HPLC 1 BASIC PRINCIPLES LAAQBLC 001 B Invention
HPLC 1 BASIC PRINCIPLES LAAQ-B-LC 001 B
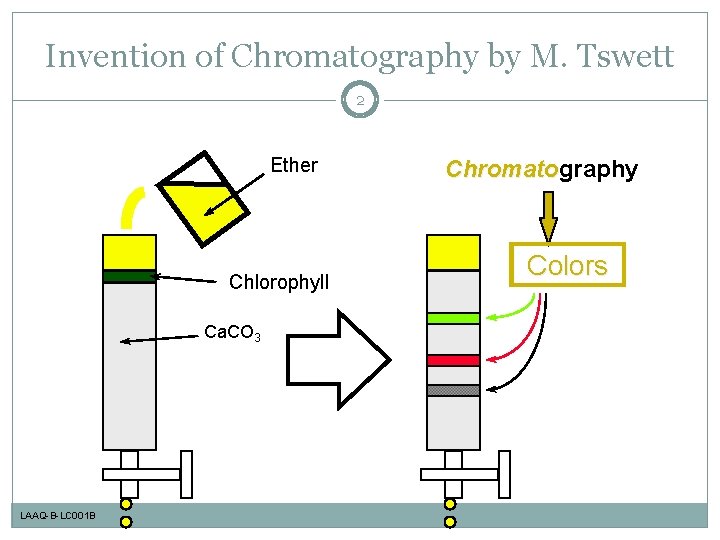
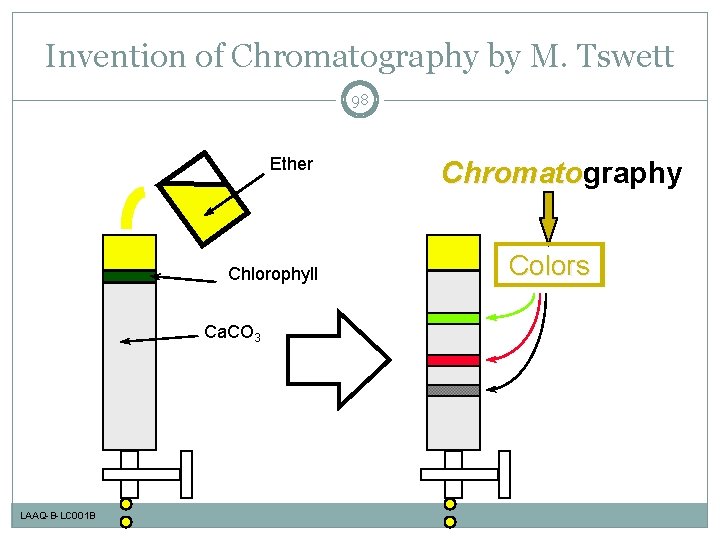
Invention of Chromatography by M. Tswett 2 Ether Chlorophyll Ca. CO 3 LAAQ-B-LC 001 B Chromatography Chromato Colors
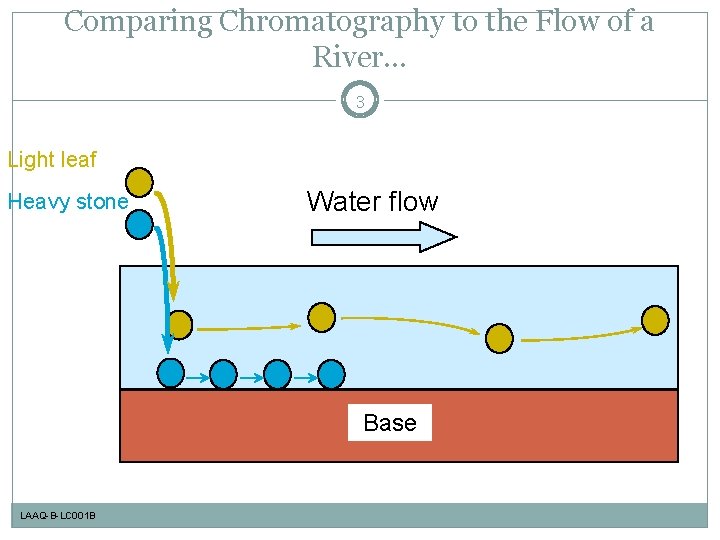
Comparing Chromatography to the Flow of a River. . . 3 Light leaf Heavy stone Water flow Base LAAQ-B-LC 001 B
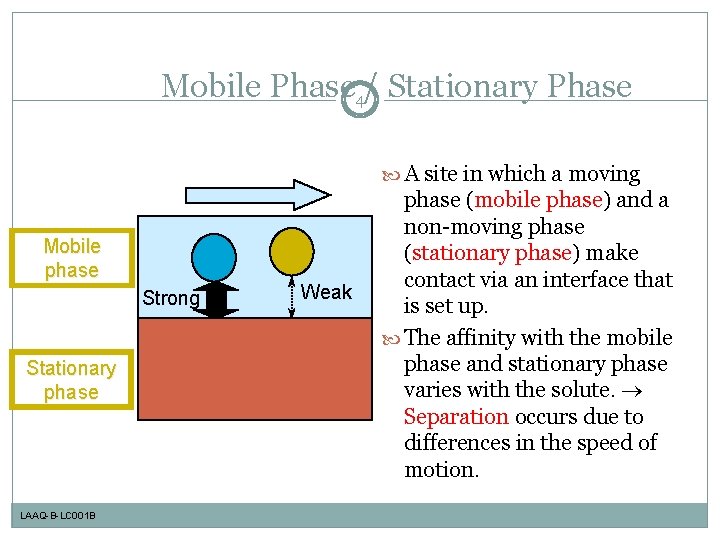
Mobile Phase 4/ Stationary Phase A site in which a moving Mobile phase Strong Stationary phase LAAQ-B-LC 001 B Weak phase (mobile phase) and a non-moving phase (stationary phase) make contact via an interface that is set up. The affinity with the mobile phase and stationary phase varies with the solute. Separation occurs due to differences in the speed of motion.
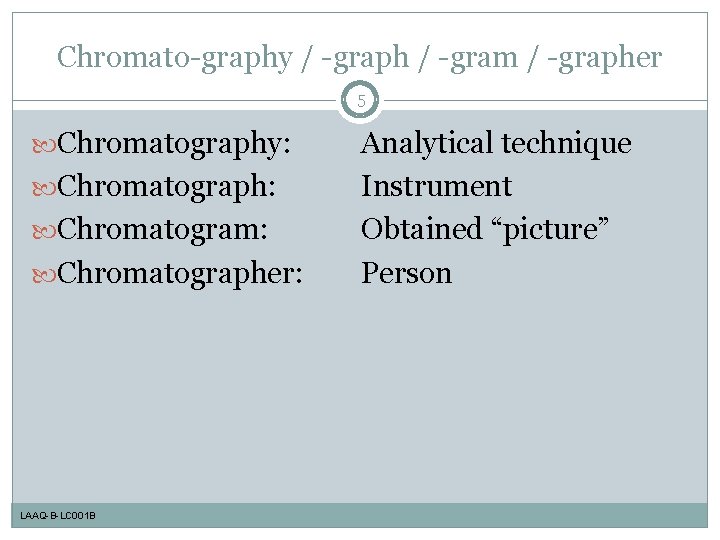
Chromato-graphy / -graph / -gram / -grapher 5 Chromatography: Chromatograph: Chromatogram: Chromatographer: LAAQ-B-LC 001 B Analytical technique Instrument Obtained “picture” Person
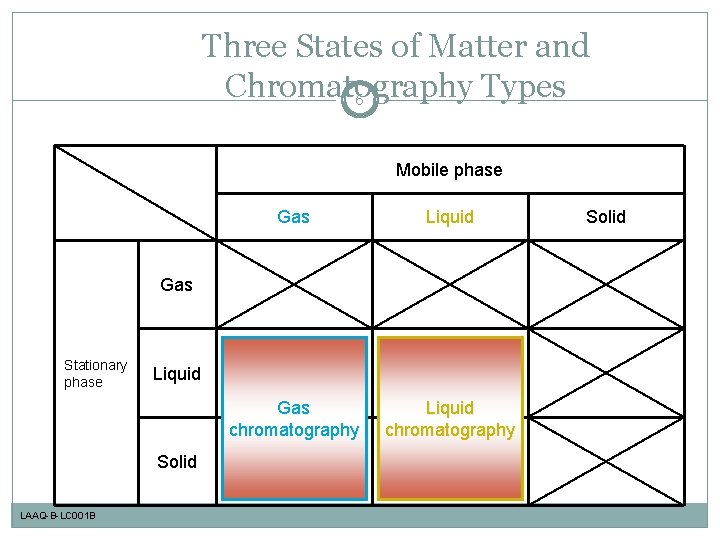
Three States of Matter and Chromatography Types 6 Mobile phase Gas Liquid Gas chromatography Liquid chromatography Gas Stationary phase Liquid Solid LAAQ-B-LC 001 B Solid
Liquid Chromatography 7 Chromatography in which the mobile phase is a liquid. The liquid used as the mobile phase is called the “eluent”. The stationary phase is usually a solid or a liquid. In general, it is possible to analyze any substance that can be stably dissolved in the mobile phase. LAAQ-B-LC 001 B
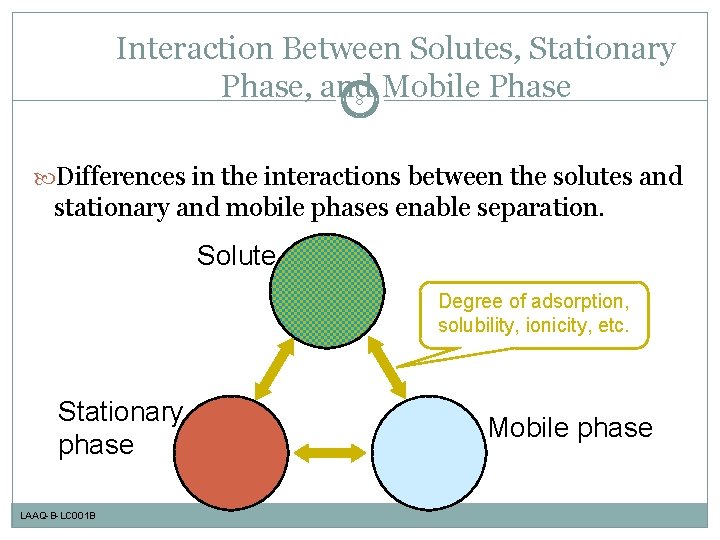
Interaction Between Solutes, Stationary Phase, and Mobile Phase 8 Differences in the interactions between the solutes and stationary and mobile phases enable separation. Solute Degree of adsorption, solubility, ionicity, etc. Stationary phase LAAQ-B-LC 001 B Mobile phase
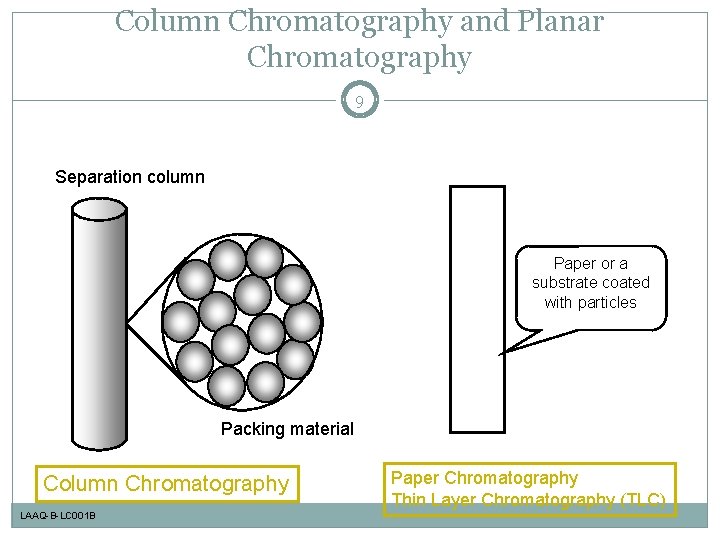
Column Chromatography and Planar Chromatography 9 Separation column Paper or a substrate coated with particles Packing material Column Chromatography LAAQ-B-LC 001 B Paper Chromatography Thin Layer Chromatography (TLC)
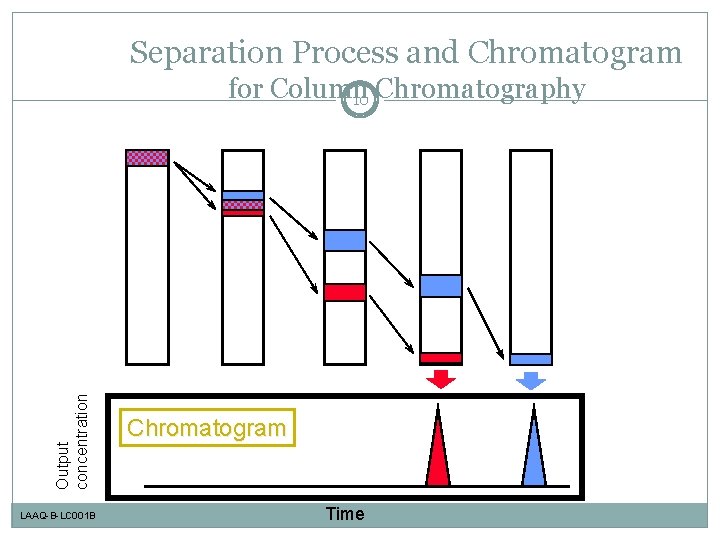
Separation Process and Chromatogram Output concentration for Column 10 Chromatography LAAQ-B-LC 001 B Chromatogram Time
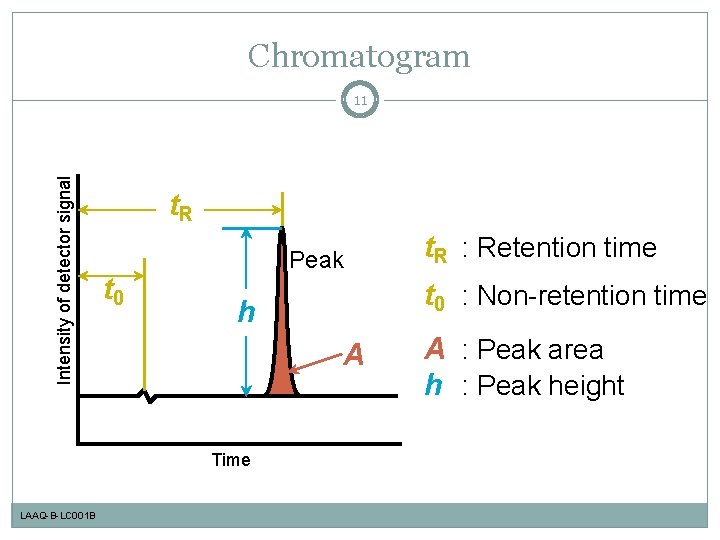
Chromatogram Intensity of detector signal 11 t. R t 0 Peak LAAQ-B-LC 001 B t 0 : Non-retention time h A Time t. R : Retention time A : Peak area h : Peak height
From Liquid Chromatography to High Performance Liquid Chromatography 12 Higher degree of separation! Refinement of packing material (3 to 10 µm) Reduction of analysis time! Delivery of eluent by pump Demand for special equipment that can withstand high pressures The arrival of high performance liquid chromatography! LAAQ-B-LC 001 B
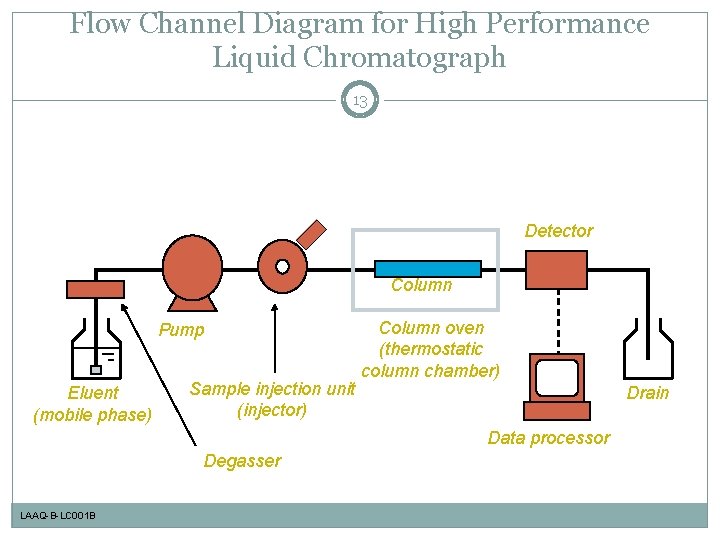
