http sciencespot net http www privatehand comflashelements html





- Slides: 27

http: //sciencespot. net/

http: //www. privatehand. com/flash/elements. html http: //boing. net/2009/09/08/they-might-be-giants-2. html http: //www. youtube. com/watch? v=WK 7 wu. Tw. Ai. BU

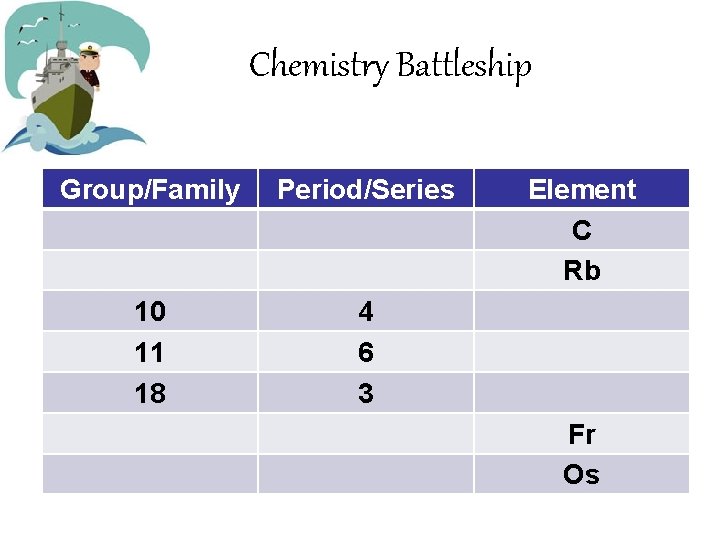
Chemistry Battleship • Groups or Families: Vertical Columns of the Periodic Table • Period or Series: Horizontal Rows of the Periodic table • Example: Group 9, Period 5 • Example: Family 12, Series 4

Chemistry Battleship Group/Family Period/Series 10 11 18 4 6 3 Element C Rb Fr Os

