dac
Nda full dac Planar atomic density fcc 111
Planar atomic density fcc 111 What is agricultural density ap human geography
What is agricultural density ap human geography Crude population density vs physiological density
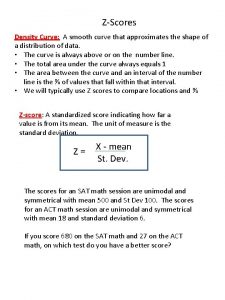
Crude population density vs physiological density Skewed left density curve
Skewed left density curve Biotic potential and environmental resistance
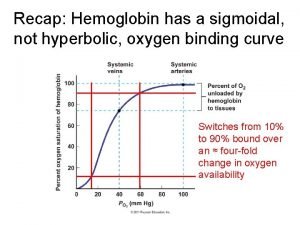
Biotic potential and environmental resistance Hyperbolic oxygen chamber
Hyperbolic oxygen chamber S curve and j curve
S curve and j curve Pleasure curve in complete denture
Pleasure curve in complete denture Delivery gas png
Delivery gas png Img pic ru
Img pic ru Crab game title png
Crab game title png Url(/images/studyanswers/blurredtext-medium.png)
Url(/images/studyanswers/blurredtext-medium.png) Ramki png
Ramki png Create png
Create png Ubahlah menjadi bentuk persen
Ubahlah menjadi bentuk persen No estoy yo que soy tu madre
No estoy yo que soy tu madre Png forest products bulolo
Png forest products bulolo Module 00107 basic communication skills
Module 00107 basic communication skills