Flow Channel Diagram for High Performance Liquid Chromatograph 13 Detector Column Pump Eluent (mobile phase) Sample injection unit (injector) Column oven (thermostatic column chamber) Drain Data processor Degasser LAAQ-B-LC 001 B
Advantages of High Performance Liquid Chromatography 14 High separation capacity, enabling the batch analysis of multiple components Superior quantitative capability and reproducibility Moderate analytical conditions Unlike GC, the sample does not need to be vaporized. Generally high sensitivity Low sample consumption Easy preparative separation and purification of samples LAAQ-B-LC 001 B
Fields in Which High Performance Liquid Chromatography Is Used 15 Food products Biogenic substances Sugars, lipids, nucleic acids, amino acids, proteins, peptides, steroids, amines, etc. Medical products Drugs, antibiotics, etc. Environmental samples Inorganic ions Hazardous organic substances, etc. Organic industrial products LAAQ-B-LC 001 B Vitamins, food additives, sugars, organic acids, amino acids, etc. Synthetic polymers, additives, surfactants, etc.

HPLC Hardware: Part 1 16 SOLVENT DELIVERY SYSTEM, DEGASSER, SAMPLE INJECTION UNIT, COLUMN OVEN LAAQ-B-LC 001 B
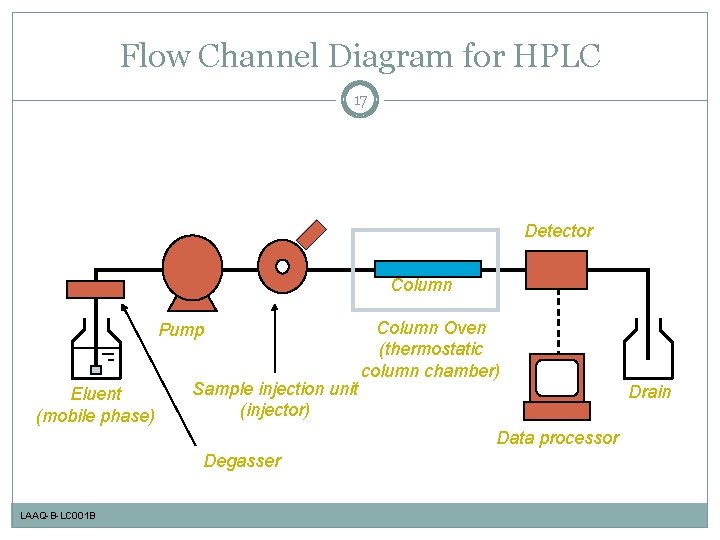
Flow Channel Diagram for HPLC 17 Detector Column Pump Eluent (mobile phase) Sample injection unit (injector) Column Oven (thermostatic column chamber) Drain Data processor Degasser LAAQ-B-LC 001 B
Solvent Delivery Pump 18 Performance Requirements Capacity to withstand high load pressures. Pulsations that accompany pressure fluctuations are small. Flow rate does not fluctuate. Solvent replacement is easy. The flow rate setting range is wide and the flow rate is accurate. LAAQ-B-LC 001 B
Solvent Delivery Pump: Representative Pumping Methods 19 Syringe pump Plunger pump Diaphragm pump LAAQ-B-LC 001 B
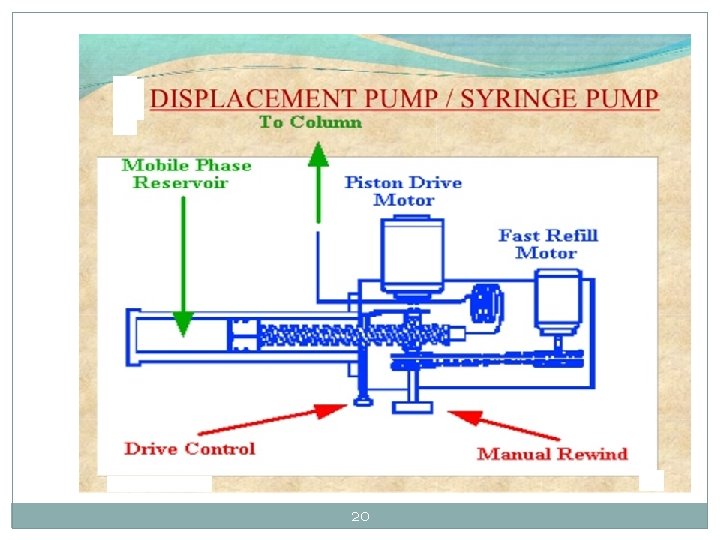
20
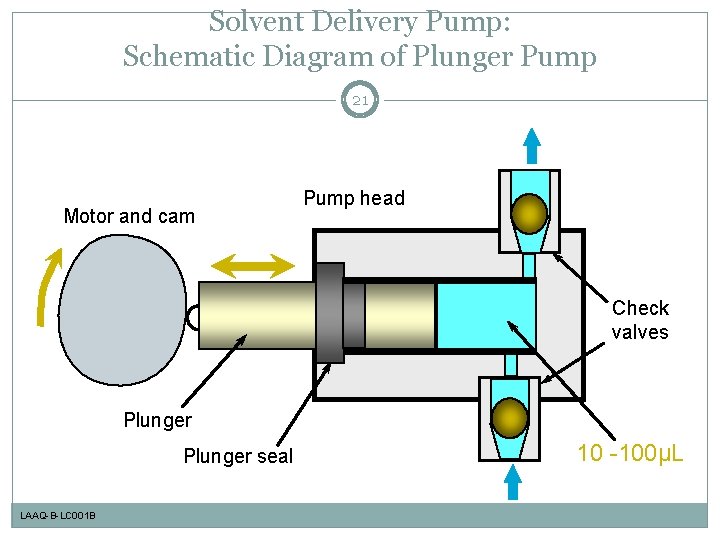
Solvent Delivery Pump: Schematic Diagram of Plunger Pump 21 Motor and cam Pump head Check valves Plunger seal LAAQ-B-LC 001 B 10 -100µL
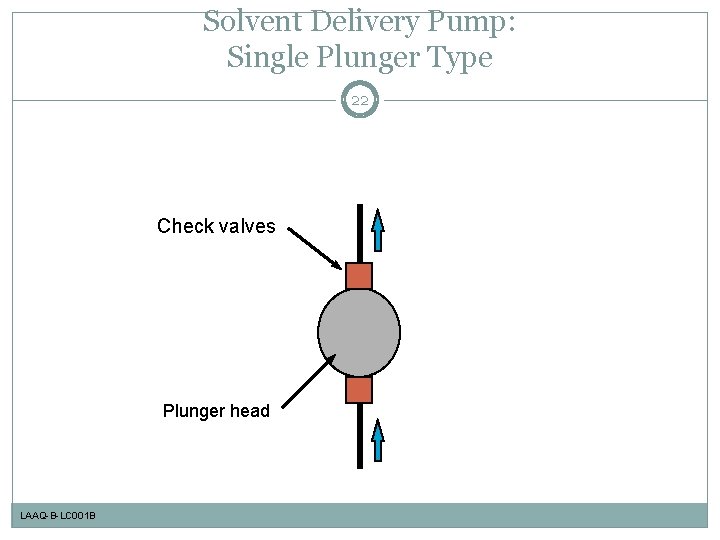
Solvent Delivery Pump: Single Plunger Type 22 Check valves Plunger head LAAQ-B-LC 001 B
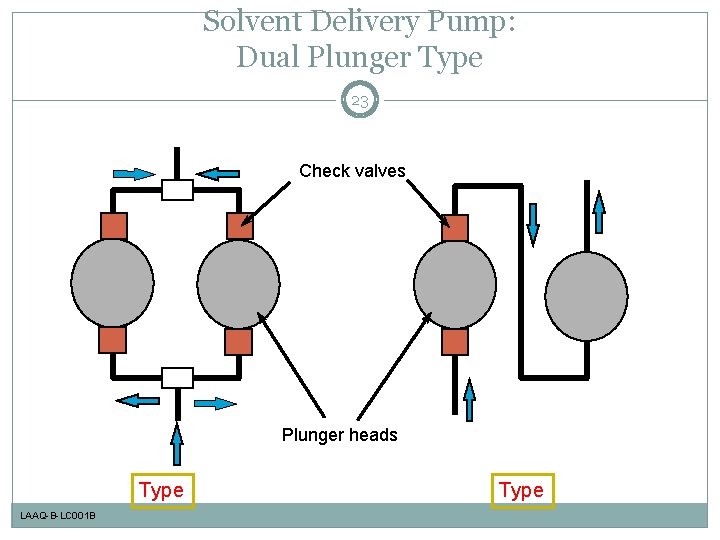
Solvent Delivery Pump: Dual Plunger Type 23 Check valves Plunger heads Type LAAQ-B-LC 001 B Type
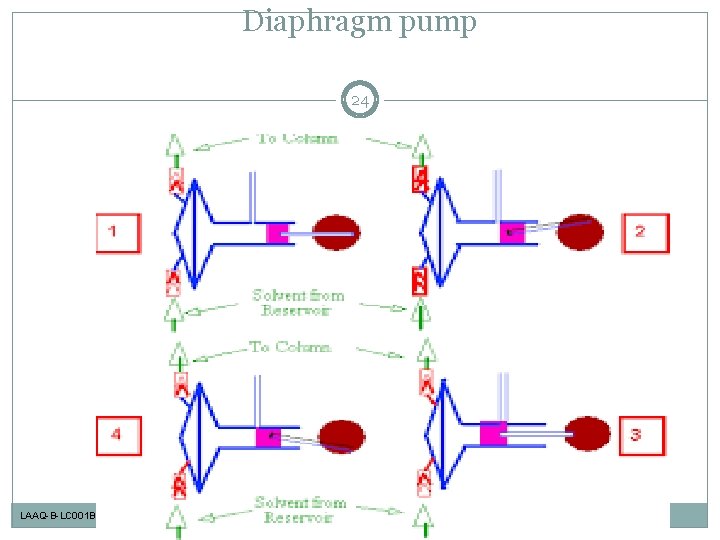
Diaphragm pump 24 LAAQ-B-LC 001 B
Gradient System 25 Isocratic system Constant eluent composition Gradient system Varying eluent composition HPGE (High Pressure Gradient) LPGE (Low Pressure Gradient) LAAQ-B-LC 001 B
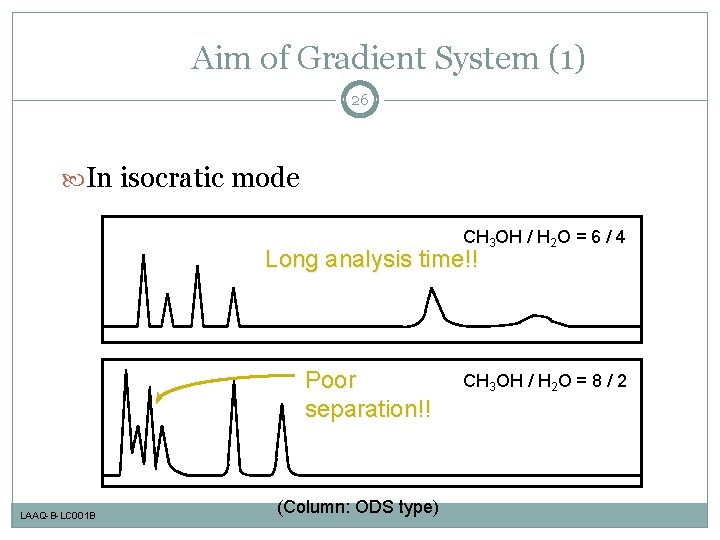
Aim of Gradient System (1) 26 In isocratic mode CH 3 OH / H 2 O = 6 / 4 Long analysis time!! Poor separation!! LAAQ-B-LC 001 B (Column: ODS type) CH 3 OH / H 2 O = 8 / 2
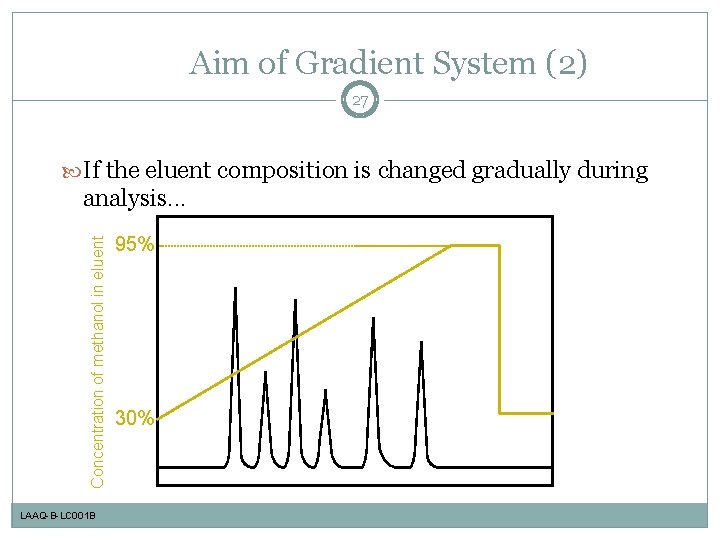
