HSPS 1 0 Level 3 The Bohr Model

HS-PS 1 -0 Level 3
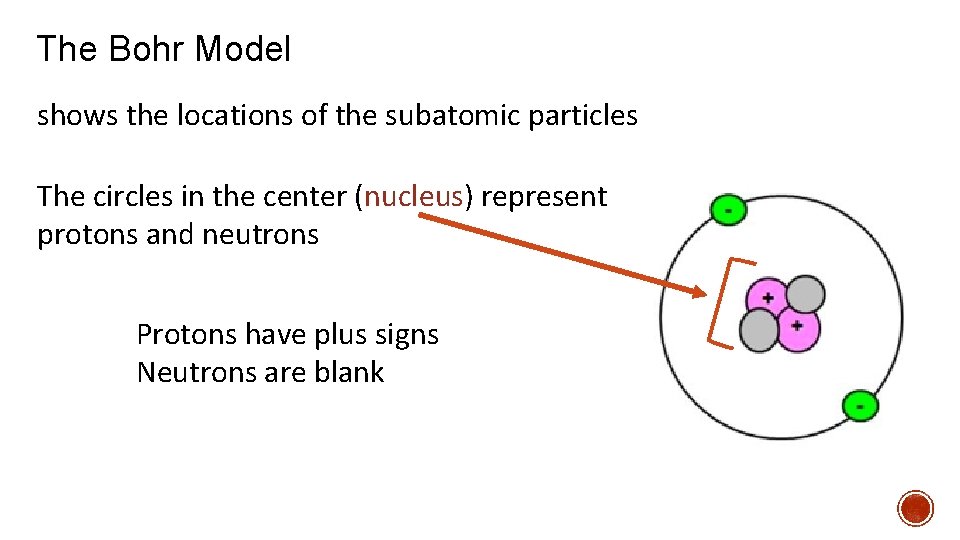
The Bohr Model shows the locations of the subatomic particles The circles in the center (nucleus) represent protons and neutrons Protons have plus signs Neutrons are blank
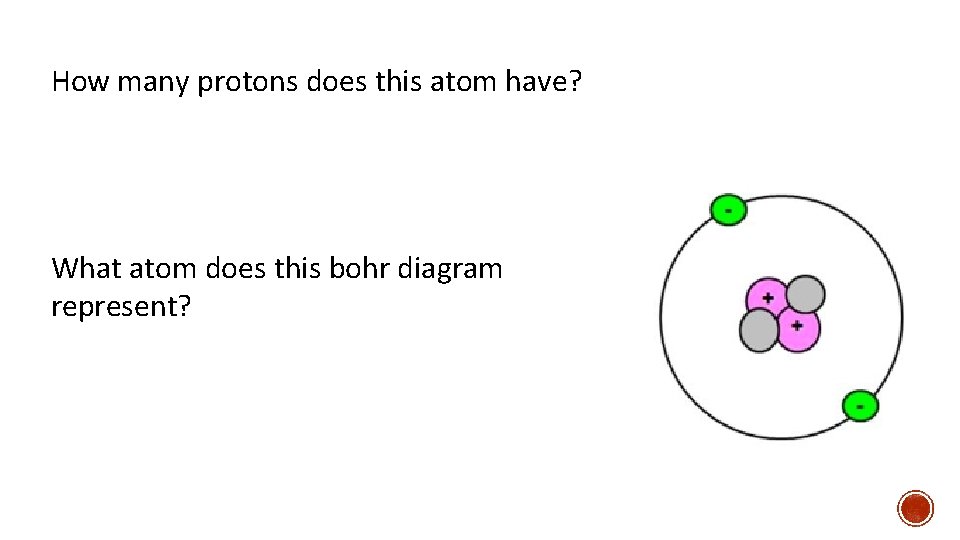
How many protons does this atom have? What atom does this bohr diagram represent?
