HPLCHigh performance liquid chromatography 2 Mobile Phase Stationary

HPLC(High performance liquid chromatography) ㈜ 우존 신명선
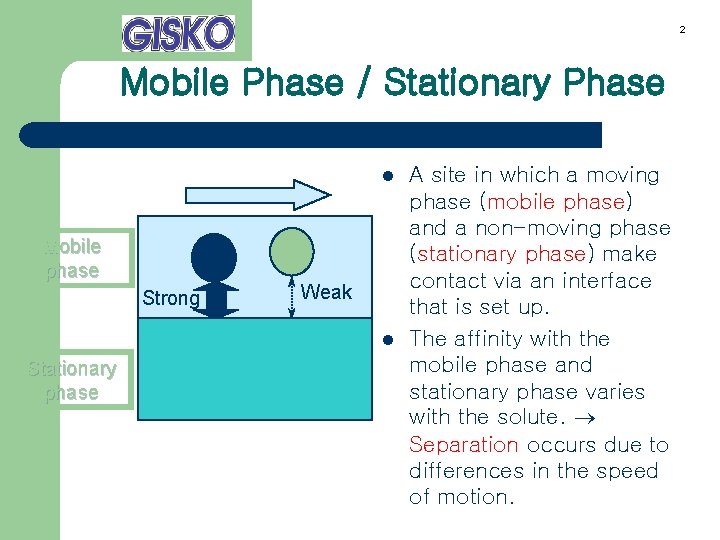
2 Mobile Phase / Stationary Phase l Mobile phase Strong Weak l Stationary phase A site in which a moving phase (mobile phase) and a non-moving phase (stationary phase) make contact via an interface that is set up. The affinity with the mobile phase and stationary phase varies with the solute. Separation occurs due to differences in the speed of motion.

Liquid Chromatography l Chromatography in which the mobile phase is a liquid. – l l The liquid used as the mobile phase is called the “eluent”. The stationary phase is usually a solid or a liquid. In general, it is possible to analyze any substance that can be stably dissolved in the mobile phase. 3
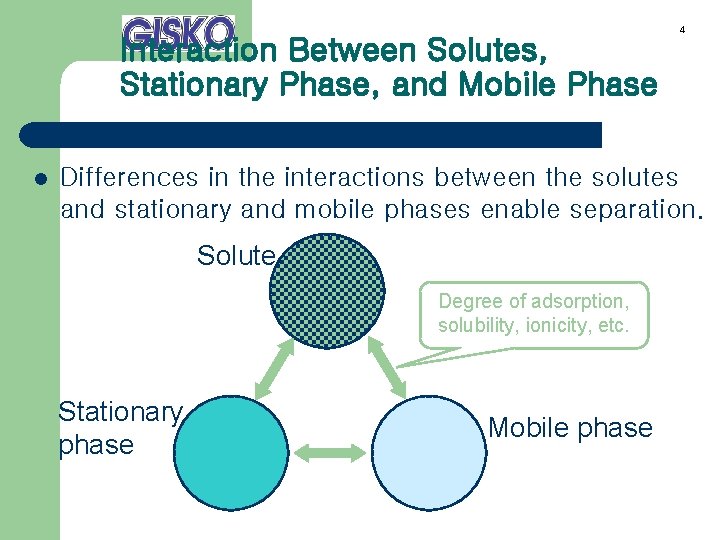
Interaction Between Solutes, Stationary Phase, and Mobile Phase l 4 Differences in the interactions between the solutes and stationary and mobile phases enable separation. Solute Degree of adsorption, solubility, ionicity, etc. Stationary phase Mobile phase
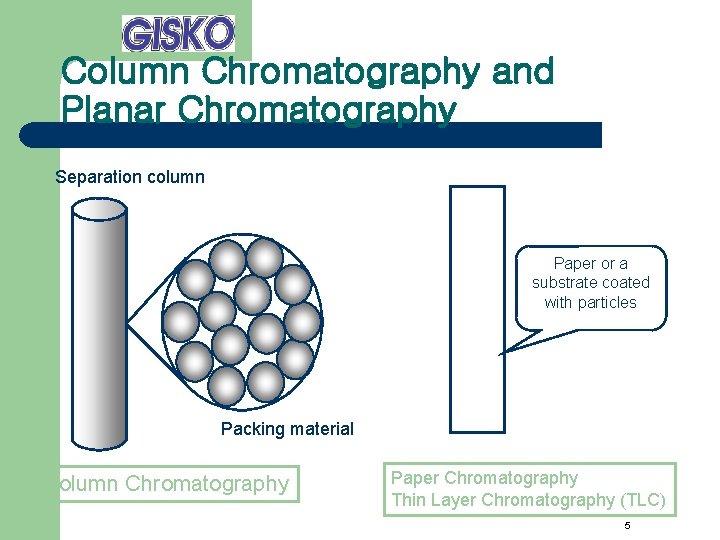
Column Chromatography and Planar Chromatography Separation column Paper or a substrate coated with particles Packing material Column Chromatography Paper Chromatography Thin Layer Chromatography (TLC) 5
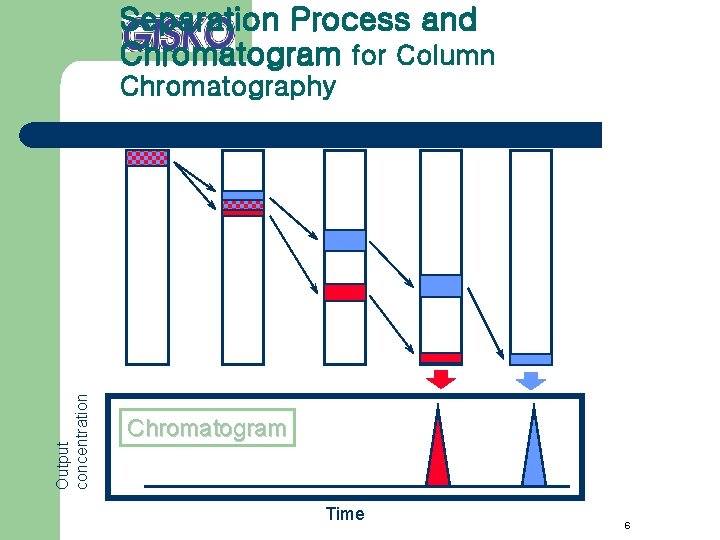
Separation Process and Chromatogram for Column Output concentration Chromatography Chromatogram Time 6
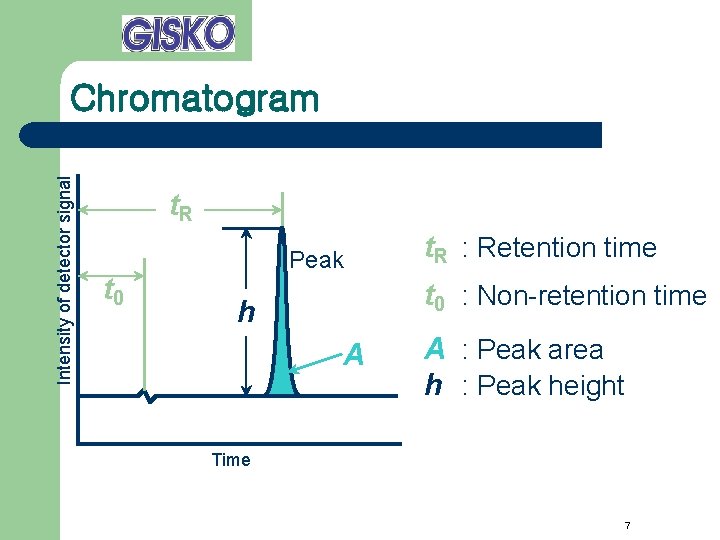
Intensity of detector signal Chromatogram t. R t 0 Peak t. R : Retention time t 0 : Non-retention time h A A : Peak area h : Peak height Time 7
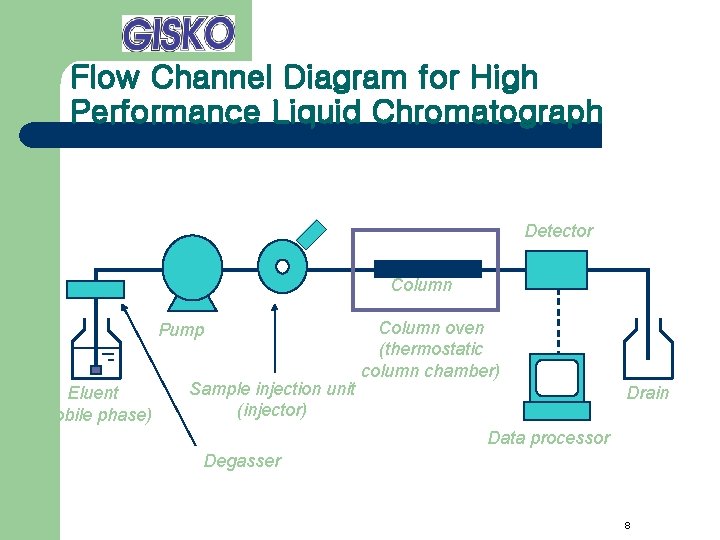