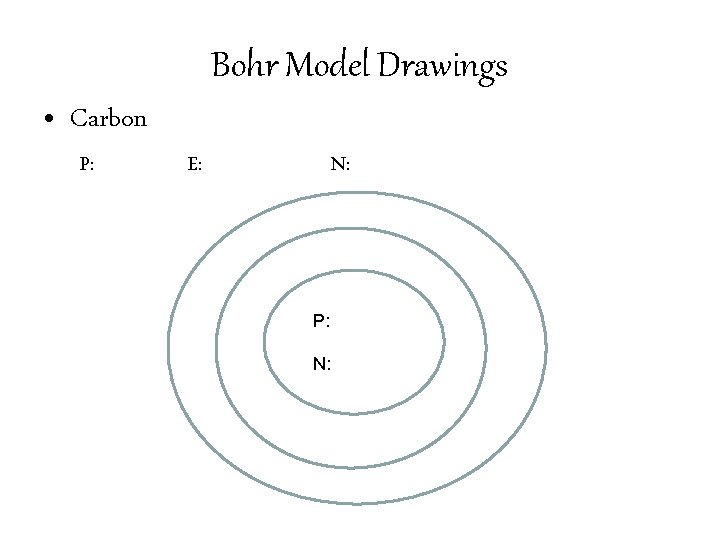
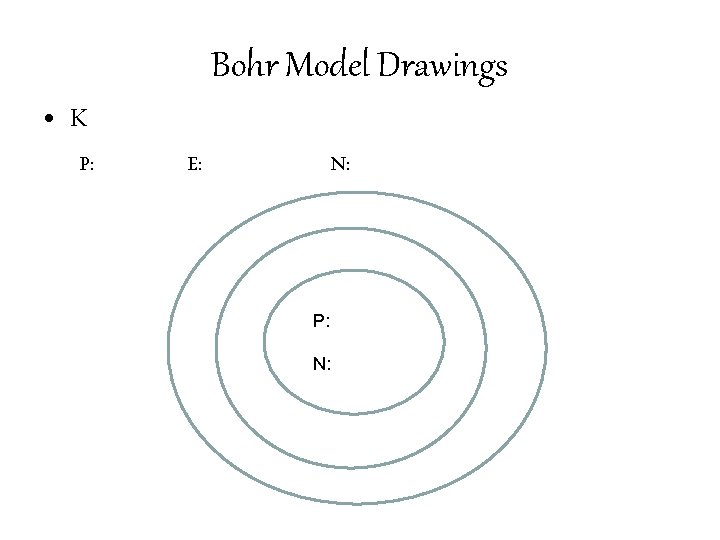
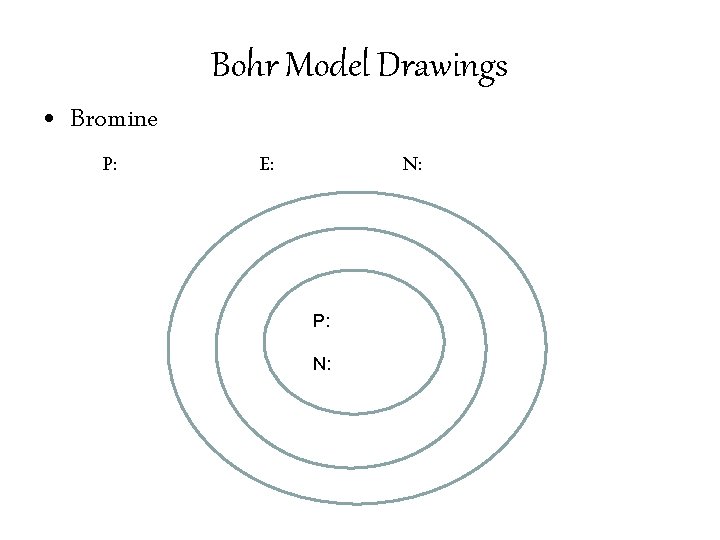
Bohr Model Drawings • Identify the number of Protons, Neutrons, and Electrons in an element. • Write the number of Protons and Neutrons in the center of the nucleus. • Draw the number of electrons in each of the orbitals. • 1 st Orbital: 2 electrons • 2 nd Orbital: 8 electrons • 3 rd Orbital: 32 electrons

Bohr Model Drawings • Carbon P: E: N: P: N:

Bohr Model Drawings • K P: E: N: P: N:

Bohr Model Drawings • Bromine P: E: N: P: N:

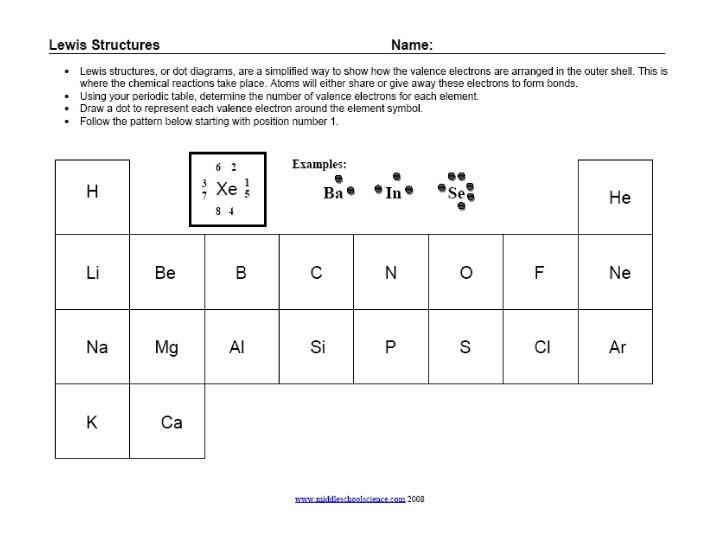
Lewis Structures 1) Find your element on the periodic table. 2) Determine the number of valence electrons. 3) This is how many electrons you will draw.

