HTA Activities in Australia and New Zealand Catherine


























- Slides: 31

HTA Activities in Australia and New Zealand Catherine Voutier Centre for Clinical Effectiveness

The overview An overview of Health Technology Assessment (HTA) in Australia and New Zealand. In this presentation, the organisations currently involved in HTA activities will be described, including a brief history, along with the various projects they are involved in.

Why do clinical librarians need to know about HTA? l l Many clinicians do not know the term or understand how it affects their decision-making HTA also covers interventions where there may be a low level of evidence HTA considers the context in which the intervention is to be utilised Has a great impact in the development of other activities

What is Health Technology Assessment (HTA)? l l l l Systematic evaluation by interdisciplinary groups Explicit analytical frameworks drawing from a variety of methods Properties, effects, and/or impacts of health care technology (drugs, surgical techniques, tools etc) Direct, intended consequences of technologies Indirect, unintended consequences Its main purpose is to inform technology-related policymaking in health care. HTA 101 Glossary National Information Center on Health Services Research and Health Care Technology (NICHSR), NLM. http: //www. nlm. nih. gov/nichsr/hta 101/ta 101014. html Accessed 23. 06

Evolution of HTA in Australasia l l l l Pharmaceutical Benefits Advisory Committee (PBAC) – established 1954 National Health Technology Advisory Panel (NHTAP) – early 1980’s Australian Health Technology Assessment Committee (AHTAC) – 1986 (Pharmaceutical Management Agency (PHARMAC) – 1993 (fully estab. 2000) NZHTA established - 1997 Medical Services Advisory Committee (MSAC) - 1997/98 Other HTA groups begin forming

NZHTA – New Zealand Health Technology Assessment l l Established in 1997 by the New Zealand Ministry of Health to assist NZ Health and Disability Services Produces a range of assessments such as systematic reviews, technical briefs, and horizon scanning for Health. PACT. Conducts assessments for MSAC, the Accident Compensation Corporation, NZ Guidelines Group and others Involved in regional activities: Clinical Decision Support Unit, Clinical Practice Committee Employs a medical librarian C


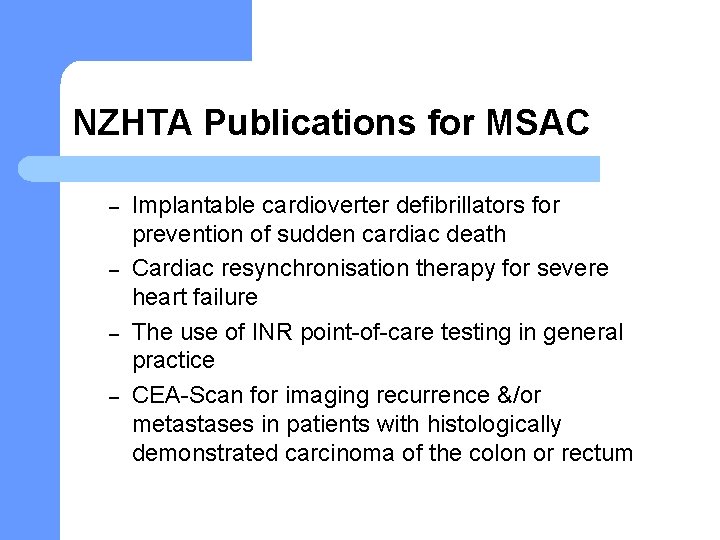
NZHTA Publications for MSAC – – Implantable cardioverter defibrillators for prevention of sudden cardiac death Cardiac resynchronisation therapy for severe heart failure The use of INR point-of-care testing in general practice CEA-Scan for imaging recurrence &/or metastases in patients with histologically demonstrated carcinoma of the colon or rectum

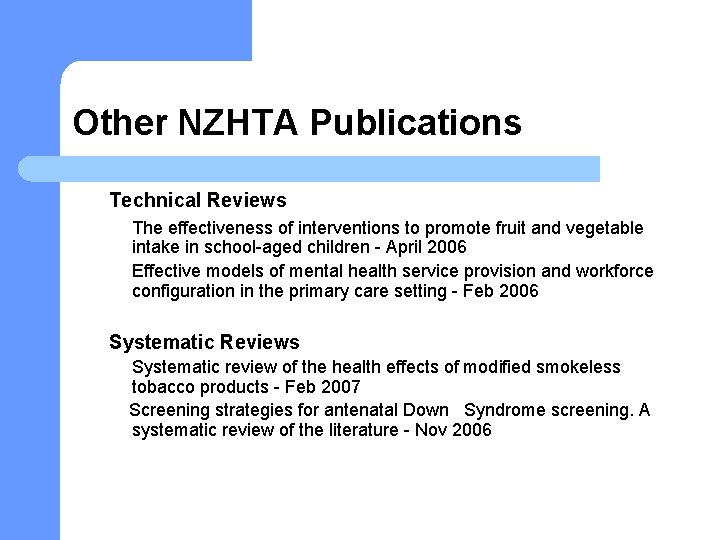
Other NZHTA Publications Technical Reviews The effectiveness of interventions to promote fruit and vegetable intake in school-aged children - April 2006 Effective models of mental health service provision and workforce configuration in the primary care setting - Feb 2006 Systematic Reviews Systematic review of the health effects of modified smokeless tobacco products - Feb 2007 Screening strategies for antenatal Down Syndrome screening. A systematic review of the literature - Nov 2006