Flow Channel Diagram for High Performance Liquid Chromatograph Detector Column Pump Eluent (mobile phase) Sample injection unit (injector) Column oven (thermostatic column chamber) Drain Data processor Degasser 8

HPLC Hardware: Part 1 Solvent Delivery System, Degasser, Sample Injection Unit, Column Oven

Solvent Delivery Pump l Performance Requirements – – – Capacity to withstand high load pressures. Pulsations that accompany pressure fluctuations are small. Flow rate does not fluctuate. Solvent replacement is easy. The flow rate setting range is wide and the flow rate is accurate. 10
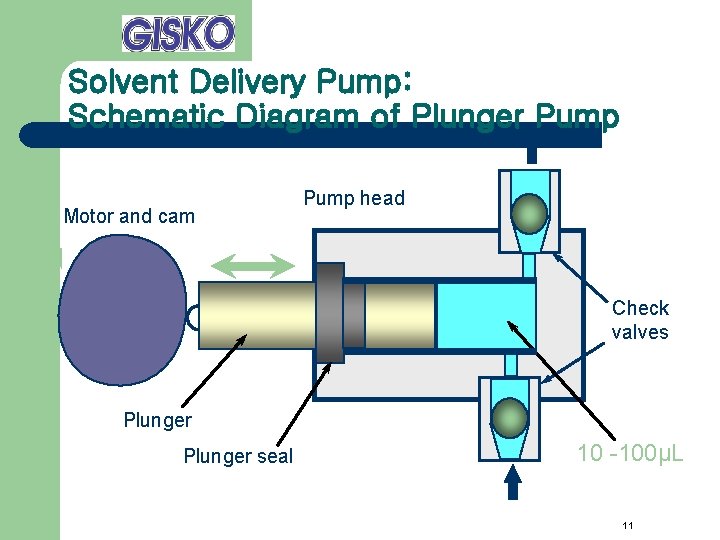
Solvent Delivery Pump: Schematic Diagram of Plunger Pump Motor and cam Pump head Check valves Plunger seal 10 -100µL 11
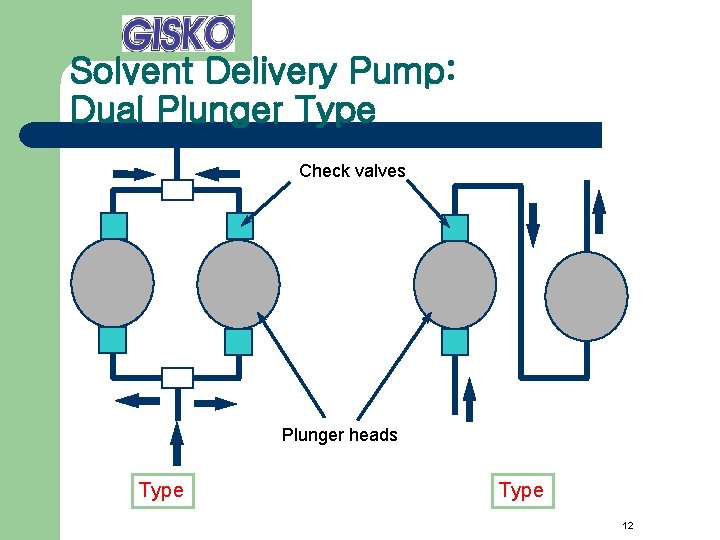
Solvent Delivery Pump: Dual Plunger Type Check valves Plunger heads Type 12
Gradient System l Isocratic system – l Constant eluent composition Gradient system – Varying eluent composition l l HPGE (High Pressure Gradient) LPGE (Low Pressure Gradient) 13
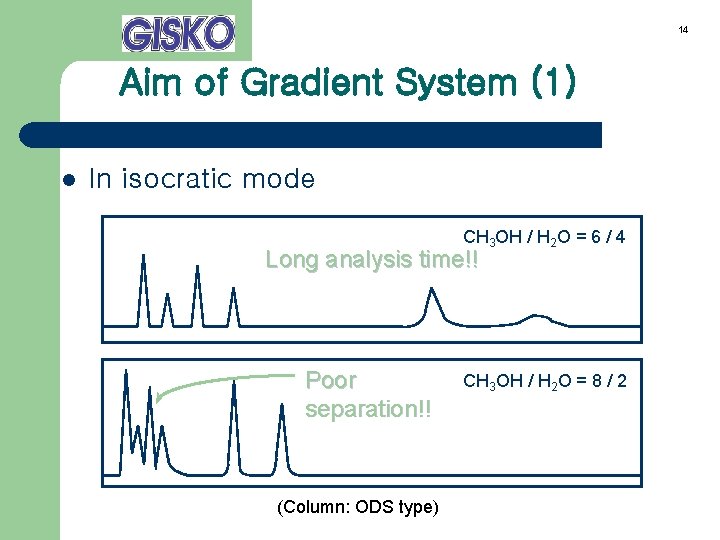
14 Aim of Gradient System (1) l In isocratic mode CH 3 OH / H 2 O = 6 / 4 Long analysis time!! Poor separation!! (Column: ODS type) CH 3 OH / H 2 O = 8 / 2
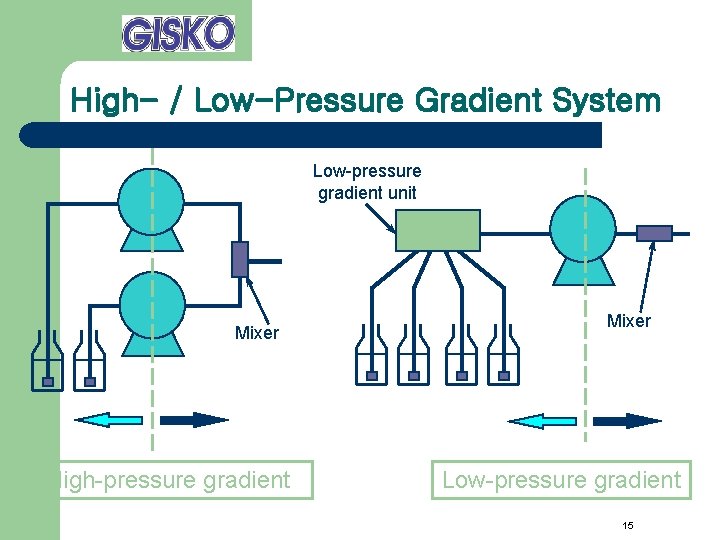
High- / Low-Pressure Gradient System Low-pressure gradient unit Mixer High-pressure gradient Mixer Low-pressure gradient 15
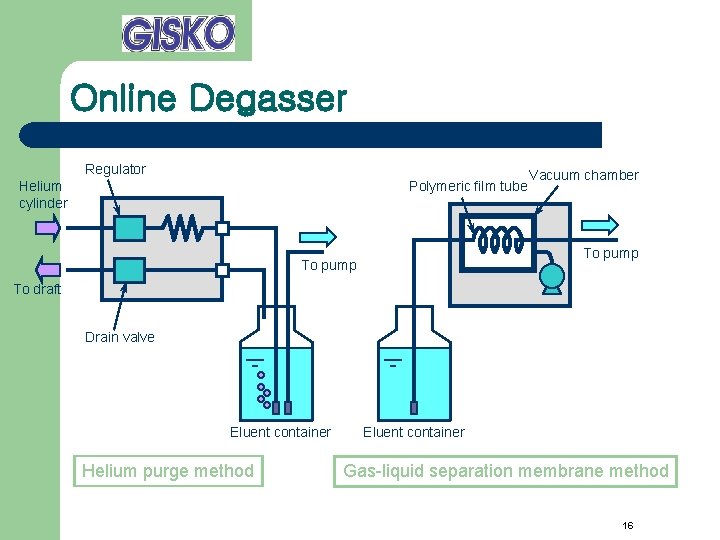
Online Degasser Regulator Helium cylinder Polymeric film tube Vacuum chamber To pump To draft Drain valve Eluent container Helium purge method Eluent container Gas-liquid separation membrane method 16
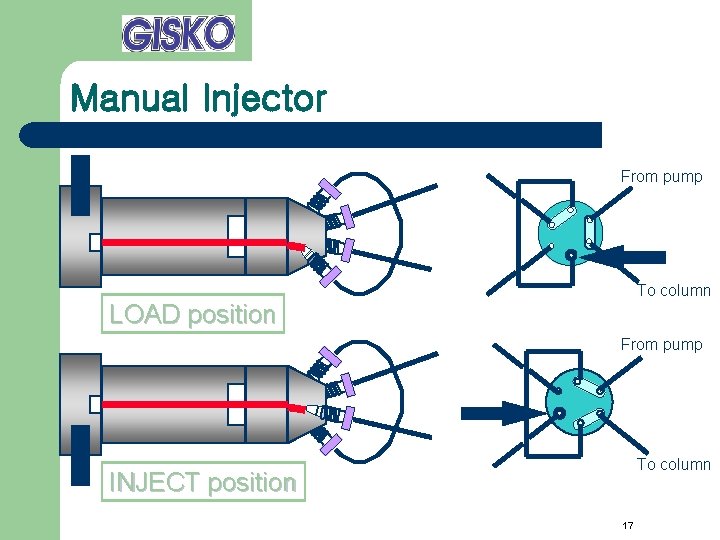
Manual Injector From pump To column LOAD position From pump To column INJECT position 17
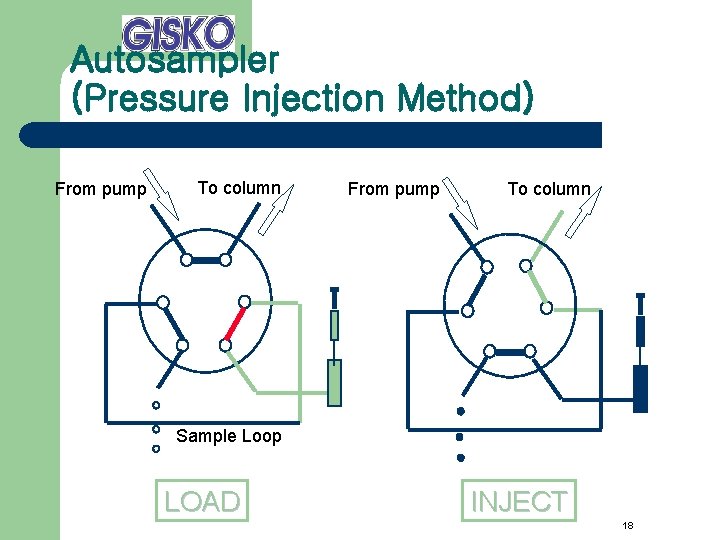