Aim of Gradient System (2) 27 If the eluent composition is changed gradually during Concentration of methanol in eluent analysis. . . LAAQ-B-LC 001 B 95% 30%
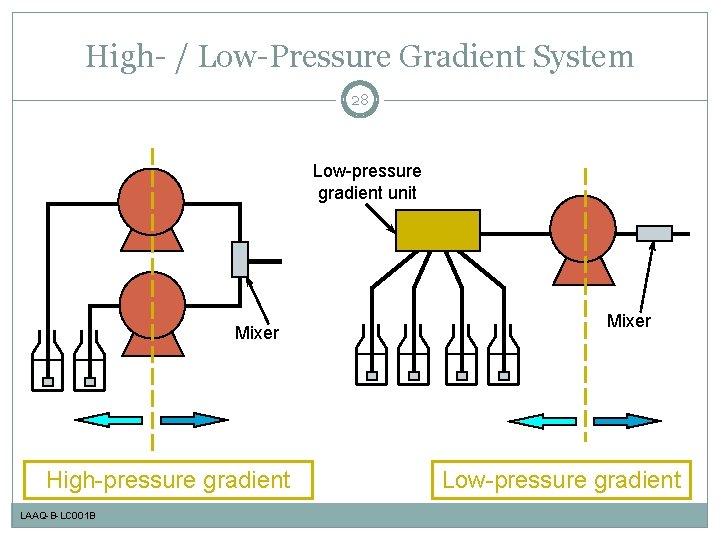
High- / Low-Pressure Gradient System 28 Low-pressure gradient unit Mixer High-pressure gradient LAAQ-B-LC 001 B Mixer Low-pressure gradient
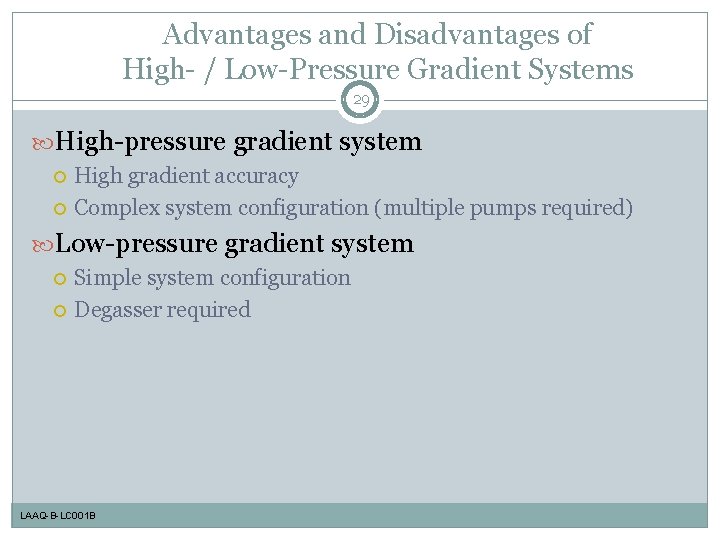
Advantages and Disadvantages of High- / Low-Pressure Gradient Systems 29 High-pressure gradient system High gradient accuracy Complex system configuration (multiple pumps required) Low-pressure gradient system Simple system configuration Degasser required LAAQ-B-LC 001 B

Degasser 30 Problems caused by dissolved air in the eluent Unstable delivery by pump More noise and large baseline drift in detector cell In order to avoid these problems, the eluent must be degassed. LAAQ-B-LC 001 B
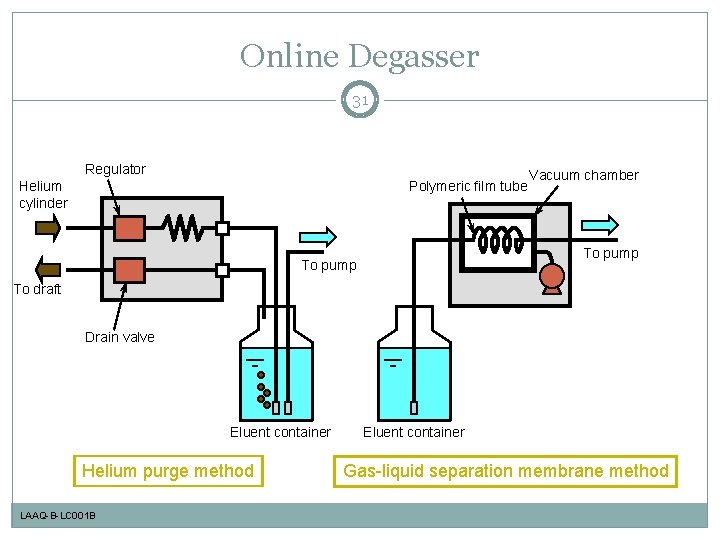
Online Degasser 31 Regulator Helium cylinder Polymeric film tube Vacuum chamber To pump To draft Drain valve Eluent container Helium purge method LAAQ-B-LC 001 B Eluent container Gas-liquid separation membrane method
Sample Injection Unit (Injector) 32 Performance Requirements No sample remaining in unit Minimal broadening of sample band Free adjustment of injection volume Minimal loss Superior durability and pressure resistance LAAQ-B-LC 001 B
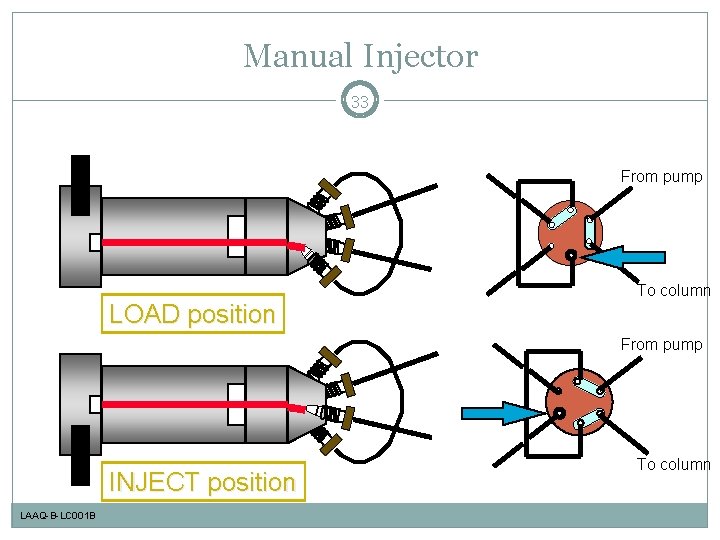
Manual Injector 33 From pump LOAD position To column From pump INJECT position LAAQ-B-LC 001 B To column
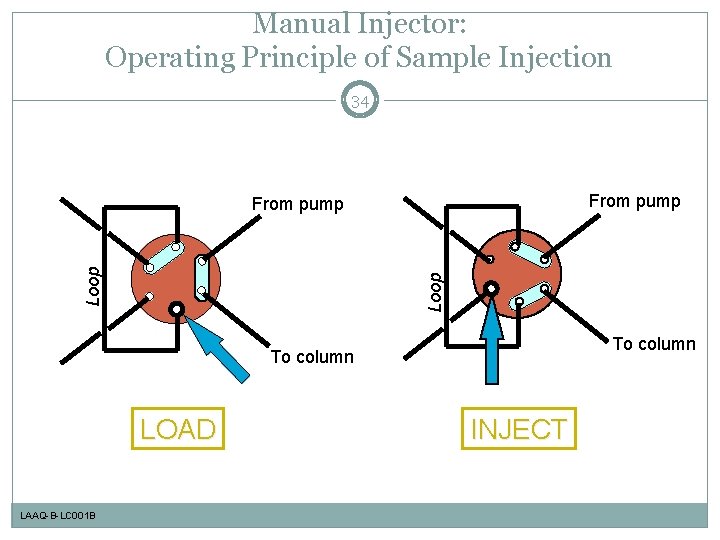
Manual Injector: Operating Principle of Sample Injection 34 From pump Loop From pump To column LOAD LAAQ-B-LC 001 B INJECT
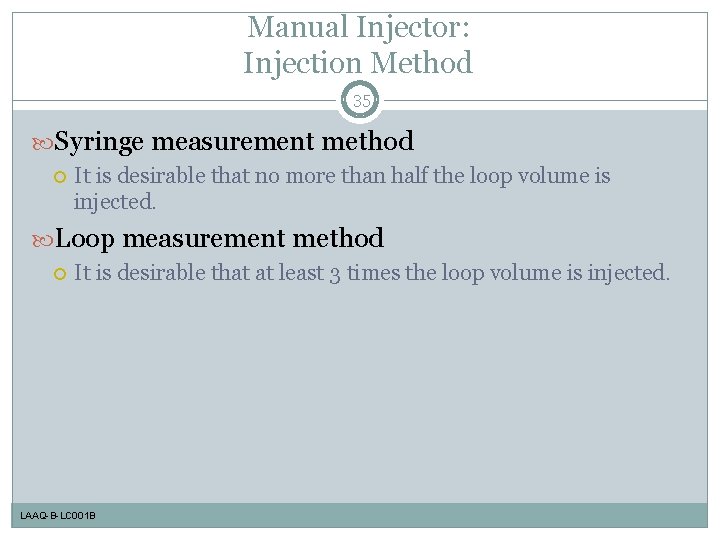
Manual Injector: Injection Method 35 Syringe measurement method It is desirable that no more than half the loop volume is injected. Loop measurement method It is desirable that at least 3 times the loop volume is injected. LAAQ-B-LC 001 B
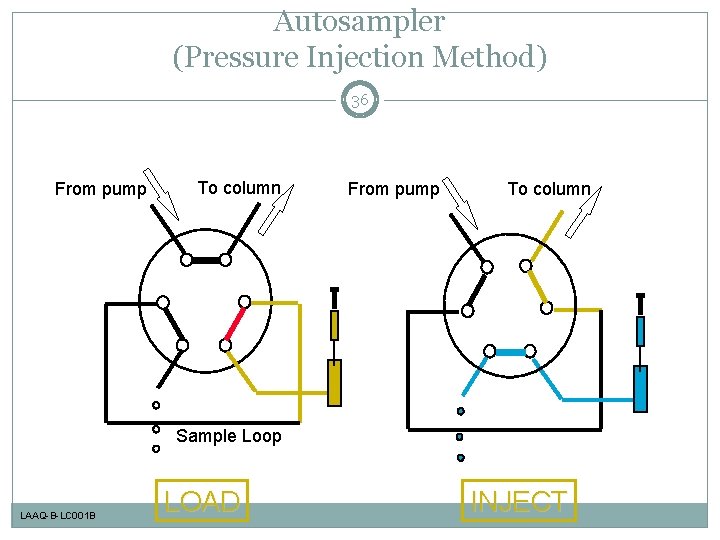
Autosampler (Pressure Injection Method) 36 From pump To column Sample Loop LAAQ-B-LC 001 B LOAD INJECT
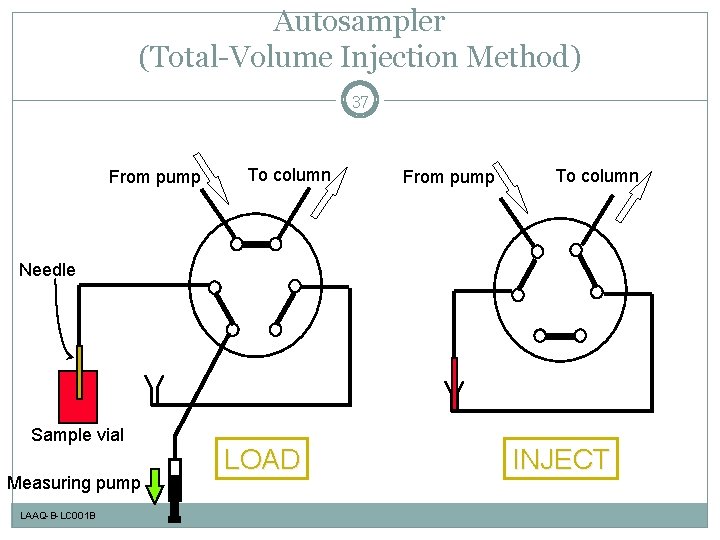
Autosampler (Total-Volume Injection Method) 37 From pump To column Needle Sample vial Measuring pump LAAQ-B-LC 001 B LOAD INJECT
Column Oven 38 Air circulation heating type Block heating type Aluminum block heater Insulated column jacket type Water bath LAAQ-B-LC 001 B
Column 39 The column is one of the most important components of the HPLC chromatograph because the separation of the sample components is achieved when those components pass through the column. The High performance liquid chromatography apparatus is made out of stainless steel tubes with a diameter of 3 to 5 mm and a length ranging from 10 to 30 cm. LAAQ-B-LC 001 B Normally, columns are filled with silica gel because its particle shape, surface properties, and pore structure help to get a good separation. Silica is wetted by nearly every potential mobile phase, is inert to most compounds and has a high surface activity which can be modified easily with water and other agents. Silica can be used to separate a wide variety of chemical compounds, & its chromatographic behavior is generally predictable and reproducible.