MSAC – Medical Services Advisory Committee l l Established in 1997 -98, the Committee advises the Minster of Health and Ageing of the cost-effectiveness, safety and effectiveness of new procedures and technologies presented for Medicare funding Compliments the activities of: – – – Medicare Benefits Consultative Committee Pathology Services Table Committee Consultative Committee on Diagnostic Imaging

MSAC – Terms of Reference l Advises the Minister on – – – l Strength of evidence of new and emerging technologies/procedures and to what extent public funding should be supported What technologies/procedures should be given interim funding in order to collect enough evidence to determine effectiveness Report references of new and emerging technologies and procedures Undertake HTAs referred by the Australian Health Ministers’ Advisory Council (AHMAC) and report to same


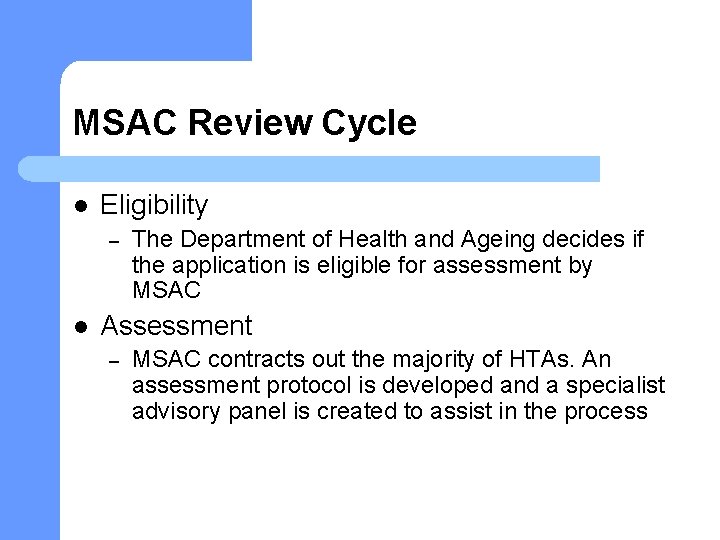
MSAC Review Cycle l Eligibility – l The Department of Health and Ageing decides if the application is eligible for assessment by MSAC Assessment – MSAC contracts out the majority of HTAs. An assessment protocol is developed and a specialist advisory panel is created to assist in the process

MSAC Review Cycle l Formulation of Advice to the Minister – l Decision – l MSAC presents the assessment, Department opinion and MSAC recommendations to the Minister The Minister, with the material presented, either rejects or accepts the recommendations. To date, all recommendations have been endorsed Implementation – The appropriate committee decides on funding levels

MSAC - Publications l Applications (under review) – – – l Autologous cartilage implantation (ACI) Hepatitis B DNA testing Lumber non-fusion posterior stabilisation devices Completed Assessments – – Advanced breast biopsy instrumentation Visual electrodiagnosis

Health. PACT – MSAC subcommittee l Health. PACT – Health Policy Advisory Committee on Technology – – Terms of reference: to assist the introduction of new and emerging medical technologies into the public sector, provide a forum for national and international discussion, inform AHMAC, and supported by the National Horizon Scanning Unit. Comprises members from MSAC, ASERNIP-S, Do. HA and the New Zealand Ministry of Health Is a key operator of the Australia and New Zealand Horizon Scanning Network (ANZHSN), an initiative of MSAC, the Australian Government Department of Health and Ageing and all Australian Health Ministers' Advisory Council (AHMAC) jurisdictions.