Autosampler (Pressure Injection Method) From pump To column Sample Loop LOAD INJECT 18
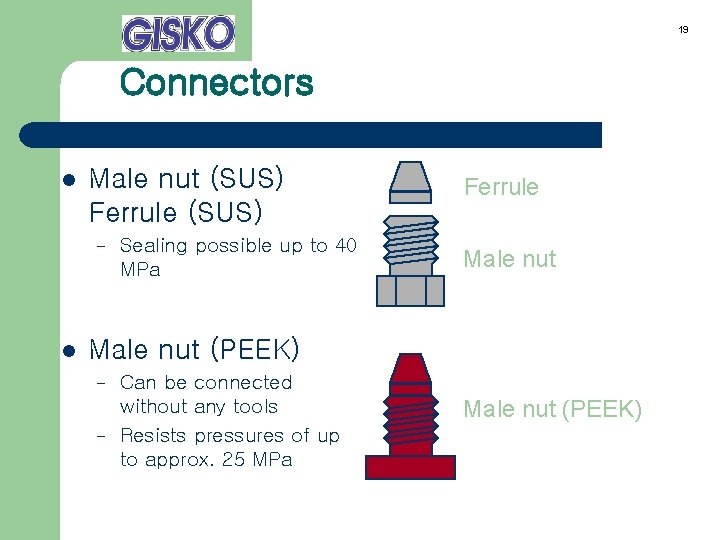
19 Connectors l Male nut (SUS) Ferrule (SUS) – l Sealing possible up to 40 MPa Ferrule Male nut (PEEK) – – Can be connected without any tools Resists pressures of up to approx. 25 MPa Male nut (PEEK)
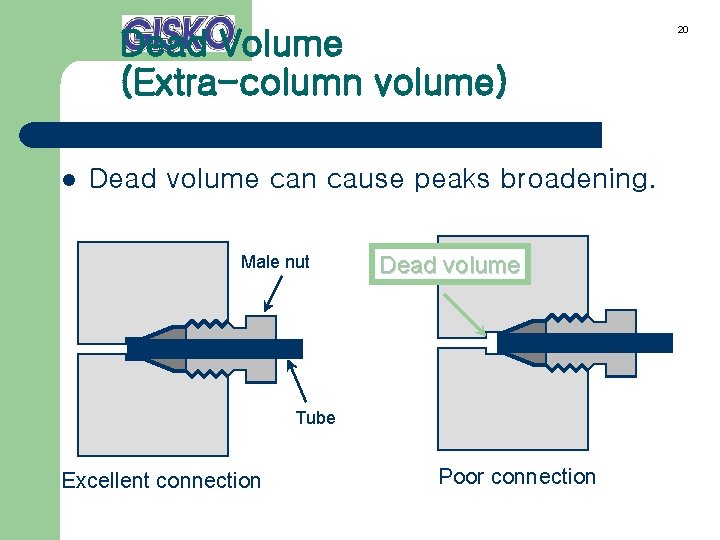
Dead Volume (Extra-column volume) l Dead volume can cause peaks broadening. Male nut Dead volume Tube Excellent connection Poor connection 20

Tubing l Material – – – Stainless steel (SUS) PEEK (polyether ketone) Fluororesin l O. D. (outer diameter) – l 1. 6 mm I. D. (inner diameter) – – 0. 1 0. 3 0. 5 0. 8 mm mm etc. 21

Mobile Phase l Water – – “Ultrapure water” can be used with confidence. Commercial “distilled water for HPLC” is also acceptable. l Organic Solvent – – – HPLC-grade solvent can be used with confidence. Special-grade solvent is acceptable depending on the detection conditions. Care is required regarding solvents containing stabilizers (e. g. , tetrahydrofuran and chloroform) 22
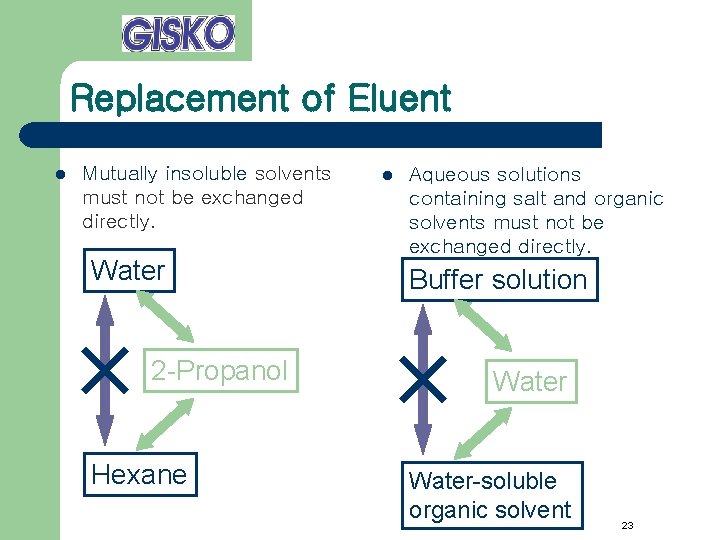
Replacement of Eluent l Mutually insoluble solvents must not be exchanged directly. Water 2 -Propanol Hexane l Aqueous solutions containing salt and organic solvents must not be exchanged directly. Buffer solution Water-soluble organic solvent 23
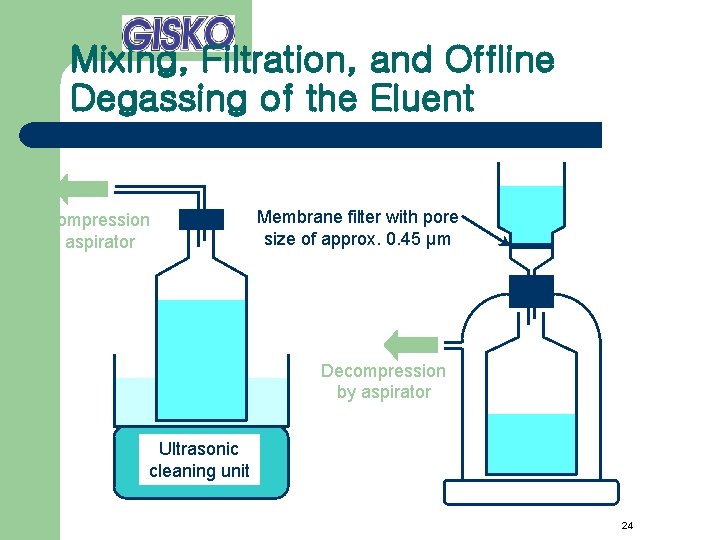
Mixing, Filtration, and Offline Degassing of the Eluent Decompression by aspirator Membrane filter with pore size of approx. 0. 45 µm Decompression by aspirator Ultrasonic cleaning unit 24
Reversed Phase Chromatography Part 1 Basic Principles
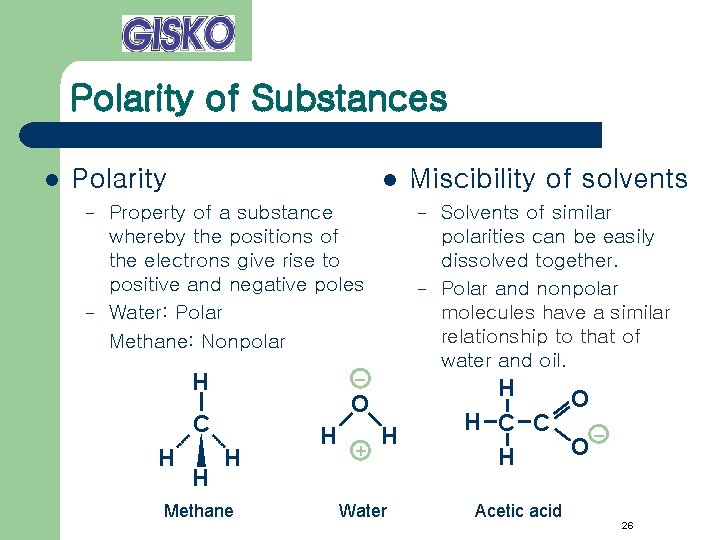