Tubing and Preparation for Solvent Delivery 40 PRIOR TO ANALYSIS LAAQ-B-LC 001 B
Several column types Normal phase Reverse phase Size exclusion Ion exchange LAAQ-B-LC 001 B
Normal phase In this column type, the retention is governed by the interaction of the polar parts of the stationary phase and solute. For retention to occur in normal phase, the packing must be more polar than the mobile phase with respect to the sample LAAQ-B-LC 001 B

Reverse phase In this column the packing material is relatively nonpolar and the solvent is polar with respect to the sample. Retention is the result of the interaction of the nonpolar components of the solutes and the nonpolar stationary phase. Typical stationary phases are nonpolar hydrocarbons, waxy liquids, or bonded hydrocarbons (such as C 18, C 8, etc. ) and the solvents are polar aqueous-organic mixtures such as methanol-water or acetonitrilewater. LAAQ-B-LC 001 B
Size exclusion In size exclusion the HPLC column is consisted of substances which have controlled pore sizes and is able to be filtered in an ordinarily phase according to its molecular size. Small molecules penetrate into the pores within the packing while larger molecules only partially penetrate the pores. The large molecules elute before the smaller molecules. LAAQ-B-LC 001 B
Ion exchange In this column type the sample components are separated based upon attractive ionic forces between molecules carrying charged groups of opposite charge to those charges on the stationary phase. Separations are made between a polar mobile liquid, usually water containing salts or small amounts of alcohols, and a stationary phase containing either acidic or basic fixed sites. LAAQ-B-LC 001 B
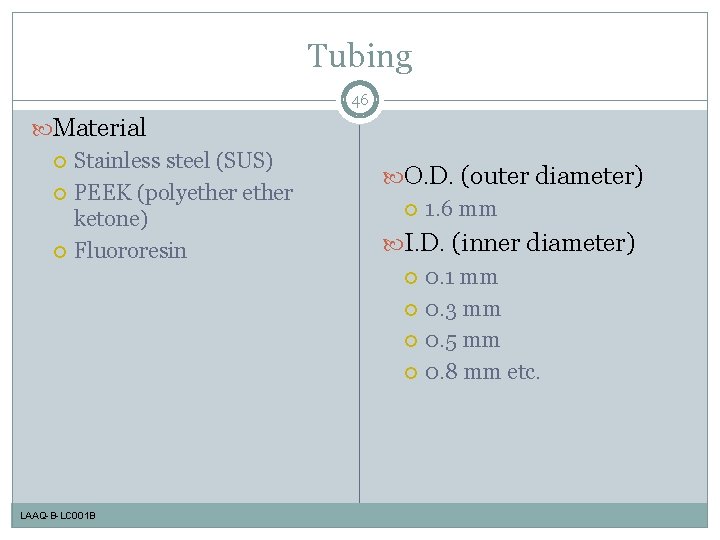
Tubing 46 Material Stainless steel (SUS) PEEK (polyether ketone) Fluororesin LAAQ-B-LC 001 B O. D. (outer diameter) 1. 6 mm I. D. (inner diameter) 0. 1 mm 0. 3 mm 0. 5 mm 0. 8 mm etc.
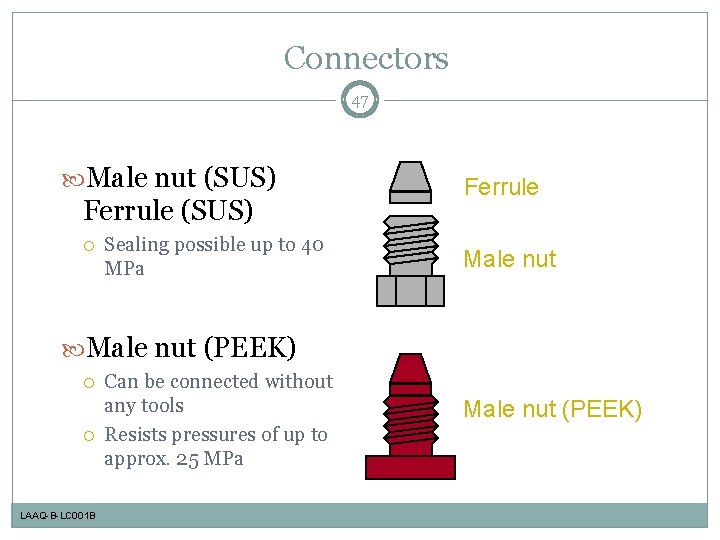
Connectors 47 Male nut (SUS) Ferrule (SUS) Sealing possible up to 40 MPa Ferrule Male nut (PEEK) LAAQ-B-LC 001 B Can be connected without any tools Resists pressures of up to approx. 25 MPa Male nut (PEEK)
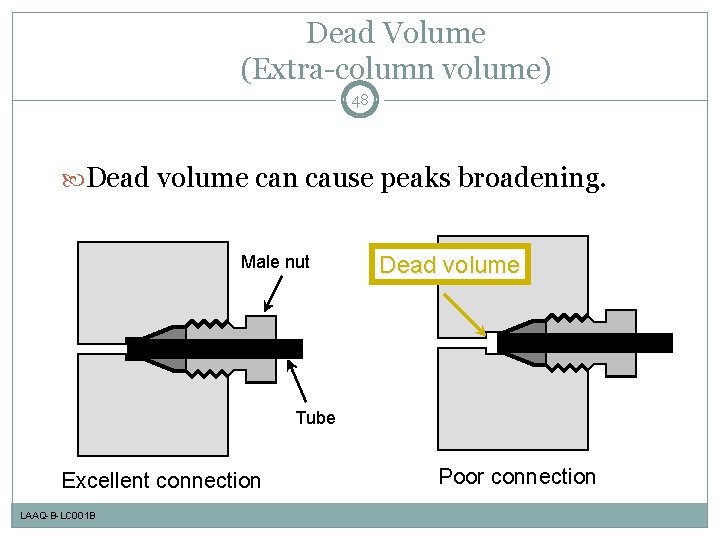
Dead Volume (Extra-column volume) 48 Dead volume can cause peaks broadening. Male nut Dead volume Tube Excellent connection LAAQ-B-LC 001 B Poor connection
Mobile Phase 49 Water “Ultrapure water” can be used with confidence. Commercial “distilled water for HPLC” is also acceptable. Organic Solvent LAAQ-B-LC 001 B HPLC-grade solvent can be used with confidence. Special-grade solvent is acceptable depending on the detection conditions. Care is required regarding solvents containing stabilizers (e. g. , tetrahydrofuran and chloroform)