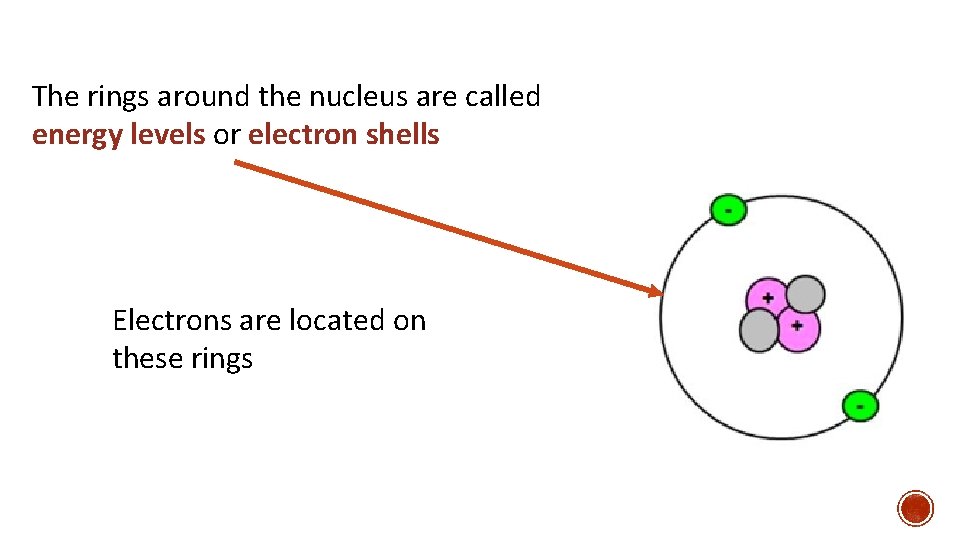
The rings around the nucleus are called energy levels or electron shells Electrons are located on these rings
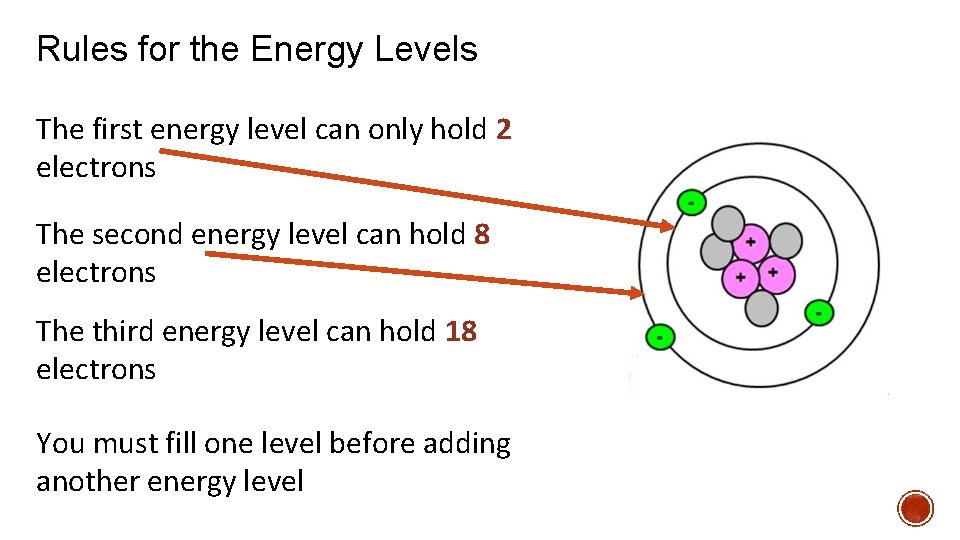
Rules for the Energy Levels The first energy level can only hold 2 electrons The second energy level can hold 8 electrons The third energy level can hold 18 electrons You must fill one level before adding another energy level
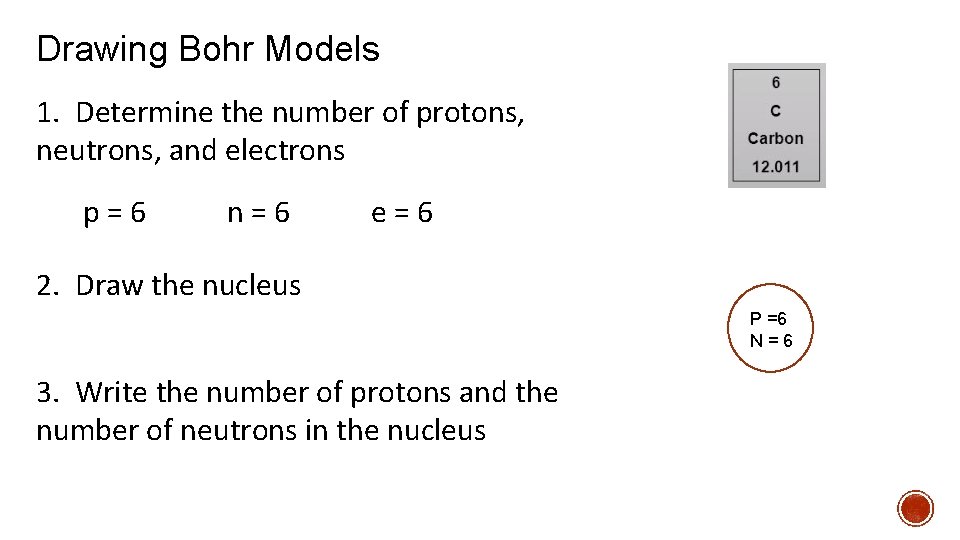
Drawing Bohr Models 1. Determine the number of protons, neutrons, and electrons p=6 n=6 e=6 