Polarity of Substances l Polarity – – l Property of a substance whereby the positions of the electrons give rise to positive and negative poles Water: Polar Methane: Nonpolar H H H – – – O C H Methane H + Miscibility of solvents H Water Solvents of similar polarities can be easily dissolved together. Polar and nonpolar molecules have a similar relationship to that of water and oil. H O H C C – O H Acetic acid 26
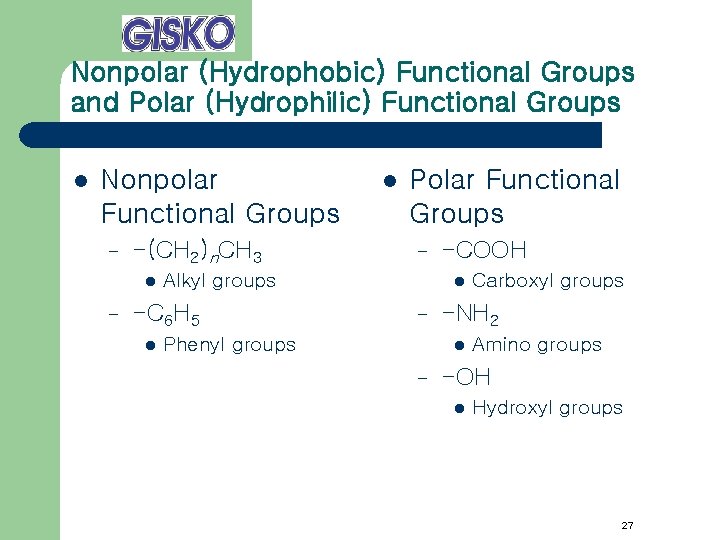
Nonpolar (Hydrophobic) Functional Groups and Polar (Hydrophilic) Functional Groups l Nonpolar Functional Groups – -(CH 2)n. CH 3 l – Polar Functional Groups – Alkyl groups -C 6 H 5 l l -COOH l – Phenyl groups -NH 2 l – Carboxyl groups Amino groups -OH l Hydroxyl groups 27
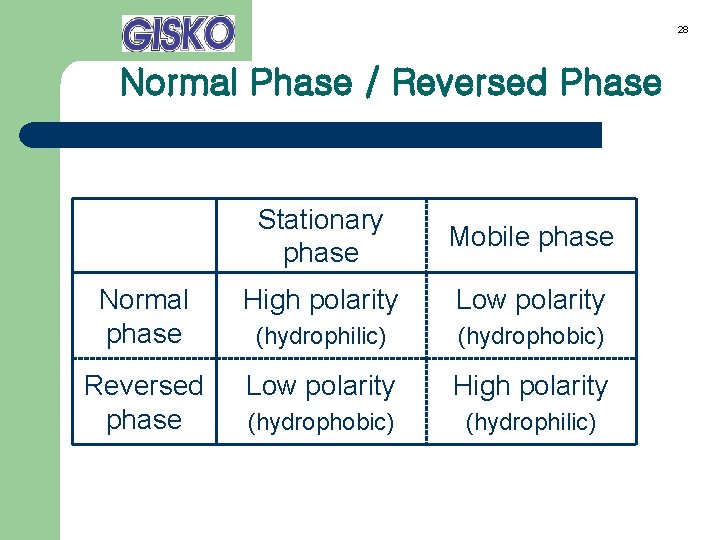
28 Normal Phase / Reversed Phase Stationary phase Mobile phase Normal phase High polarity Low polarity (hydrophilic) (hydrophobic) Reversed phase Low polarity High polarity (hydrophobic) (hydrophilic)

Reversed Phase Chromatography l Stationary phase: Low polarity – l Octadecyl group-bonded silical gel (ODS) Mobile phase: High polarity – – Water, methanol, acetonitrile Salt is sometimes added. 29
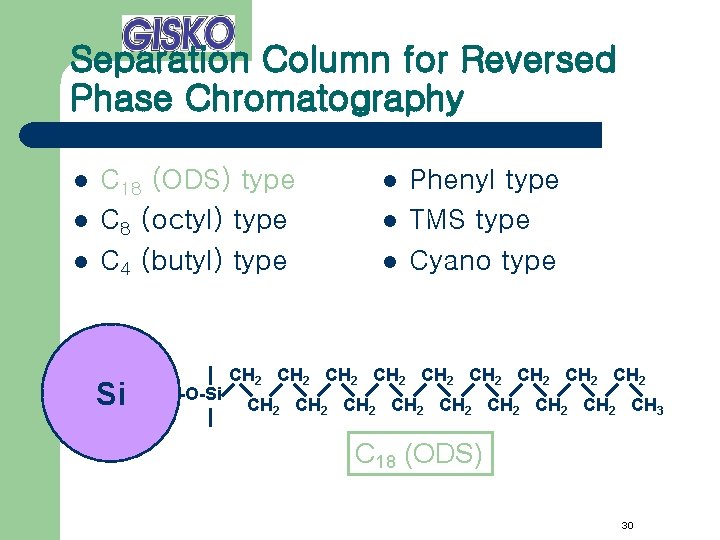
Separation Column for Reversed Phase Chromatography l l l C 18 (ODS) type C 8 (octyl) type C 4 (butyl) type Si -O-Si l l l Phenyl type TMS type Cyano type CH 2 CH 2 CH 2 CH 2 CH 2 CH 3 C 18 (ODS) 30
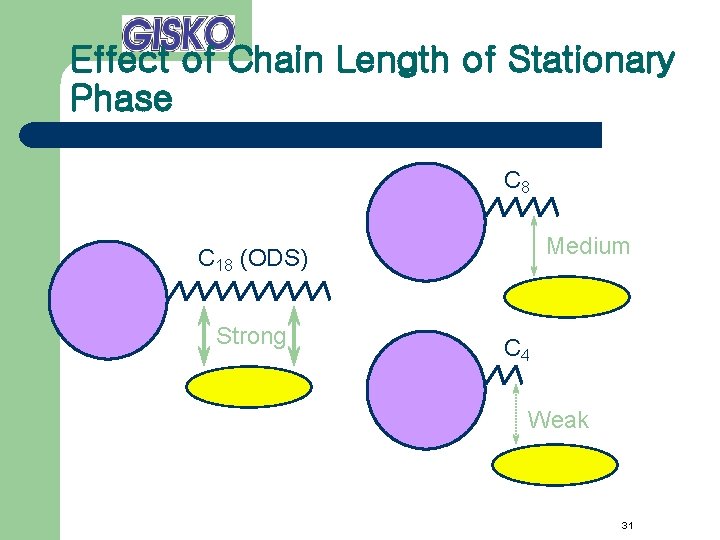
Effect of Chain Length of Stationary Phase C 8 Medium C 18 (ODS) Strong C 4 Weak 31
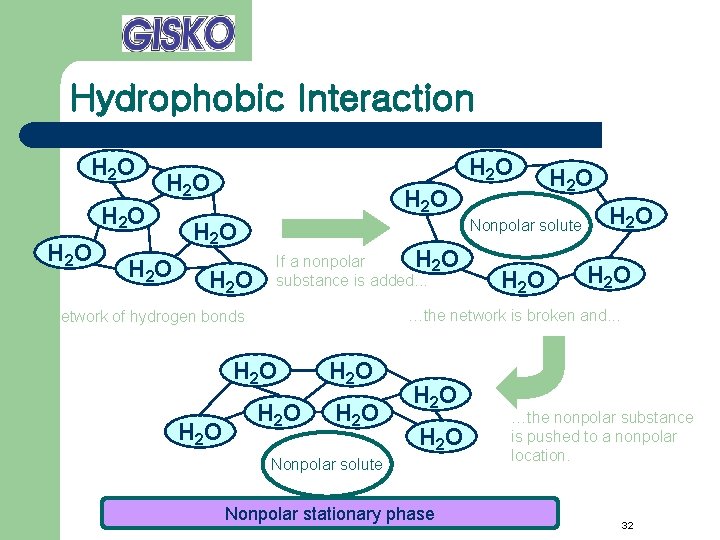
Hydrophobic Interaction H 2 O H 2 O H 2 O Nonpolar solute If a nonpolar H 2 O substance is added. . . H 2 O …the network is broken and. . . Network of hydrogen bonds H 2 O H 2 O Nonpolar solute Nonpolar stationary phase …the nonpolar substance is pushed to a nonpolar location. 32
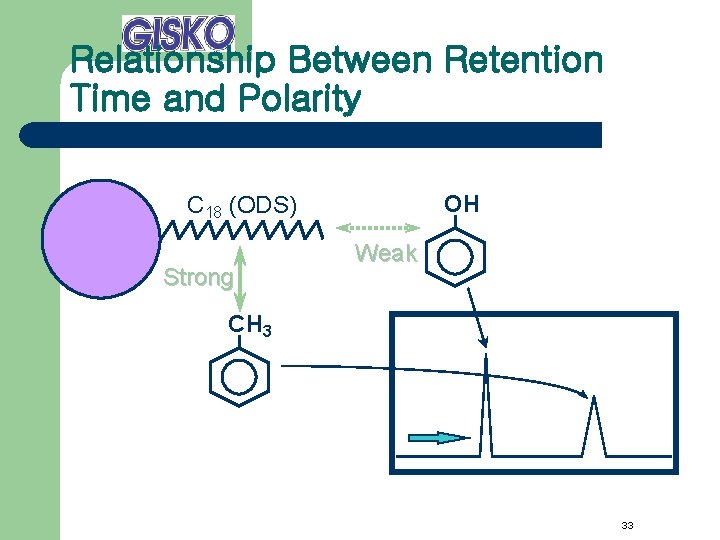