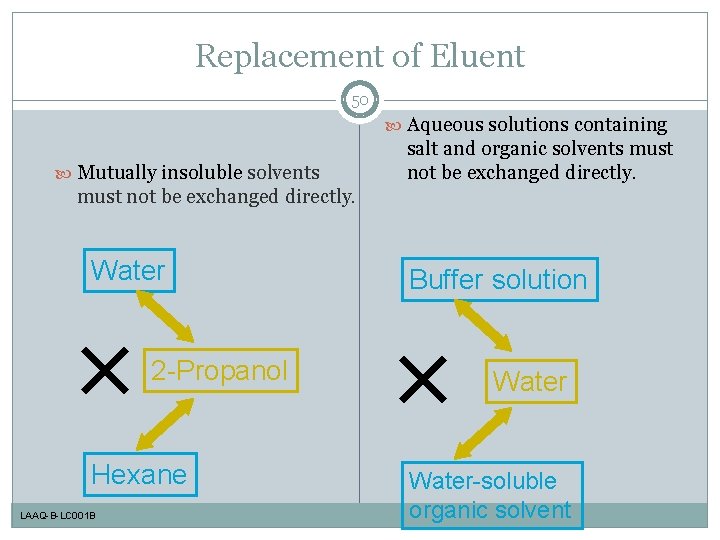
Replacement of Eluent 50 Aqueous solutions containing Mutually insoluble solvents salt and organic solvents must not be exchanged directly. Water 2 -Propanol Hexane LAAQ-B-LC 001 B Buffer solution Water-soluble organic solvent
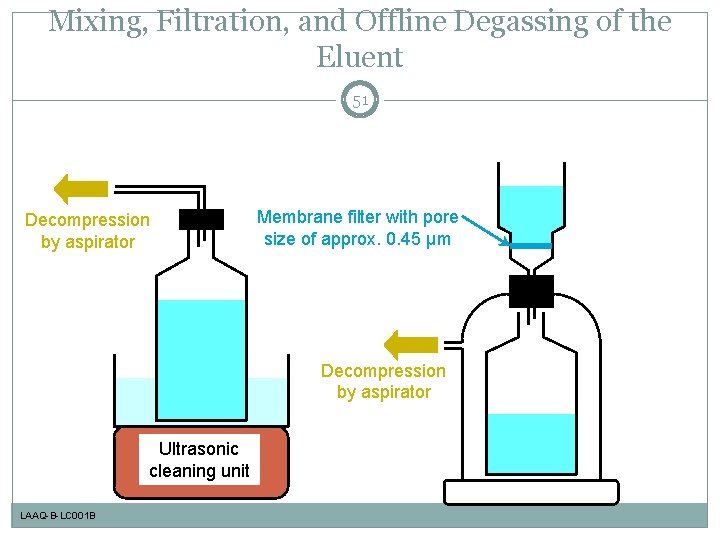
Mixing, Filtration, and Offline Degassing of the Eluent 51 Decompression by aspirator Membrane filter with pore size of approx. 0. 45 µm Decompression by aspirator Ultrasonic cleaning unit LAAQ-B-LC 001 B
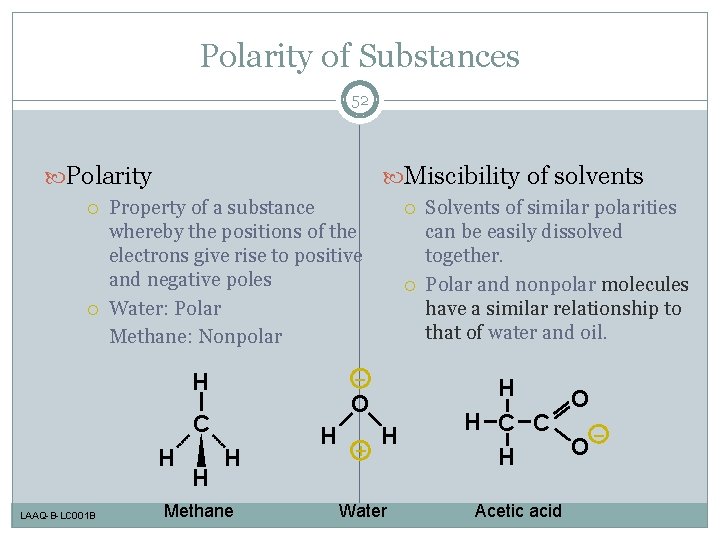
Polarity of Substances 52 Polarity Miscibility of solvents Property of a substance whereby the positions of the electrons give rise to positive and negative poles Water: Polar Methane: Nonpolar H LAAQ-B-LC 001 B H – O C H H Methane H + H Water Solvents of similar polarities can be easily dissolved together. Polar and nonpolar molecules have a similar relationship to that of water and oil. H O H C C – O H Acetic acid
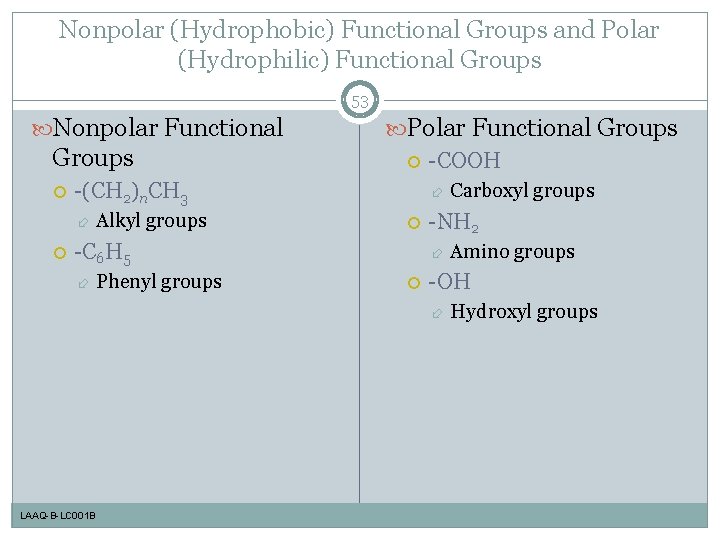
Nonpolar (Hydrophobic) Functional Groups and Polar (Hydrophilic) Functional Groups 53 Nonpolar Functional Groups -(CH 2)n. CH 3 Polar Functional Groups -COOH Alkyl groups -C 6 H 5 Phenyl groups -NH 2 Amino groups -OH LAAQ-B-LC 001 B Carboxyl groups Hydroxyl groups

Partition Chromatography 54 A liquid (or a substance regarded as a liquid) is used as the stationary phase, and the solute is separated according to whether it dissolves more readily in the stationary or mobile phase. Liquid-liquid chromatography LAAQ-B-LC 001 B
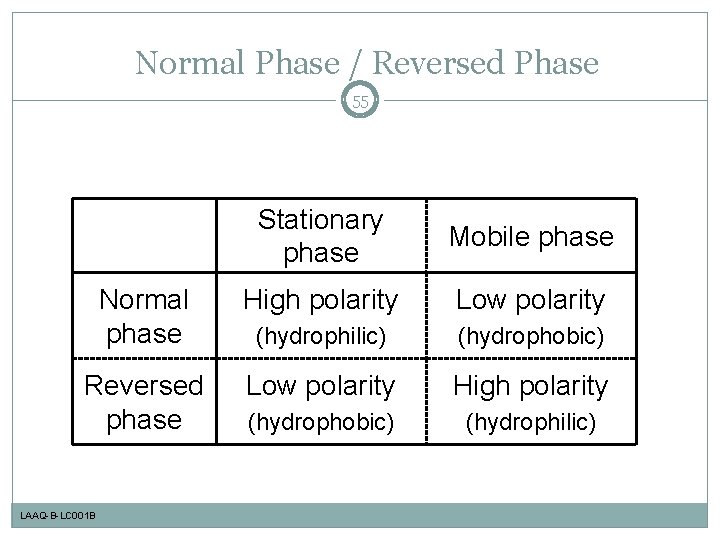
Normal Phase / Reversed Phase 55 Stationary phase Mobile phase Normal phase High polarity Low polarity (hydrophilic) (hydrophobic) Reversed phase Low polarity High polarity (hydrophobic) (hydrophilic) LAAQ-B-LC 001 B
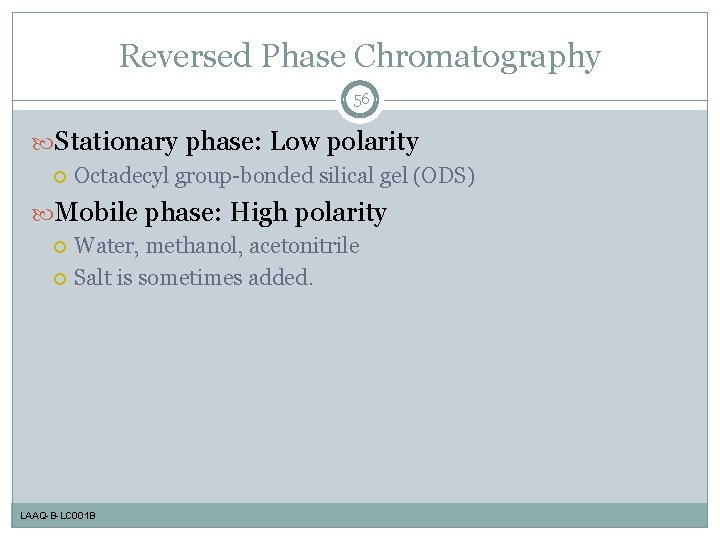
Reversed Phase Chromatography 56 Stationary phase: Low polarity Octadecyl group-bonded silical gel (ODS) Mobile phase: High polarity Water, methanol, acetonitrile Salt is sometimes added. LAAQ-B-LC 001 B
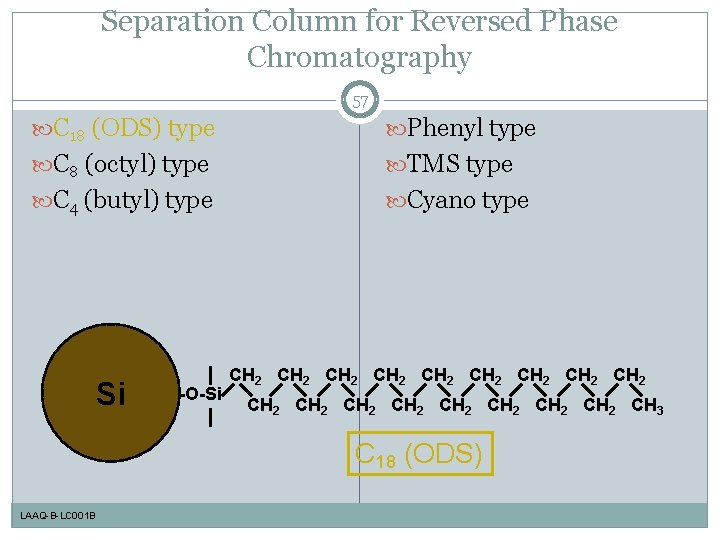
Separation Column for Reversed Phase Chromatography 57 C 18 (ODS) type Phenyl type C 8 (octyl) type TMS type C 4 (butyl) type Cyano type Si -O-Si CH 2 CH 2 CH 2 CH 2 CH 2 CH 3 C 18 (ODS) LAAQ-B-LC 001 B
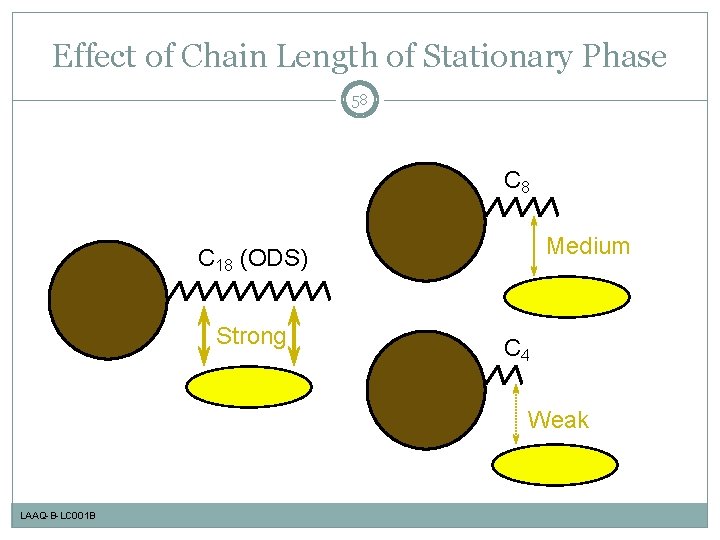
Effect of Chain Length of Stationary Phase 58 C 8 Medium C 18 (ODS) Strong C 4 Weak LAAQ-B-LC 001 B
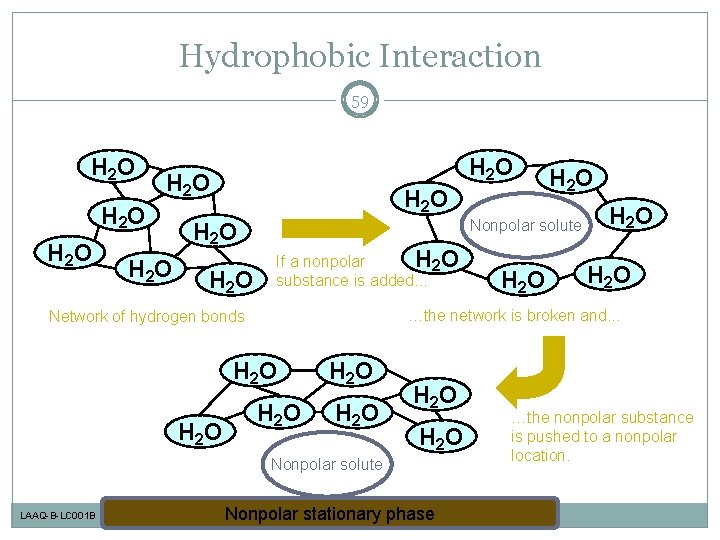
Hydrophobic Interaction 59 H 2 O H 2 O H 2 O Nonpolar solute If a nonpolar H 2 O substance is added. . . H 2 O H 2 O Nonpolar solute LAAQ-B-LC 001 B H 2 O …the network is broken and. . . Network of hydrogen bonds H 2 O Nonpolar stationary phase …the nonpolar substance is pushed to a nonpolar location.