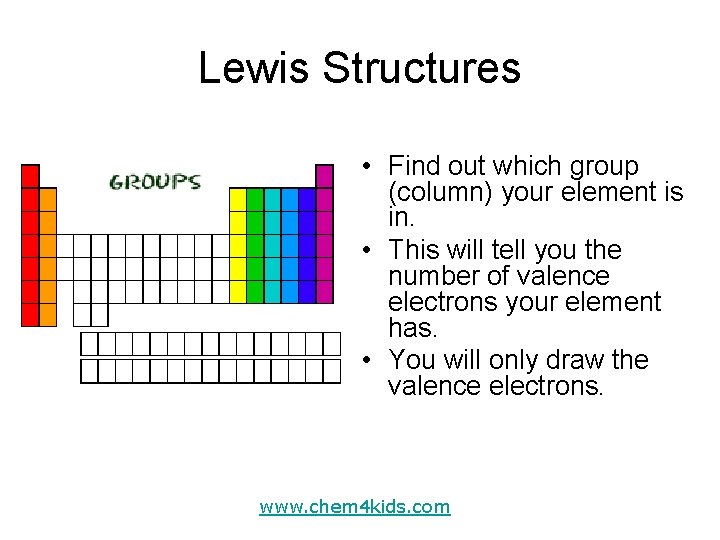
Lewis Structures • Find out which group (column) your element is in. • This will tell you the number of valence electrons your element has. • You will only draw the valence electrons. www. chem 4 kids. com

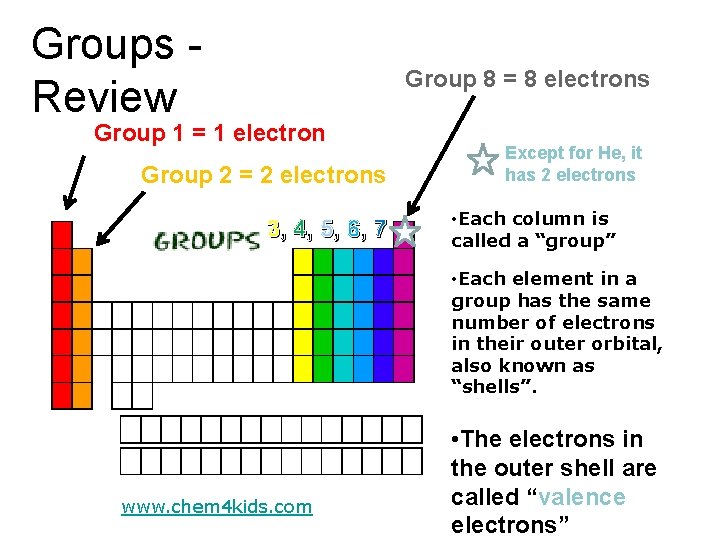
Groups Review Group 8 = 8 electrons Group 1 = 1 electron Group 2 = 2 electrons 3, 4, 5, 6, 7 Except for He, it has 2 electrons • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. www. chem 4 kids. com • The electrons in the outer shell are called “valence electrons”

Lewis Structures C 1) Write the element symbol. 2) Carbon is in the 4 th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, counterclockwise around the element symbol.