Relationship Between Retention Time and Polarity OH C 18 (ODS) Strong Weak CH 3 33

Basic Settings for Eluent Used in Reversed Phase Mode l Water (buffer solution) + water-soluble organic solvent – – – Water-soluble organic solvent: Methanol Acetonitrile Tetrahydrofuran etc. The mixing ratio of the water (buffer solution) and organic solvent has the greatest influence on separation. If a buffer solution is used, its p. H value is an important separation parameter. 34
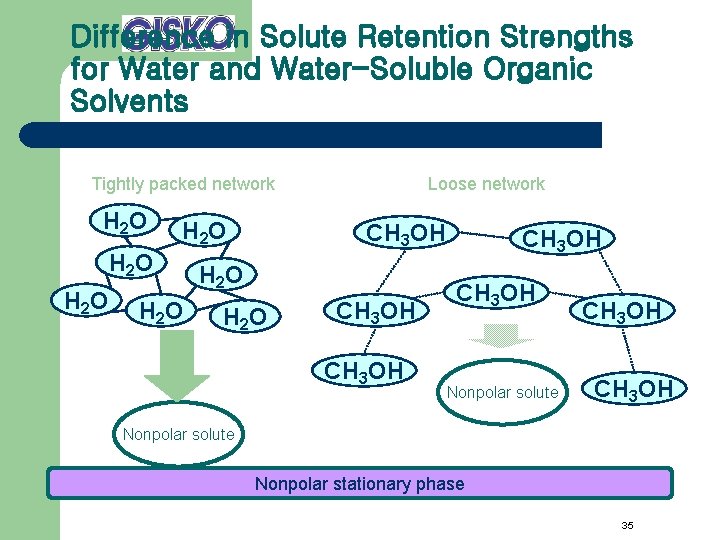
Difference in Solute Retention Strengths for Water and Water-Soluble Organic Solvents Tightly packed network H 2 O H 2 O Loose network CH 3 OH H 2 O CH 3 OH Nonpolar solute CH 3 OH Nonpolar solute Nonpolar stationary phase 35
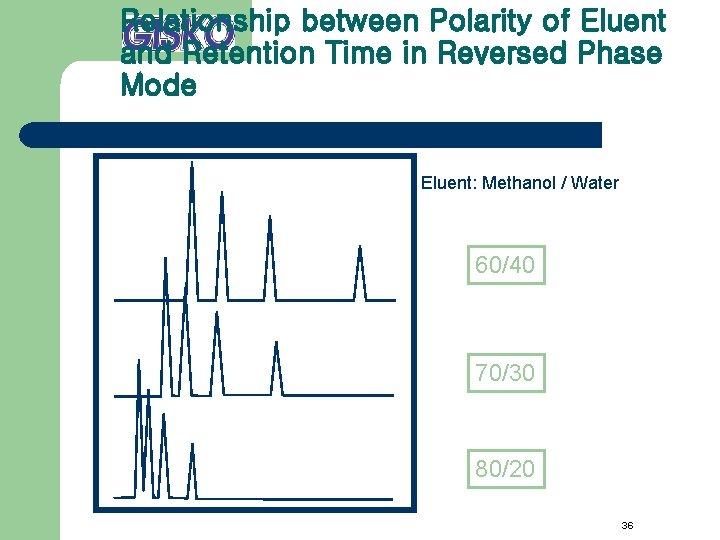
Relationship between Polarity of Eluent and Retention Time in Reversed Phase Mode Eluent: Methanol / Water 60/40 70/30 80/20 36
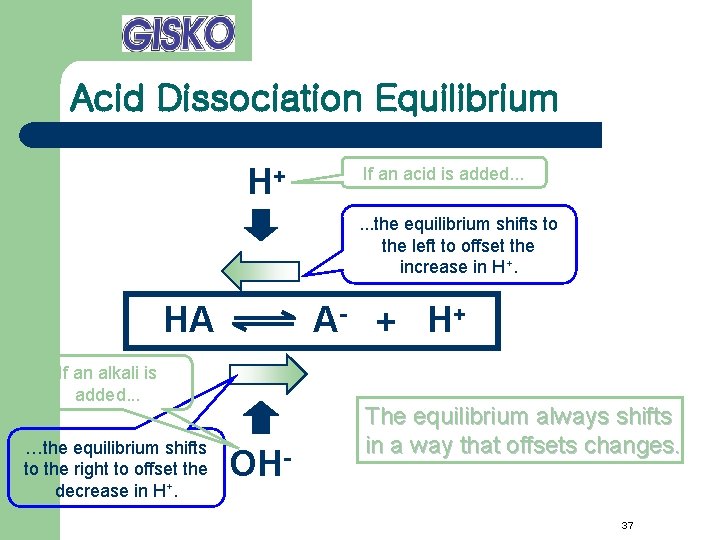
Acid Dissociation Equilibrium H+ If an acid is added. . . the equilibrium shifts to the left to offset the increase in H+. HA A- + H+ If an alkali is added. . . …the equilibrium shifts to the right to offset the decrease in H+. OH- The equilibrium always shifts in a way that offsets changes. 37
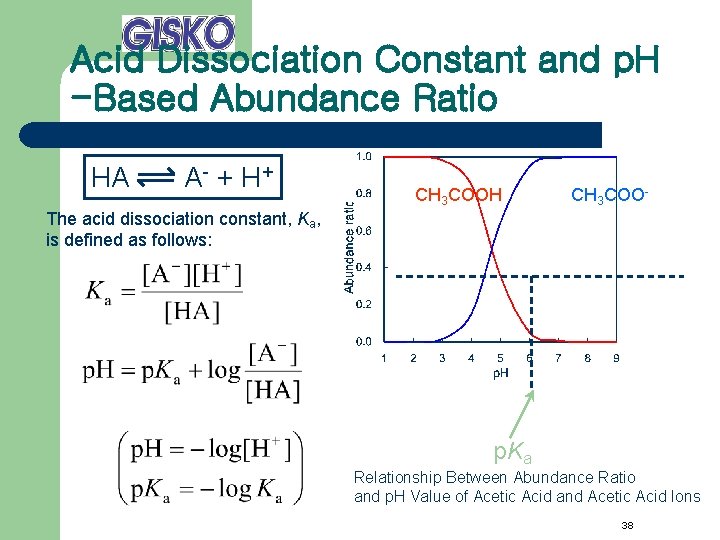
Acid Dissociation Constant and p. H -Based Abundance Ratio HA A - + H+ CH 3 COOH CH 3 COO- The acid dissociation constant, Ka, is defined as follows: p. Ka Relationship Between Abundance Ratio and p. H Value of Acetic Acid and Acetic Acid Ions 38
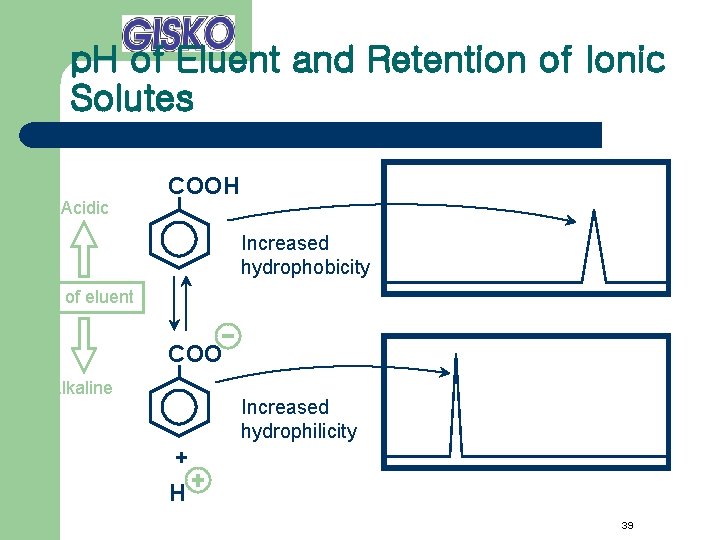
p. H of Eluent and Retention of Ionic Solutes Acidic COOH Increased hydrophobicity p. H of eluent COO Alkaline Increased hydrophilicity + H 39
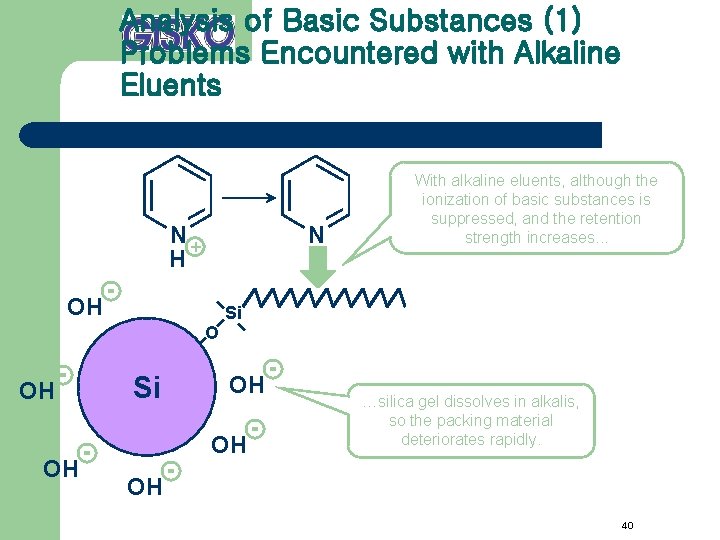