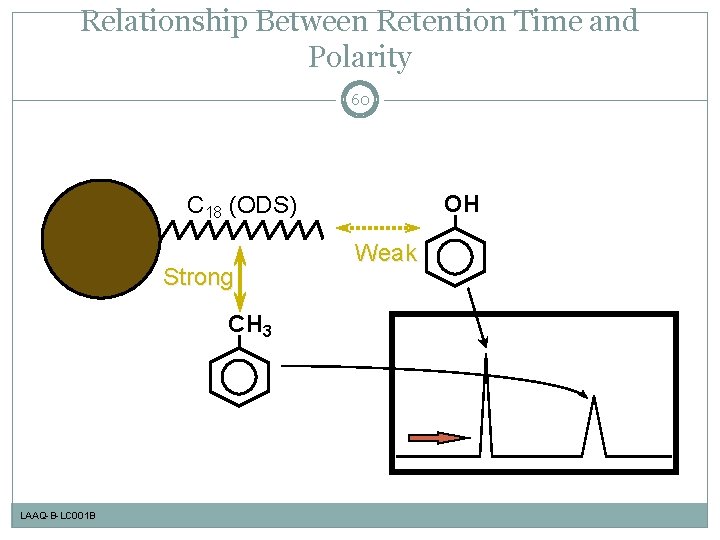
Relationship Between Retention Time and Polarity 60 OH C 18 (ODS) Strong CH 3 LAAQ-B-LC 001 B Weak

Basic Settings for Eluent Used in Reversed Phase Mode 61 Water (buffer solution) + water-soluble organic solvent Water-soluble organic solvent: Methanol Acetonitrile Tetrahydrofuran etc. The mixing ratio of the water (buffer solution) and organic solvent has the greatest influence on separation. If a buffer solution is used, its p. H value is an important separation parameter. LAAQ-B-LC 001 B
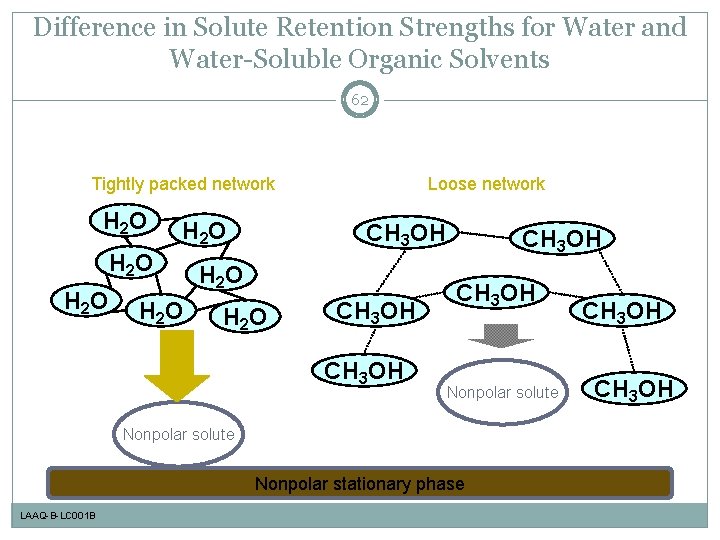
Difference in Solute Retention Strengths for Water and Water-Soluble Organic Solvents 62 Tightly packed network H 2 O H 2 O Loose network CH 3 OH H 2 O CH 3 OH Nonpolar solute Nonpolar stationary phase LAAQ-B-LC 001 B CH 3 OH
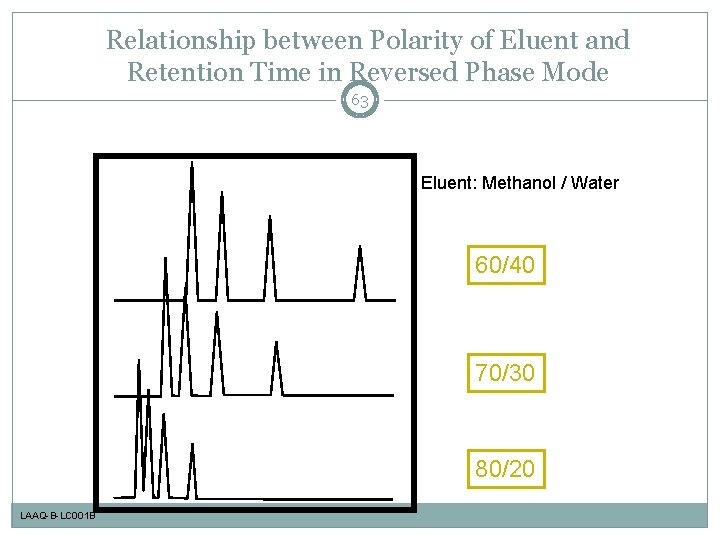
Relationship between Polarity of Eluent and Retention Time in Reversed Phase Mode 63 Eluent: Methanol / Water 60/40 70/30 80/20 LAAQ-B-LC 001 B
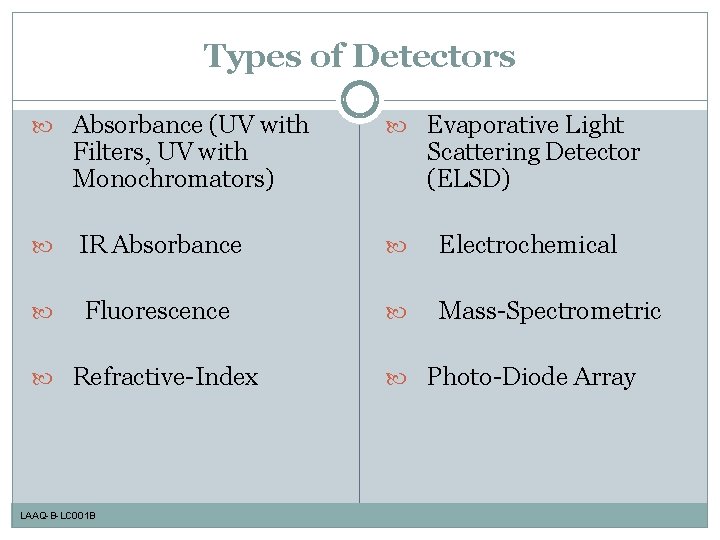
Types of Detectors Absorbance (UV with Filters, UV with Monochromators) Evaporative Light Scattering Detector (ELSD) IR Absorbance Electrochemical Fluorescence Mass-Spectrometric Refractive-Index LAAQ-B-LC 001 B Photo-Diode Array

Chromatogram Parameters 65 METHODS FOR EXPRESSING SEPARATION AND COLUMN PERFORMANCE LAAQ-B-LC 001 B
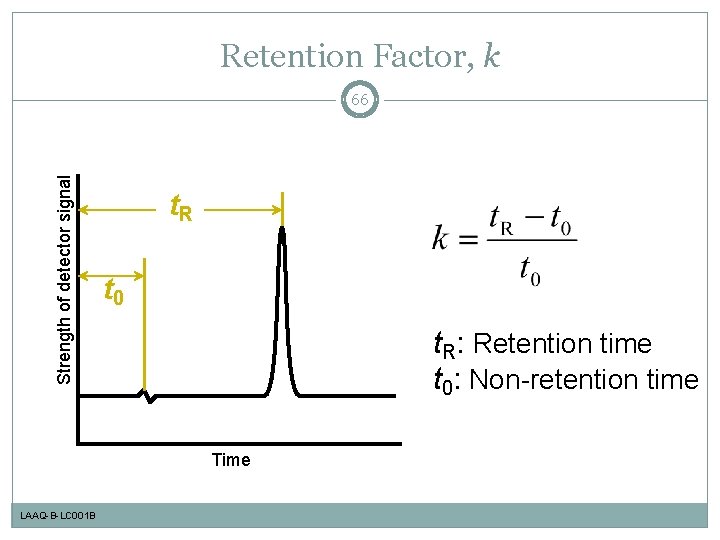
Retention Factor, k Strength of detector signal 66 t. R t 0 t. R: Retention time t 0: Non-retention time Time LAAQ-B-LC 001 B
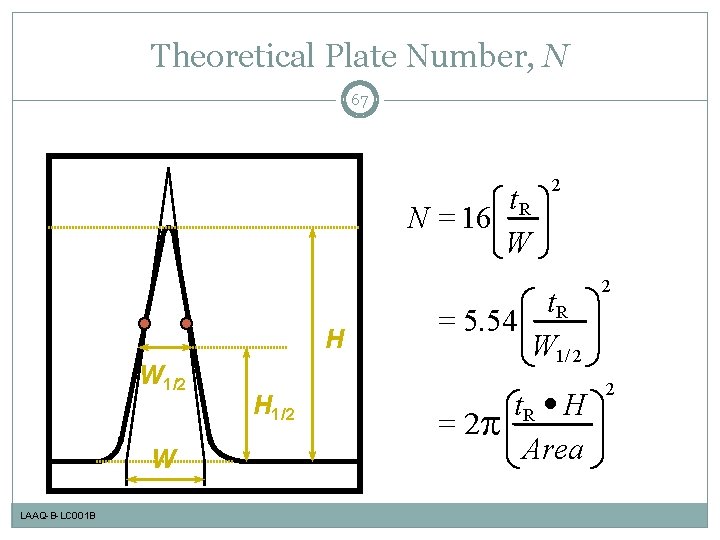
Theoretical Plate Number, N 67 t. R N = 16 W H W 1/2 W LAAQ-B-LC 001 B H 1/2 2 t. R = 5. 54 W 1/ 2 2 t. R · H Area 2 = 2
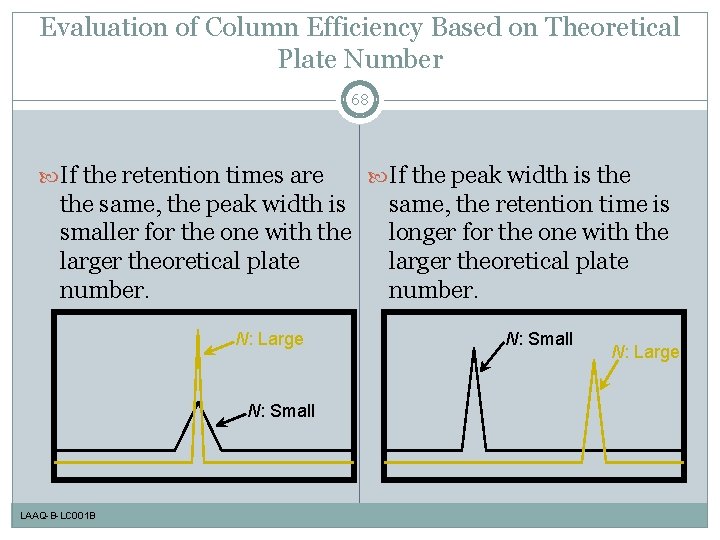
Evaluation of Column Efficiency Based on Theoretical Plate Number 68 If the retention times are the same, the peak width is smaller for the one with the larger theoretical plate number. N: Large N: Small LAAQ-B-LC 001 B If the peak width is the same, the retention time is longer for the one with the larger theoretical plate number. N: Small N: Large
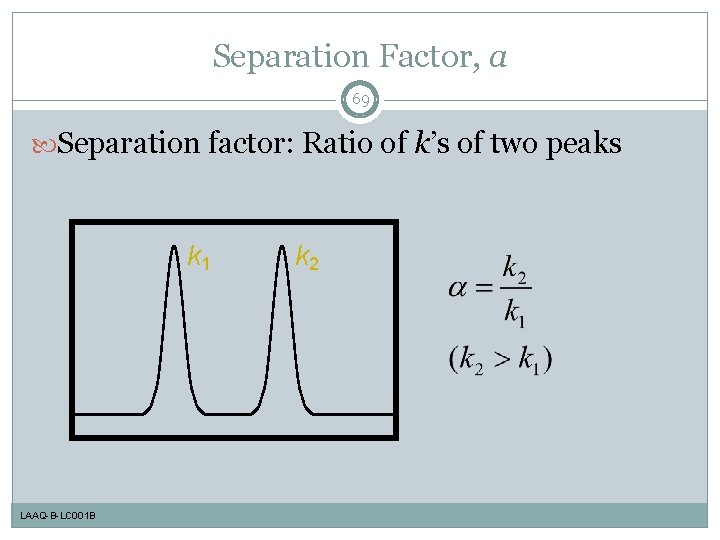
Separation Factor, a 69 Separation factor: Ratio of k’s of two peaks k 1 LAAQ-B-LC 001 B k 2
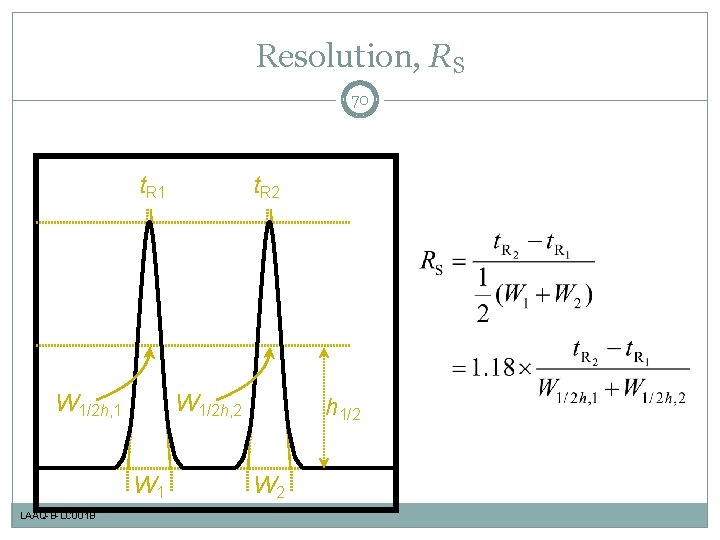
Resolution, RS 70 t. R 1 W 1/2 h, 2 W 1 LAAQ-B-LC 001 B t. R 2 h 1/2 W 2
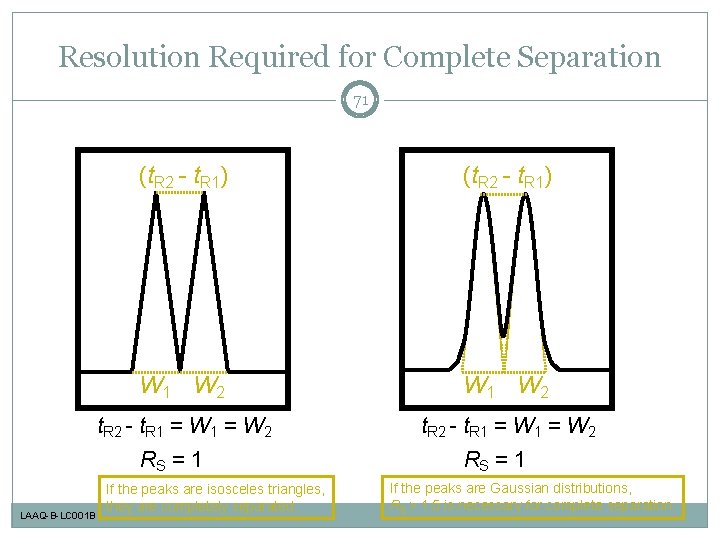
Resolution Required for Complete Separation 71 (t. R 2 - t. R 1) W 1 W 2 t. R 2 - t. R 1 = W 2 RS = 1 LAAQ-B-LC 001 B If the peaks are isosceles triangles, they are completely separated. RS = 1 If the peaks are Gaussian distributions, RS > 1. 5 is necessary for complete separation.