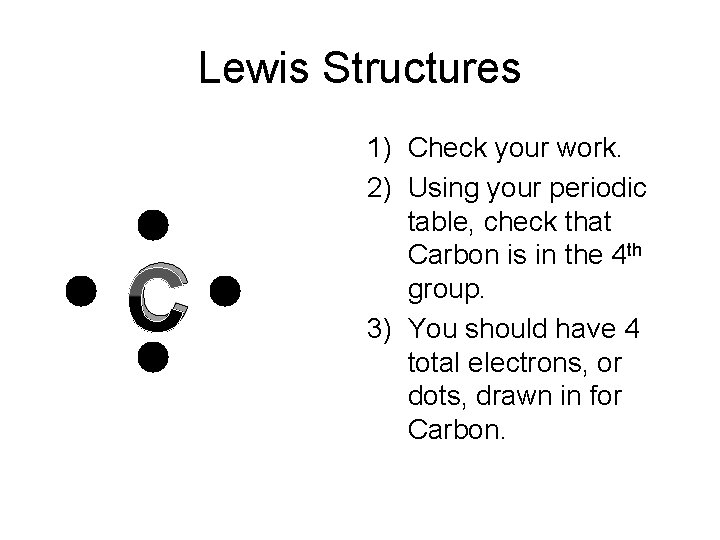
Lewis Structures C 1) Check your work. 2) Using your periodic table, check that Carbon is in the 4 th group. 3) You should have 4 total electrons, or dots, drawn in for Carbon.

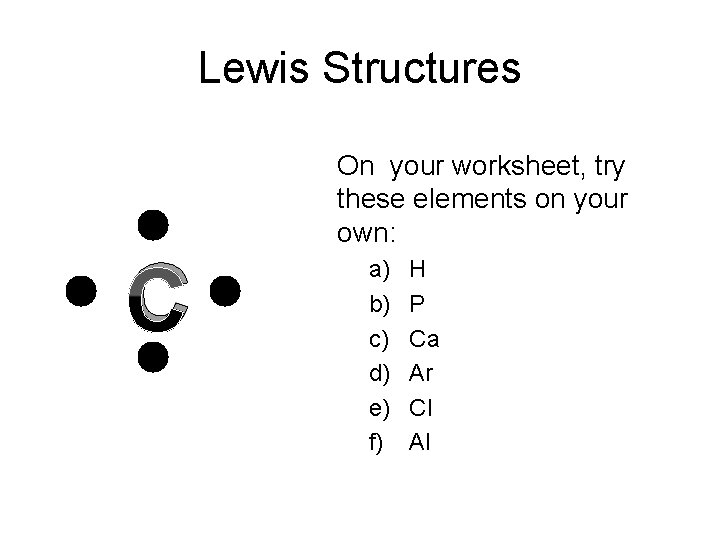
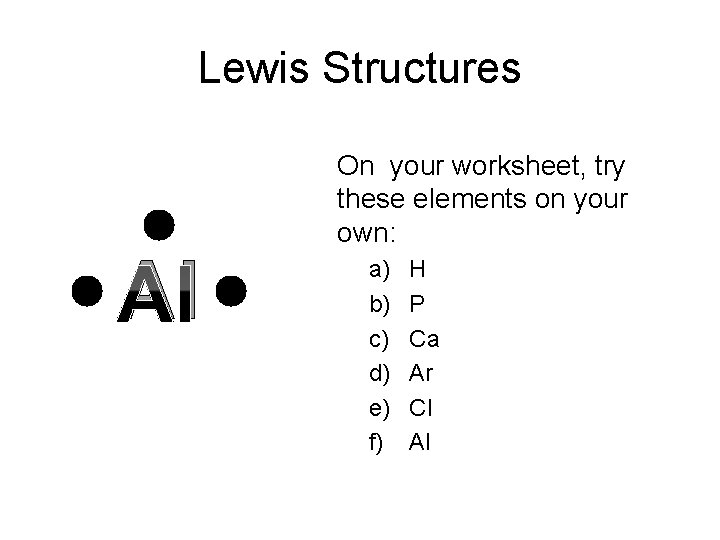
Lewis Structures C On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