Analysis of Basic Substances (1) Problems Encountered with Alkaline Eluents N+ H N OH With alkaline eluents, although the ionization of basic substances is suppressed, and the retention strength increases. . . Si O OH OH Si OH OH …silica gel dissolves in alkalis, so the packing material deteriorates rapidly. OH 40
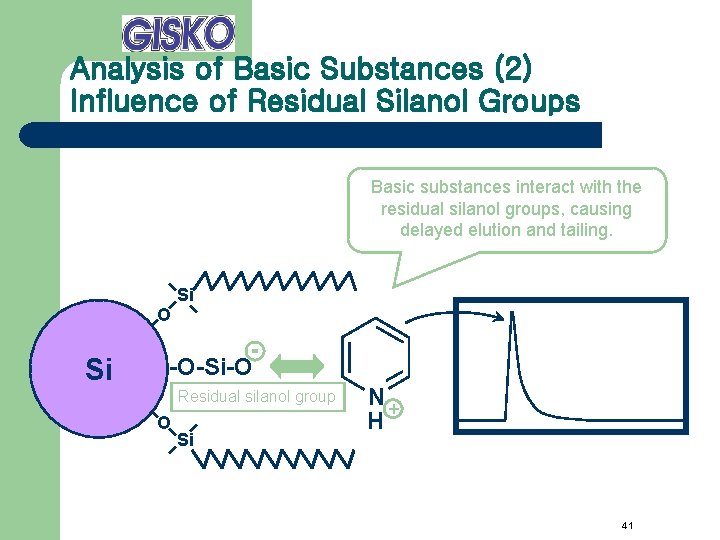
Analysis of Basic Substances (2) Influence of Residual Silanol Groups Basic substances interact with the residual silanol groups, causing delayed elution and tailing. Si O Si -O-Si-O Residual silanol group O Si N+ H 41
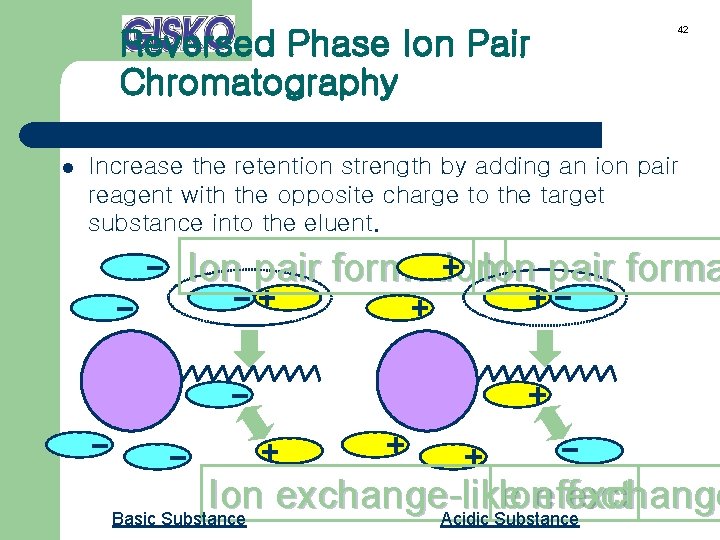
Reversed Phase Ion Pair Chromatography l 42 Increase the retention strength by adding an ion pair reagent with the opposite charge to the target substance into the eluent. Ion pair formation. Ion pair forma Ion exchange-like Ion effect exchange Basic Substance Acidic Substance
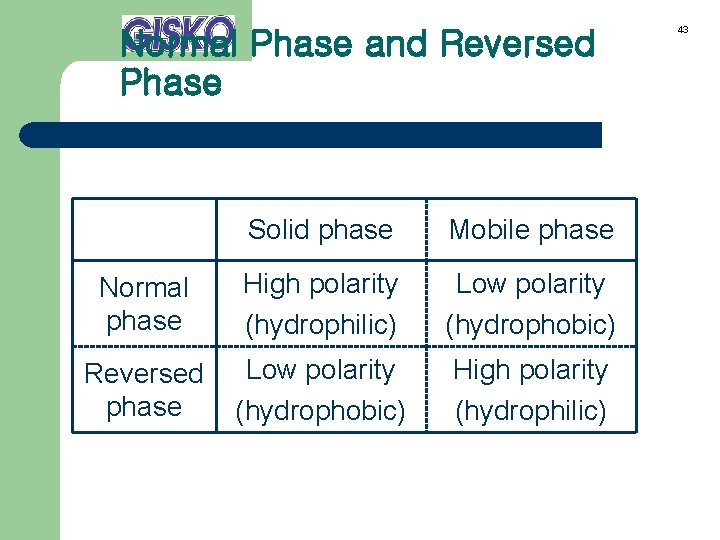
Normal Phase and Reversed Phase Solid phase Mobile phase Normal phase High polarity (hydrophilic) Low polarity (hydrophobic) Reversed phase Low polarity (hydrophobic) High polarity (hydrophilic) 43
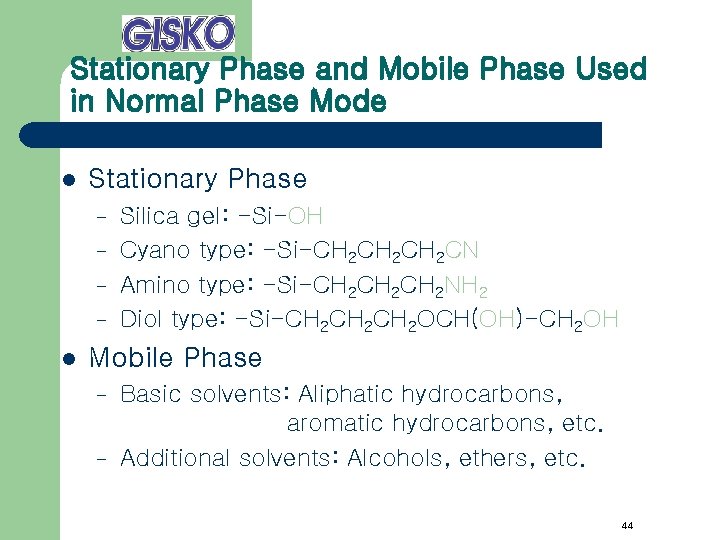
Stationary Phase and Mobile Phase Used in Normal Phase Mode l Stationary Phase – – l Silica gel: -Si-OH Cyano type: -Si-CH 2 CH 2 CN Amino type: -Si-CH 2 CH 2 NH 2 Diol type: -Si-CH 2 CH 2 OCH(OH)-CH 2 OH Mobile Phase – – Basic solvents: Aliphatic hydrocarbons, aromatic hydrocarbons, etc. Additional solvents: Alcohols, ethers, etc. 44
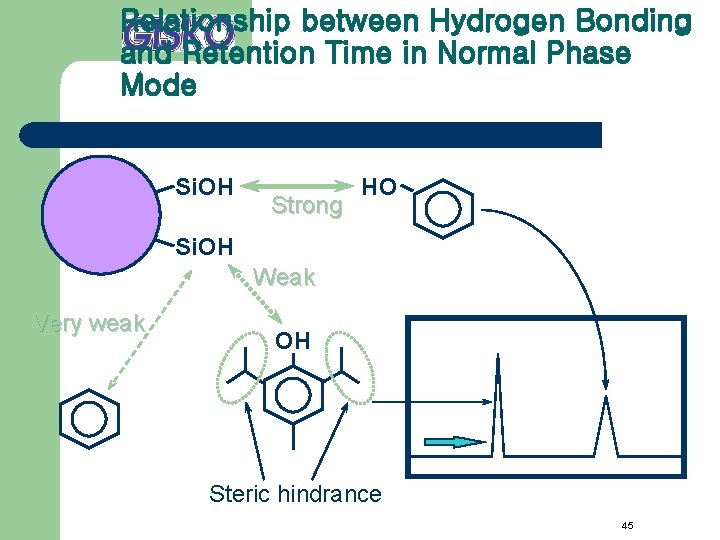
Relationship between Hydrogen Bonding and Retention Time in Normal Phase Mode Si. OH Strong HO Si. OH Weak Very weak OH Steric hindrance 45
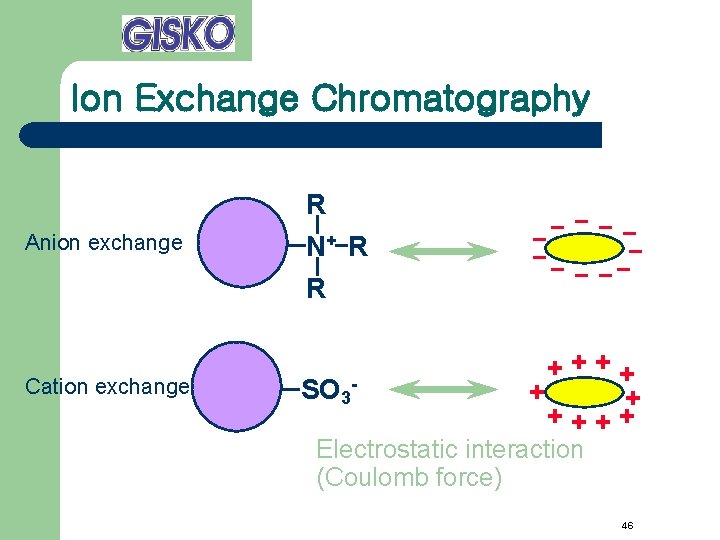
Ion Exchange Chromatography Anion exchange Cation exchange R N+ R R SO 3 - ++++ + + ++++ Electrostatic interaction (Coulomb force) 46