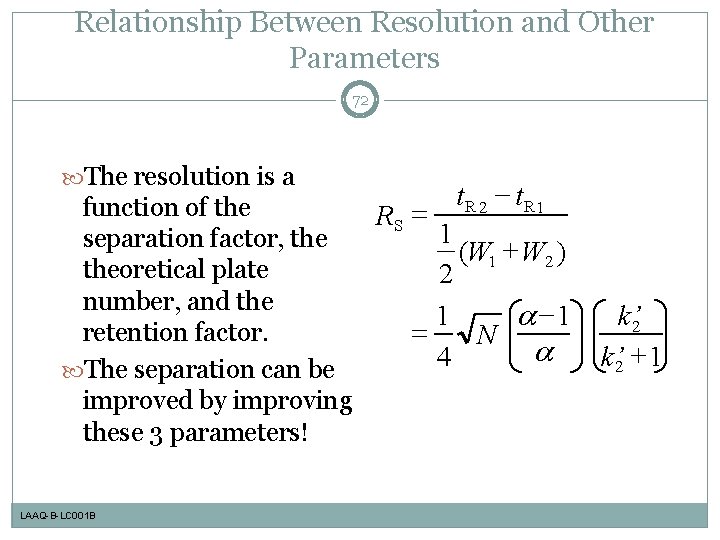
Relationship Between Resolution and Other Parameters 72 The resolution is a - t. R 2 t. R 1 function of the RS = 1 separation factor, the (W 1 + W 2 ) theoretical plate 2 number, and the -1 1 retention factor. = N 4 The separation can be improved by improving these 3 parameters! LAAQ-B-LC 001 B k’ 2 + 1
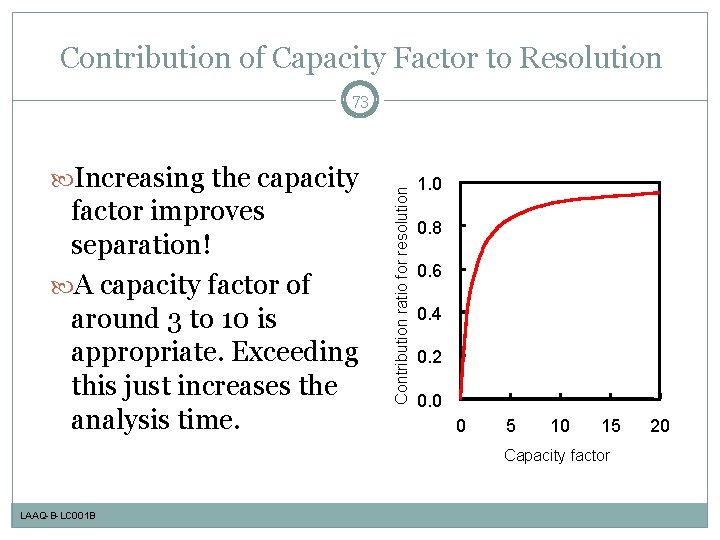
Contribution of Capacity Factor to Resolution Increasing the capacity factor improves separation! A capacity factor of around 3 to 10 is appropriate. Exceeding this just increases the analysis time. Contribution ratio for resolution 73 1. 0 0. 8 0. 6 0. 4 0. 2 0. 0 0 5 10 15 Capacity factor LAAQ-B-LC 001 B 20
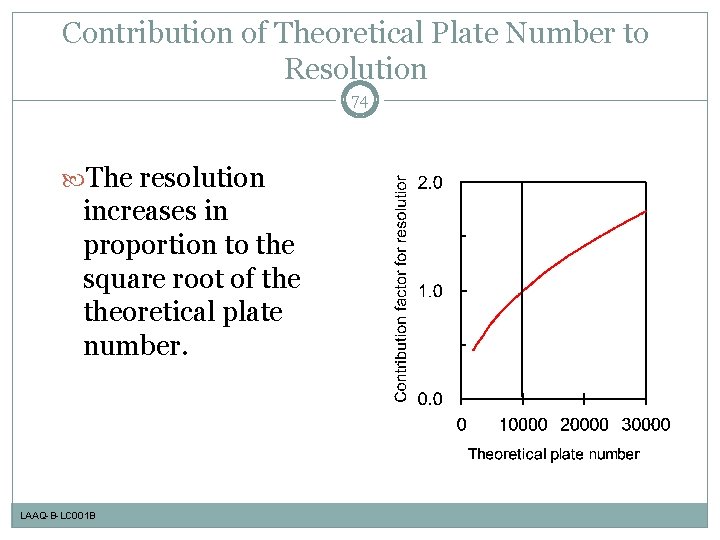
Contribution of Theoretical Plate Number to Resolution 74 The resolution increases in proportion to the square root of theoretical plate number. LAAQ-B-LC 001 B
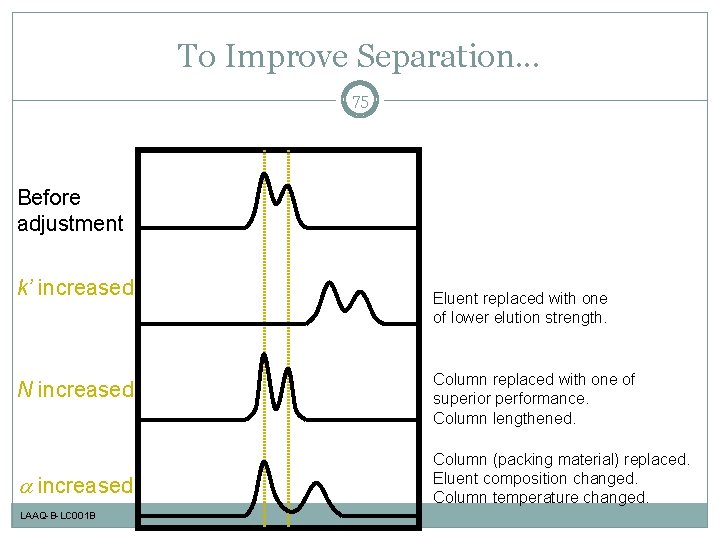
To Improve Separation. . . 75 Before adjustment k’ increased N increased LAAQ-B-LC 001 B Eluent replaced with one of lower elution strength. Column replaced with one of superior performance. Column lengthened. Column (packing material) replaced. Eluent composition changed. Column temperature changed.

p. H Buffer Solution Used for Eluent 76 SELECTION AND PREPARATION OF BUFFER SOLUTION LAAQ-B-LC 001 B
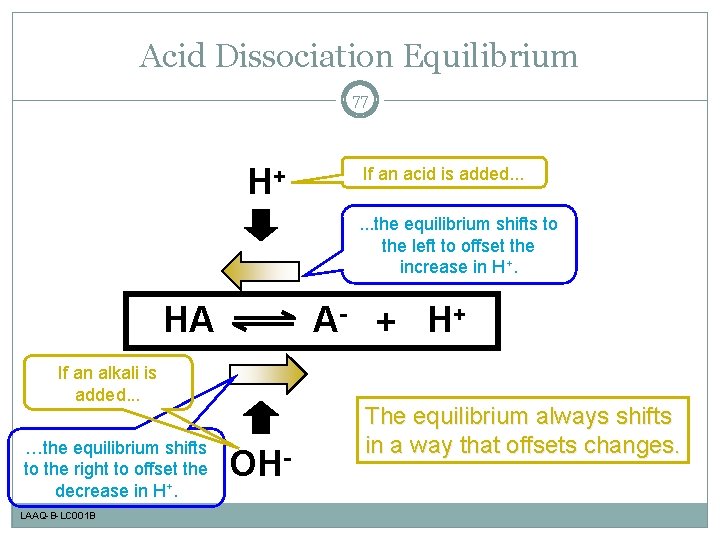
Acid Dissociation Equilibrium 77 H+ If an acid is added. . . the equilibrium shifts to the left to offset the increase in H+. HA A- + H+ If an alkali is added. . . …the equilibrium shifts to the right to offset the decrease in H+. LAAQ-B-LC 001 B OH- The equilibrium always shifts in a way that offsets changes.