PBAC- Pharmaceutical Benefits Advisory Committee l l l Established in 1954, PBAC advises the Minister of Health and Ageing on which pharmaceuticals and other products should attract pharmaceutical benefits PBAC recommendations assess the effectiveness and cost of proposed benefits as well as possible restrictions and supply issues PBAC recommendations (medicines only) are made available as Public Summary Documents (PSDs) on the Department of Health and Ageing website

IMS Pricing & Market Access (P&MA) l Established in 1995 and part of IMS Health from 2005, P&MA (formerly known as M-TAG) provides services to PBAC, MSAC and various other bodies both local and international. – l l Staff serve on an evaluation panel similar to MSAC for the Commonwealth Government Main role is to present reimbursement applications to PBAC and to date, has supplied over 200 applications. Employs a medical librarian C


HTAnalysts - Sydney l l l Similar services to IMS P&MA Public and private clients Specialises in – – l l PBAC reimbursement applications MSAC applications for medical services, devices, procedures and diagnostic tests Conducts clinical, basic and applied research http: //www. htanalysts. com/index. htm


ASERNIP-S l Australian Safety and Efficacy of New Interventional Procedures – Surgical – l Supported by Australasian College of Surgeons Assesses new and emerging surgical technologies & techniques via – – Horizon Scanning Systematic Reviews of the literature Clinical and research audits/trials Clinical guidelines


ASERNIP-S Publications l Systematic reviews: – – l Surgical simulation: a systematic review (update), August 2006 Paravertebral blocks for anaesthesia and analgesia: A Systematic Review, January 2006 NETS: – – Artificial cervical disc replacement (update) Injection snoreplasty

AHTA - Adelaide Health Technology Assessment l l l Established in 2001, AHTA provides evidence-based assessments on a variety of technologies, services and procedures Specialised systematic literature reviews for PBAC, MSAC, NICS, NHMRC and others Guideline development Horizon scanning Information needs supported by the University Library


AHTA Publications l Systematic reviews – l Critical Appraisal – – l Vertebroplasty August 2005 NHMRC review of “Clinical practice guidelines for stroke recovery rehabilitation and recovery (National Stroke Foundation)”. June 2005. NHMRC GAR consultant review of “Management of diabetic retinopathy (Australian Diabetes Society)”. August 2003 – 2005 Horizon Scanning – – Microvolt T-wave alternans for the determination of patients likely to benefit from ICD therapy (Feb 2007) Available from the Australian and New Zealand Horizon Scanning Network, http: //www. horizonscanning. gov. au/

CCE – Centre for Clinical Effectiveness l CCE and the Australasian Cochrane Centre underwent organisational restructuring in early 2005. This resulted in the CCE HTA team moving to Cochrane. CCE HTA reports can still be found on the MSAC Publications web page.

About me l Research Officer (Information) at CCE: – – – l Searching/information retrieval Assists staff in their research projects (this may include appraising guidelines, copy editing, investigating copyright issues etc) Collaborates with other librarians on various projects Questions? Please come to see me afterwards at break. I have a hearing impairment so I won’t be taking questions from the audience.

Thank you l Acknowledgements Pip Dival and Sarah Sutton for inviting me to join you here today, Becky Skidmore, Susan Bidwell (HTA librarians) and Janet Hiller (AHTA) – for their advice and encouragement.

Websites l NZHTA – l MSAC – l http: //www. surgeons. org/Content/Navigation. Menu/Research /ASERNIPS/default. htm INAHTA – l http: //www. health. adelaide. edu. au/publichealth/consult/healt h_technol_assess. html ASERNIP-S – l http: //www. msac. gov. au AHTA – l http: //nzhta. chmeds. ac. nz/index. htm http: //www. inhata. org IMS P&MA – http: //www. m-tag. net
 Lesson 1 physical geography of australia and new zealand
Lesson 1 physical geography of australia and new zealand Internal medicine society of australia and new zealand
Internal medicine society of australia and new zealand Australian vs new zealand accent
Australian vs new zealand accent New zealand attitudes and values study
New zealand attitudes and values study Hundertwasser toilets nz
Hundertwasser toilets nz New zealand official languages english
New zealand official languages english New zealand disability strategy
New zealand disability strategy Wong wai yin
Wong wai yin New zealand alpha lipid colostrum
New zealand alpha lipid colostrum Why is new zealand a biodiversity hotspot
Why is new zealand a biodiversity hotspot Aotearoa the land of the long white cloud
Aotearoa the land of the long white cloud Capital of new zealand
Capital of new zealand Natives of new zealand
Natives of new zealand Urfolk new zealand
Urfolk new zealand Slidetodoc.com
Slidetodoc.com New zealand health strategy 2016
New zealand health strategy 2016 New zealand holiday 2016
New zealand holiday 2016 Nonrhotic
Nonrhotic New zealand nurse practitioner
New zealand nurse practitioner Saurabh paranjape
Saurabh paranjape Measurement standards laboratory
Measurement standards laboratory Inner circle
Inner circle Paragraph about new zealand
Paragraph about new zealand New zealand
New zealand New zealand population
New zealand population Kumara new zealand food
Kumara new zealand food Jelaskan sistem periodik menurut oktaf new zealand
Jelaskan sistem periodik menurut oktaf new zealand Acvm new zealand
Acvm new zealand What is oceania
What is oceania Mori new zealand
Mori new zealand Zland
Zland Acn broadband
Acn broadband