2. Draw the nucleus P =6 N=6 3. Write the number of protons and the number of neutrons in the nucleus
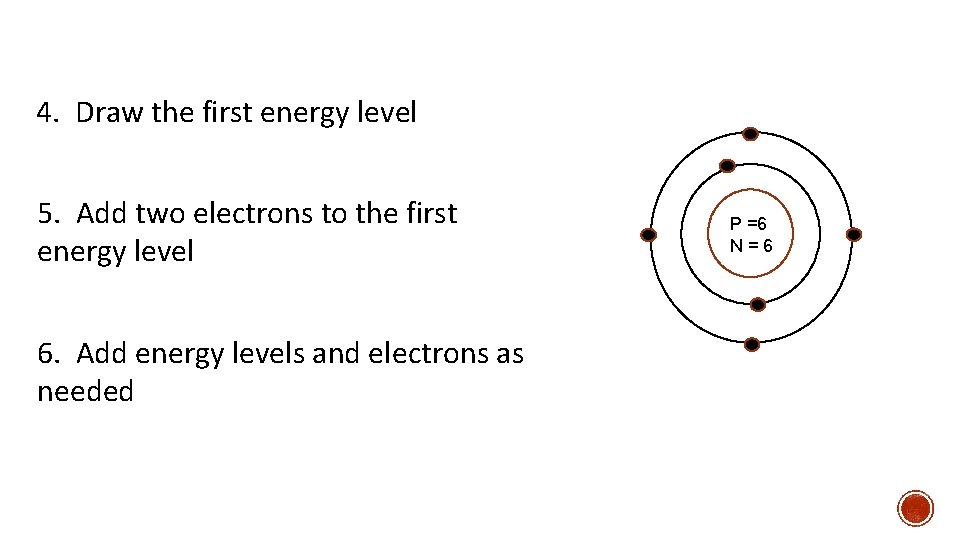
4. Draw the first energy level 5. Add two electrons to the first energy level 6. Add energy levels and electrons as needed P =6 N=6
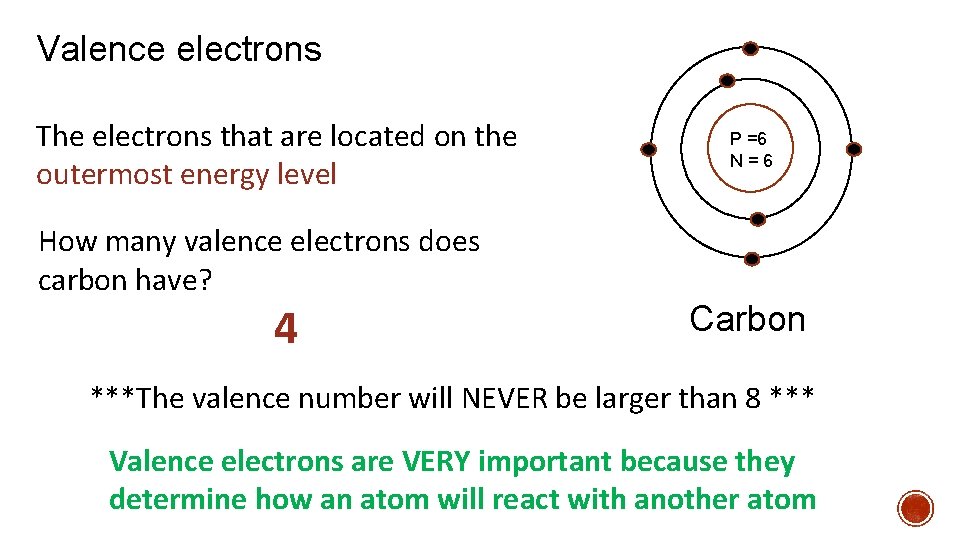
Valence electrons The electrons that are located on the outermost energy level P =6 N=6 How many valence electrons does carbon have? 4 Carbon ***The valence number will NEVER be larger than 8 *** Valence electrons are VERY important because they determine how an atom will react with another atom