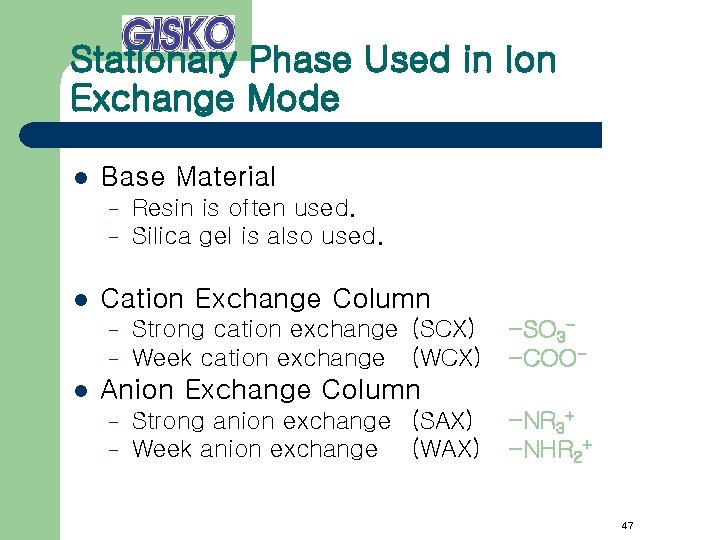
Stationary Phase Used in Ion Exchange Mode l Base Material – – l Cation Exchange Column – – l Resin is often used. Silica gel is also used. Strong cation exchange (SCX) Week cation exchange (WCX) -SO 3 -COO- Anion Exchange Column – – Strong anion exchange (SAX) Week anion exchange (WAX) -NR 3+ -NHR 2+ 47

Detection Condition Requirements l Sensitivity – l Selectivity – l l The detector must have the appropriate level of sensitivity. The detector must be able to detect the target substance without, if possible, detecting other substances. Adaptability to separation conditions Operability, etc. 48
Representative HPLC Detectors l l l l UV-VIS absorbance detector Photodiode array-type UV-VIS absorbance detector Fluorescence detector Refractive index detector Evaporative light scattering detector Electrical conductivity detector Electrochemical detector Mass spectrometer 49
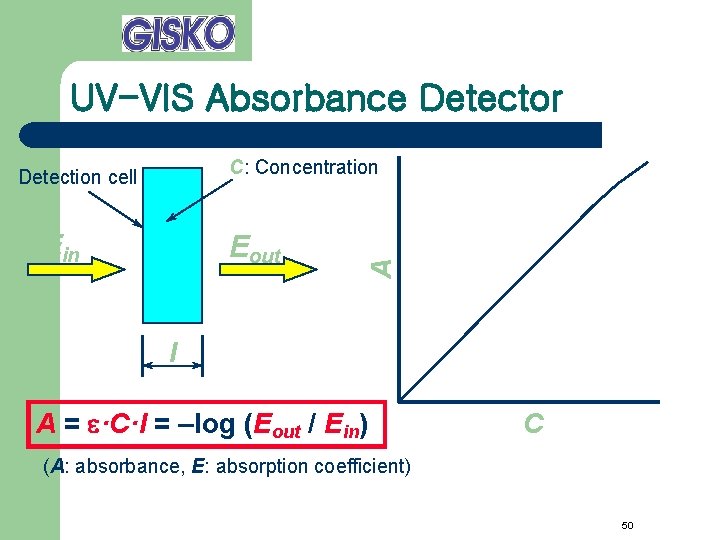
UV-VIS Absorbance Detector Ein Eout A C: Concentration Detection cell l A = e·C·l = –log (Eout / Ein) C (A: absorbance, E: absorption coefficient) 50
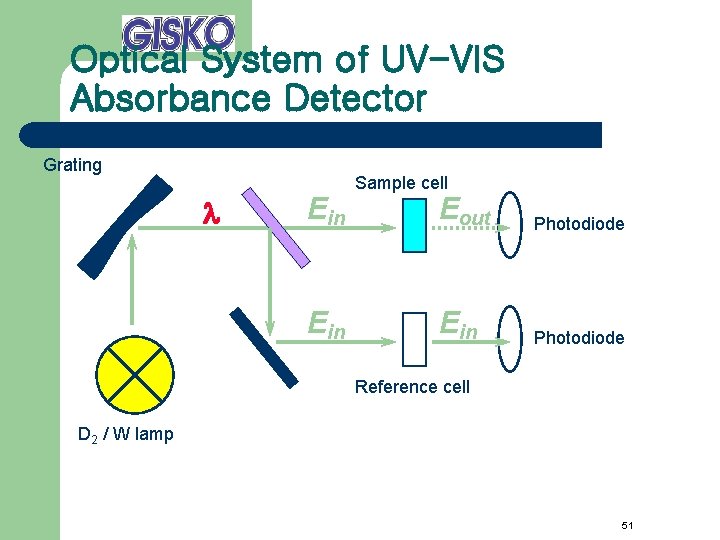
Optical System of UV-VIS Absorbance Detector Grating l Ein Sample cell Eout Photodiode Ein Photodiode Reference cell D 2 / W lamp 51
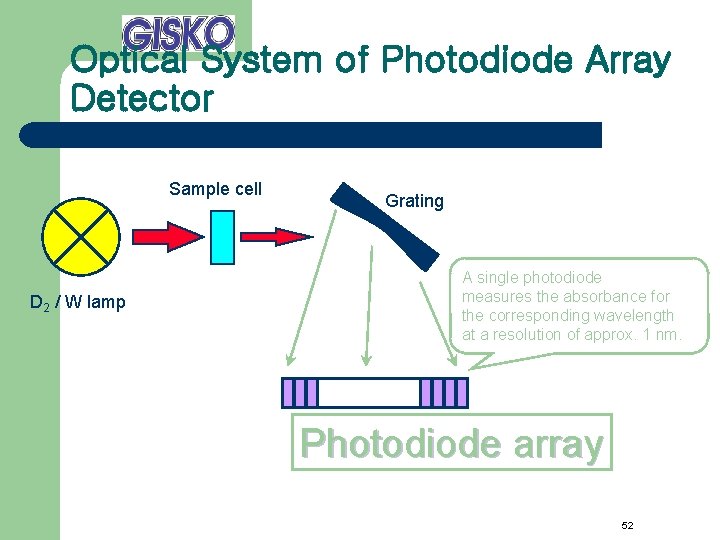
Optical System of Photodiode Array Detector Sample cell D 2 / W lamp Grating A single photodiode measures the absorbance for the corresponding wavelength at a resolution of approx. 1 nm. Photodiode array 52
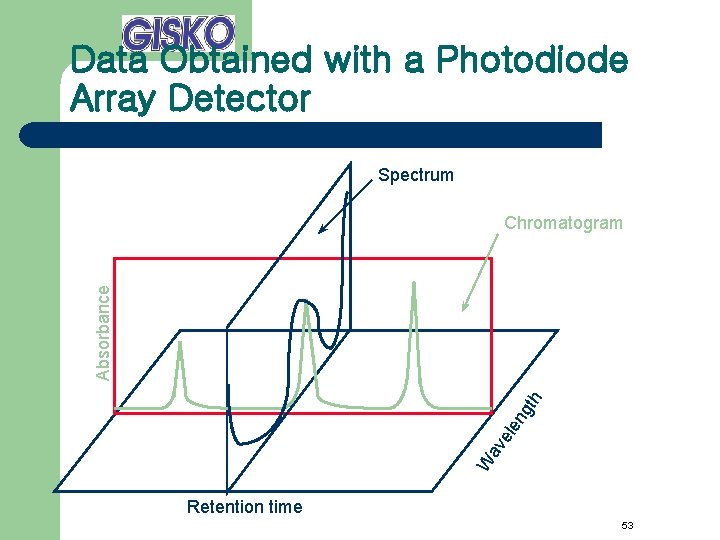
Data Obtained with a Photodiode Array Detector Spectrum W av ele ng th Absorbance Chromatogram Retention time 53
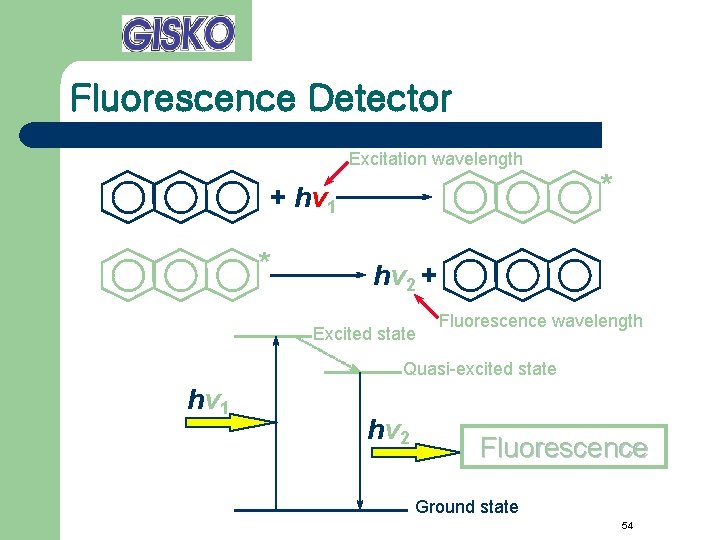