Lewis Structures H On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

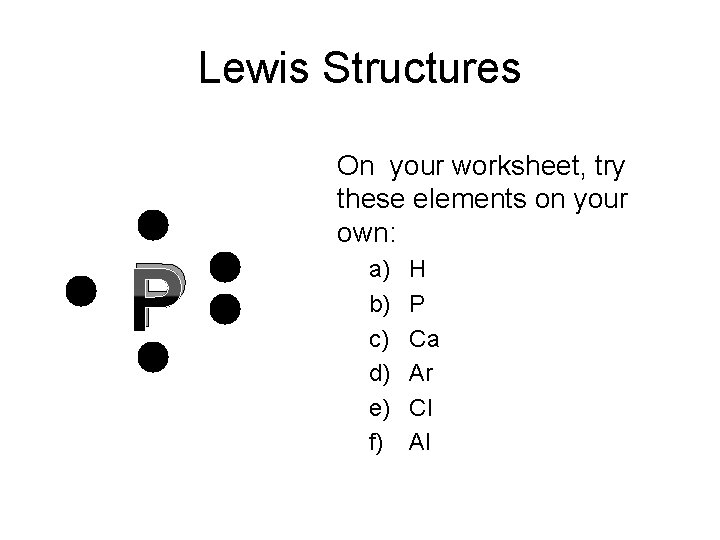
Lewis Structures P On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

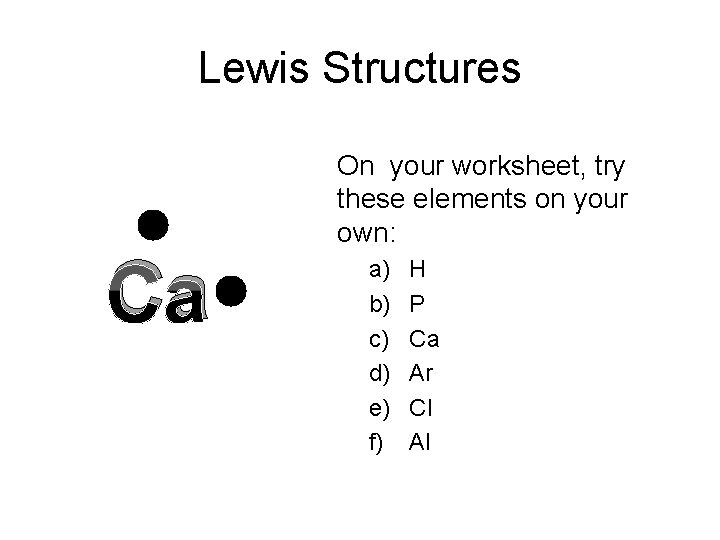
Lewis Structures Ca On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

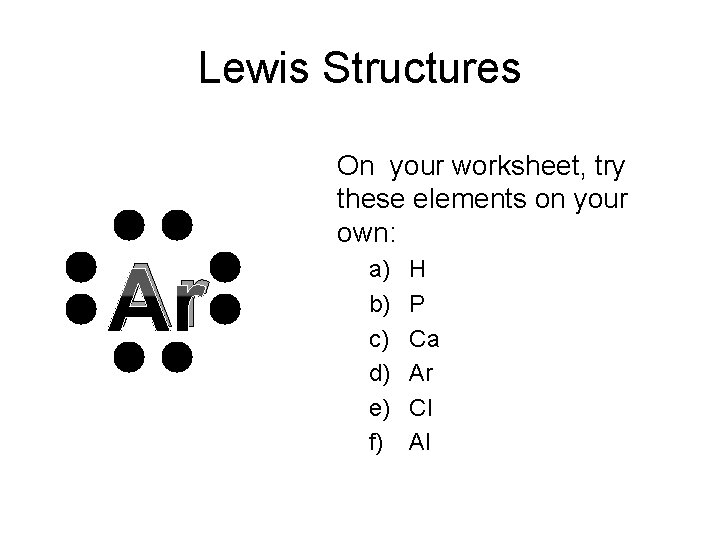
Lewis Structures Ar On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