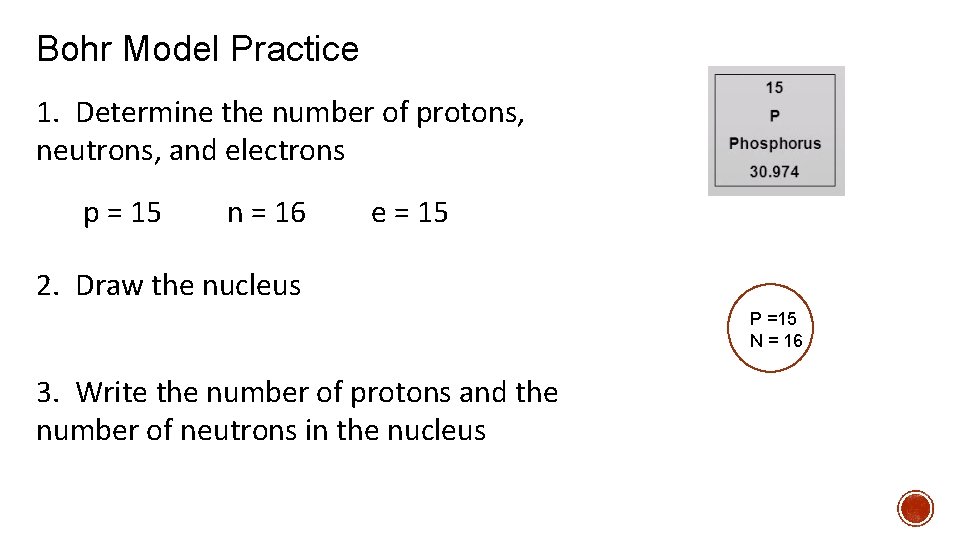
Bohr Model Practice 1. Determine the number of protons, neutrons, and electrons p = 15 n = 16 e = 15 2. Draw the nucleus P =15 N = 16 3. Write the number of protons and the number of neutrons in the nucleus
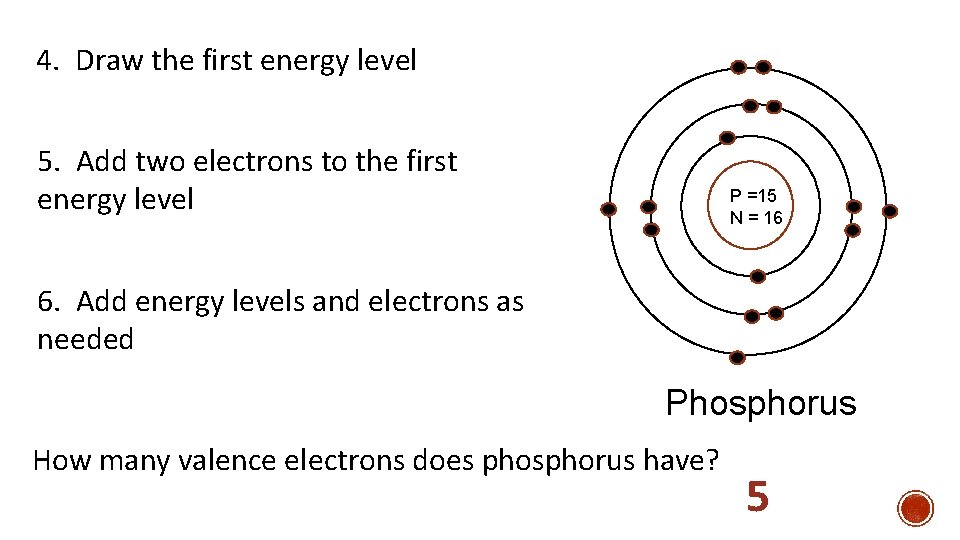
4. Draw the first energy level 5. Add two electrons to the first energy level P =15 N = 16 6. Add energy levels and electrons as needed Phosphorus How many valence electrons does phosphorus have? 5
- Slides: 10