Fluorescence Detector Excitation wavelength + hv 1 * * hv 2 + Excited state Fluorescence wavelength Quasi-excited state hv 1 hv 2 Fluorescence Ground state 54
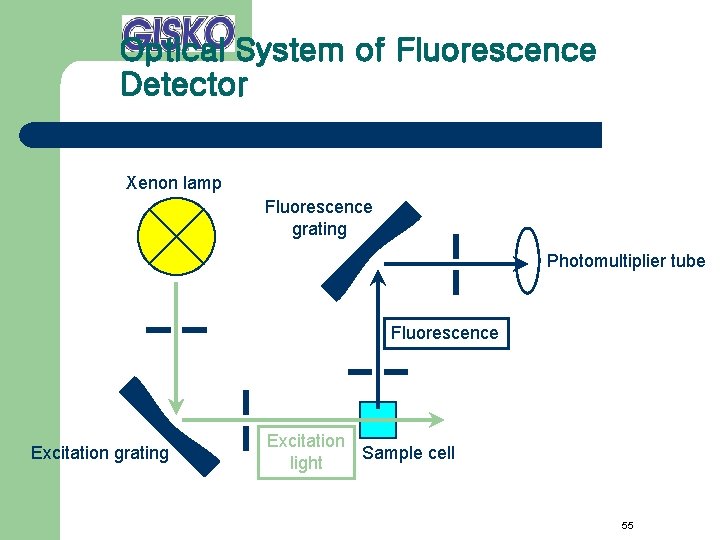
Optical System of Fluorescence Detector Xenon lamp Fluorescence grating Photomultiplier tube Fluorescence Excitation grating Excitation Sample cell light 55
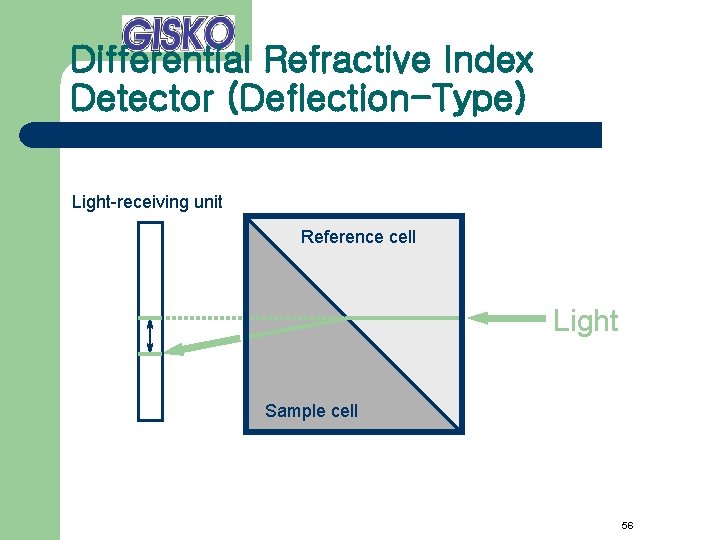
Differential Refractive Index Detector (Deflection-Type) Light-receiving unit Reference cell Light Sample cell 56
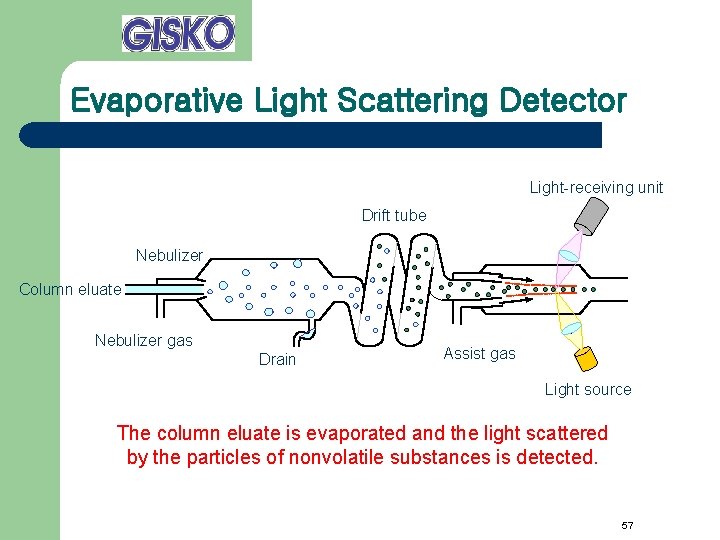
Evaporative Light Scattering Detector Light-receiving unit Drift tube Nebulizer Column eluate Nebulizer gas Drain Assist gas Light source The column eluate is evaporated and the light scattered by the particles of nonvolatile substances is detected. 57
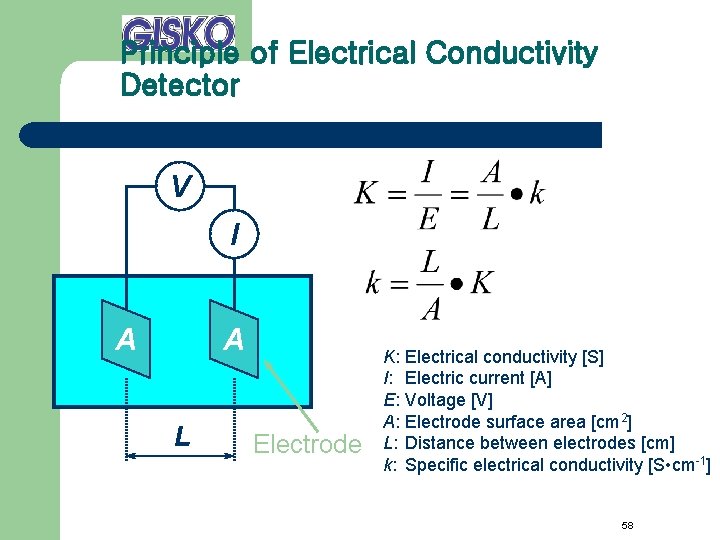
Principle of Electrical Conductivity Detector V I A A L Electrode K: I: E: A: L: k: Electrical conductivity [S] Electric current [A] Voltage [V] Electrode surface area [cm 2] Distance between electrodes [cm] Specific electrical conductivity [S • cm-1] 58
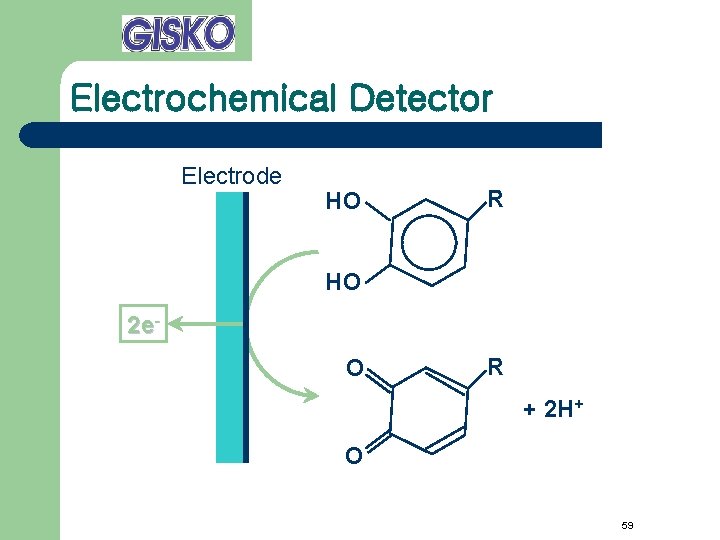
Electrochemical Detector Electrode HO R HO 2 e. O R + 2 H+ O 59
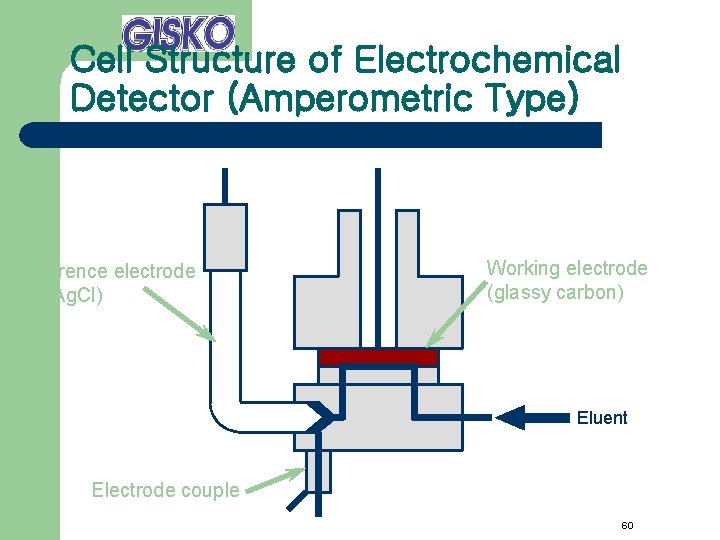
Cell Structure of Electrochemical Detector (Amperometric Type) Reference electrode (Ag/Ag. Cl) Working electrode (glassy carbon) Eluent Electrode couple 60
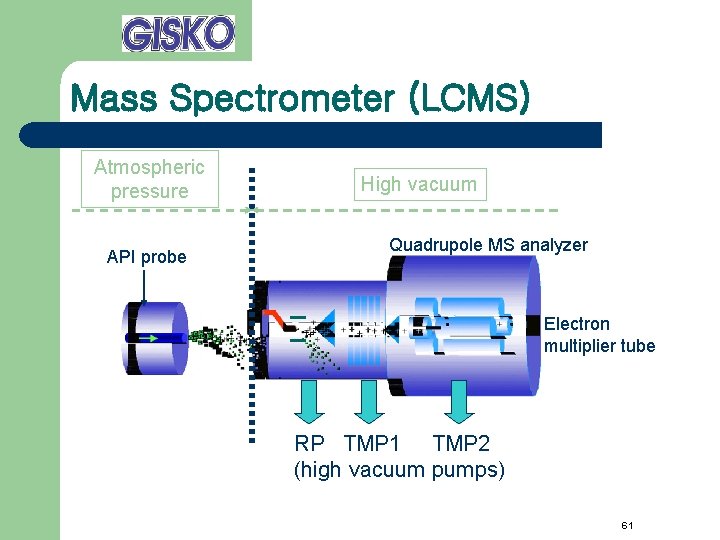
Mass Spectrometer (LCMS) Atmospheric pressure API probe High vacuum Quadrupole MS analyzer Electron multiplier tube RP TMP 1 TMP 2 (high vacuum pumps) 61
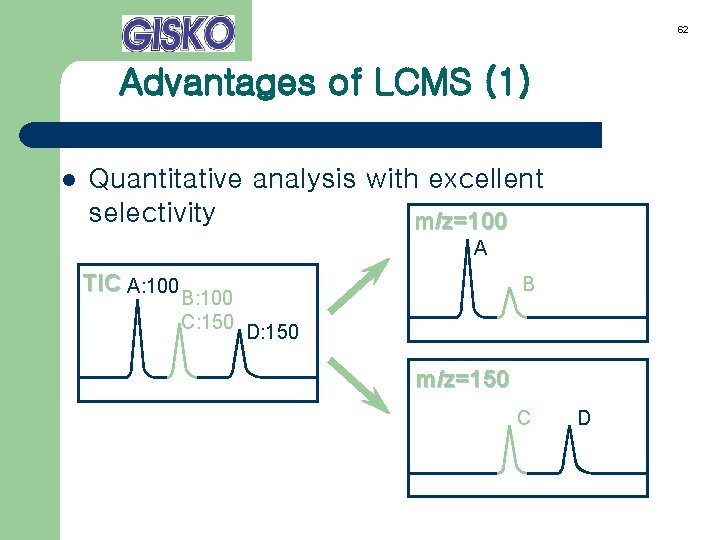
62 Advantages of LCMS (1) l Quantitative analysis with excellent selectivity m/z=100 A TIC A: 100 B B: 100 C: 150 D: 150 m/z=150 C D
- Slides: 62