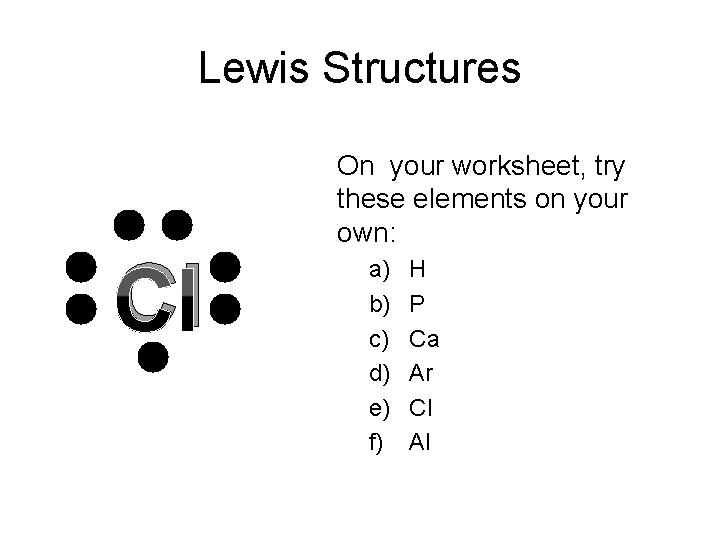
Lewis Structures Cl On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

Lewis Structures Al On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al


1. Which elements had complete outer shells? Give the name and symbol for each. ________________ _____ What do you notice about the location of these elements? 2. Which elements had only one valence electron? Give the name and symbol for each. ________________ _____ What do you notice about the location of these elements?

3. What do you notice about the number of valence electrons as you move from left to right across a row or period in the periodic table? Na Mg Al Si P S Cl Ar 4. What do you notice about the number of energy levels or shells as you move down a group or column in the periodic table? H Li Na

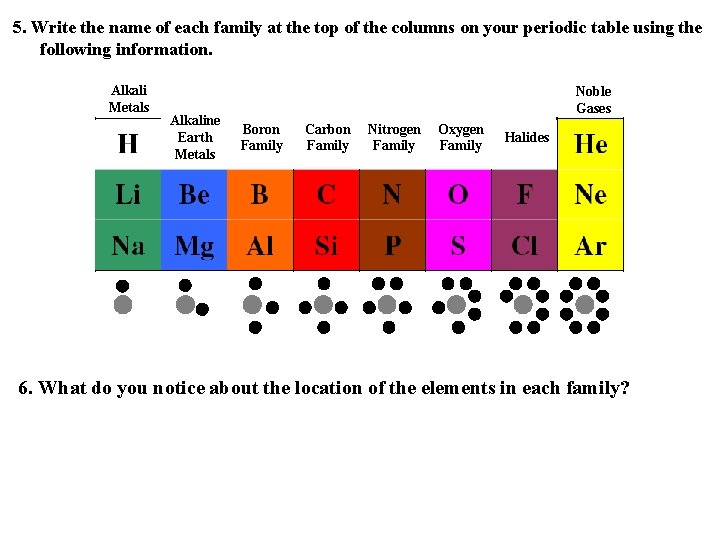
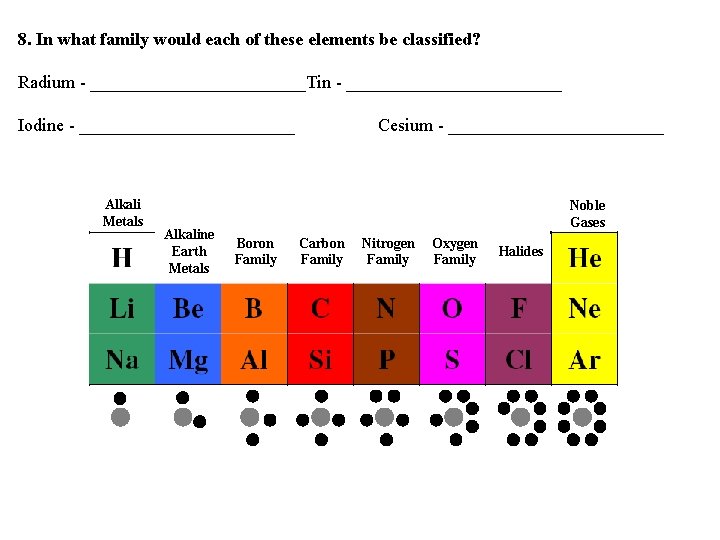
5. Write the name of each family at the top of the columns on your periodic table using the following information. Alkali Metals Alkaline Earth Metals Noble Gases Boron Family Carbon Family Nitrogen Family Oxygen Family Halides 6. What do you notice about the location of the elements in each family?

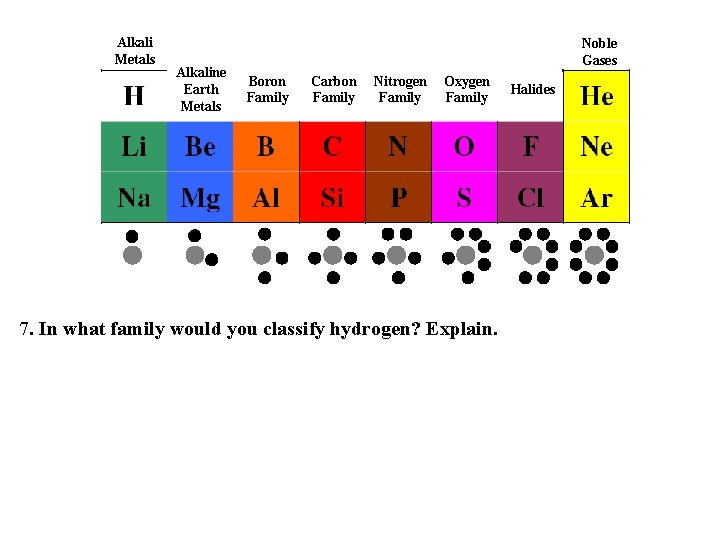
Alkali Metals Alkaline Earth Metals Noble Gases Boron Family Carbon Family Nitrogen Family Oxygen Family 7. In what family would you classify hydrogen? Explain. Halides

8. In what family would each of these elements be classified? Radium - ____________Tin - ____________ Iodine - ____________ Alkali Metals Alkaline Earth Metals Cesium - ____________ Noble Gases Boron Family Carbon Family Nitrogen Family Oxygen Family Halides

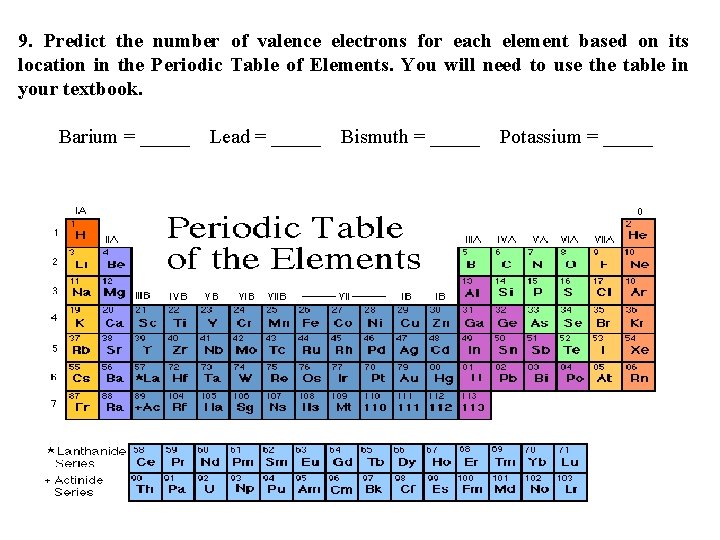
9. Predict the number of valence electrons for each element based on its location in the Periodic Table of Elements. You will need to use the table in your textbook. Barium = _____ Lead = _____ Bismuth = _____ Potassium = _____