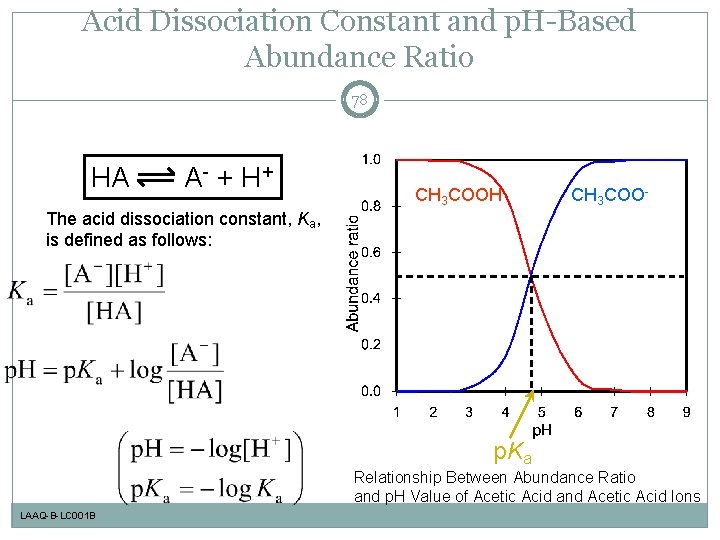
Acid Dissociation Constant and p. H-Based Abundance Ratio 78 HA A - + H+ CH 3 COOH CH 3 COO- The acid dissociation constant, Ka, is defined as follows: p. Ka Relationship Between Abundance Ratio and p. H Value of Acetic Acid and Acetic Acid Ions LAAQ-B-LC 001 B

Preparing p. H Buffer Solution 79 Use a weak acid with a p. Ka value close to the desired p. H value. Example: Preparing a buffer solution for a p. H value of around 4. 8. Use acetic acid, which has a p. Ka value of 4. 8. Make the concentrations of HA and A- roughly equal. Mix an acid with its salt. Example: Mix acetic acid and sodium acetate so that they have the same molar concentration. LAAQ-B-LC 001 B
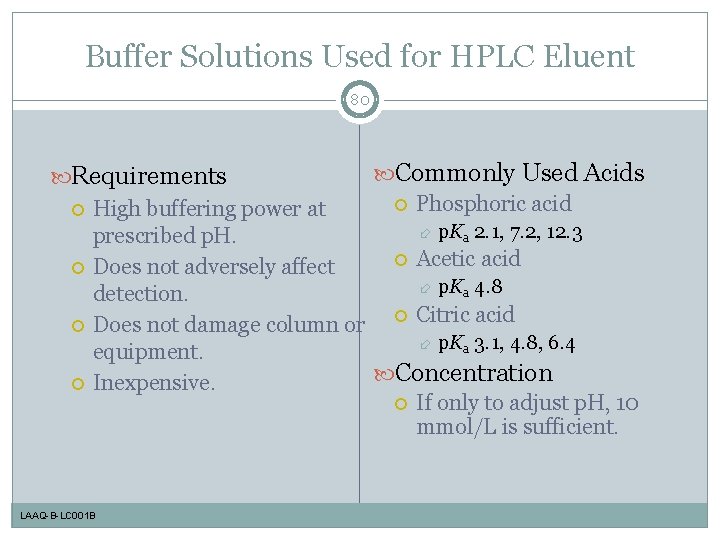
Buffer Solutions Used for HPLC Eluent 80 Commonly Used Acids Requirements Phosphoric acid High buffering power at p. Ka 2. 1, 7. 2, 12. 3 prescribed p. H. Acetic acid Does not adversely affect p. Ka 4. 8 detection. Citric acid Does not damage column or p. Ka 3. 1, 4. 8, 6. 4 equipment. Concentration Inexpensive. If only to adjust p. H, 10 mmol/L is sufficient. LAAQ-B-LC 001 B
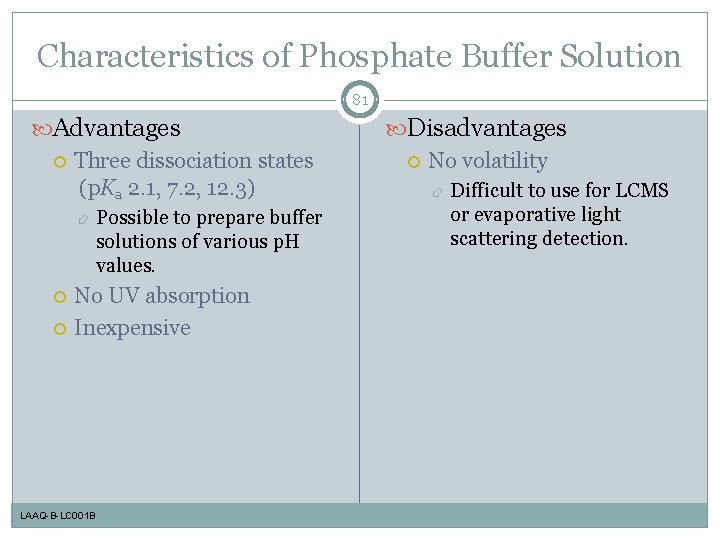
Characteristics of Phosphate Buffer Solution 81 Advantages Three dissociation states (p. Ka 2. 1, 7. 2, 12. 3) Possible to prepare buffer solutions of various p. H values. No UV absorption Inexpensive LAAQ-B-LC 001 B Disadvantages No volatility Difficult to use for LCMS or evaporative light scattering detection.

Guidelines for Setting Mobile Phase Conditions (1) Neutral (Nonionic) Substances 82 Eluent Composition Water / acetonitrile Water / methanol Separation Adjustment Changing the mixing ratio of the water and organic solvent Changing the type of organic solvent LAAQ-B-LC 001 B
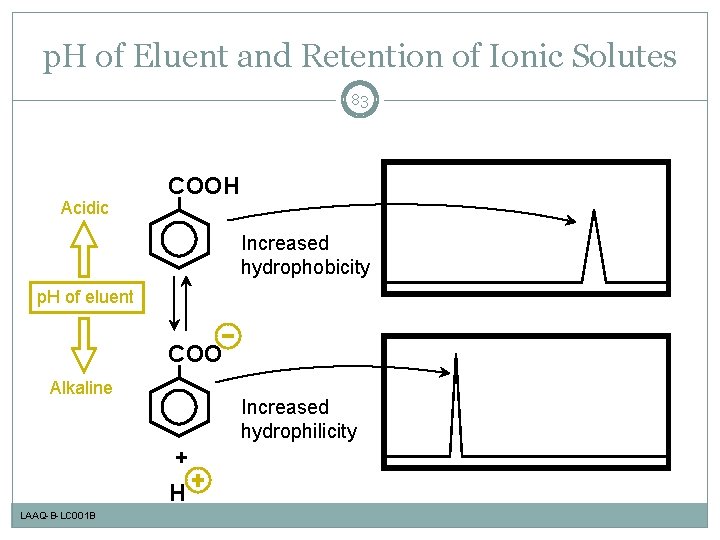
p. H of Eluent and Retention of Ionic Solutes 83 Acidic COOH Increased hydrophobicity p. H of eluent COO Alkaline Increased hydrophilicity + H LAAQ-B-LC 001 B

Guidelines for Setting Mobile Phase Conditions (2) Acidic (Anionic) Substances 84 Eluent Composition Acidic buffer solution / acetonitrile Acidic buffer solution / methanol Increase retention strength by making the eluent acidic and suppressing ionization! LAAQ-B-LC 001 B
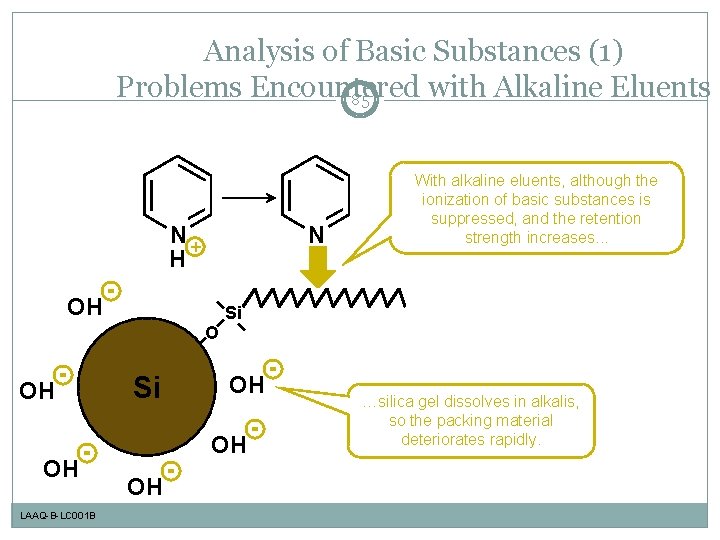
Analysis of Basic Substances (1) Problems Encountered with Alkaline Eluents 85 N+ H N OH With alkaline eluents, although the ionization of basic substances is suppressed, and the retention strength increases. . . Si O OH OH LAAQ-B-LC 001 B Si OH OH OH …silica gel dissolves in alkalis, so the packing material deteriorates rapidly.
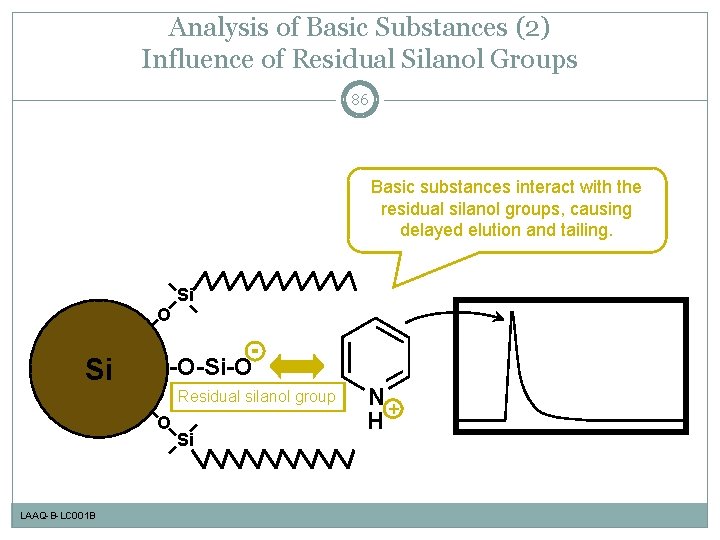
Analysis of Basic Substances (2) Influence of Residual Silanol Groups 86 Basic substances interact with the residual silanol groups, causing delayed elution and tailing. Si O Si -O-Si-O Residual silanol group O LAAQ-B-LC 001 B Si N+ H
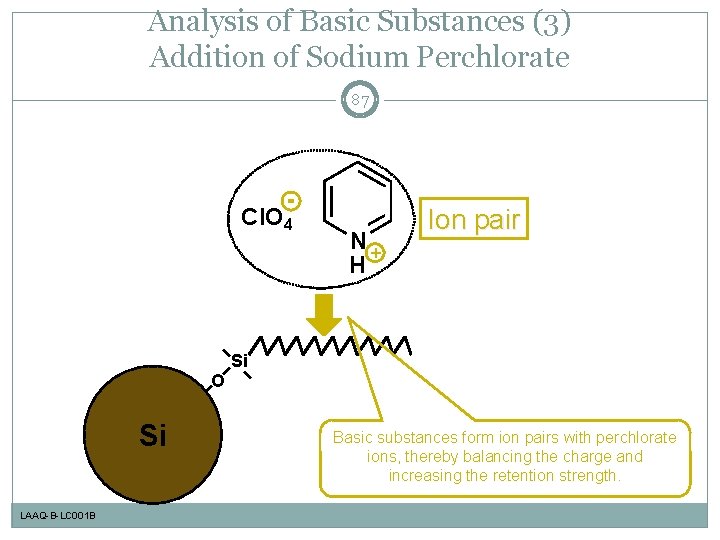
Analysis of Basic Substances (3) Addition of Sodium Perchlorate 87 Cl. O 4 N+ H Ion pair Si O Si LAAQ-B-LC 001 B Basic substances form ion pairs with perchlorate ions, thereby balancing the charge and increasing the retention strength.
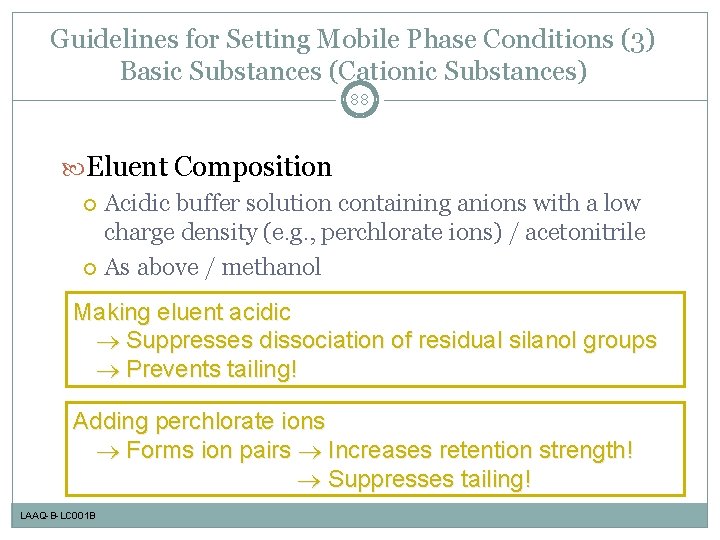
Guidelines for Setting Mobile Phase Conditions (3) Basic Substances (Cationic Substances) 88 Eluent Composition Acidic buffer solution containing anions with a low charge density (e. g. , perchlorate ions) / acetonitrile As above / methanol Making eluent acidic Suppresses dissociation of residual silanol groups Prevents tailing! Adding perchlorate ions Forms ion pairs Increases retention strength! Suppresses tailing! LAAQ-B-LC 001 B
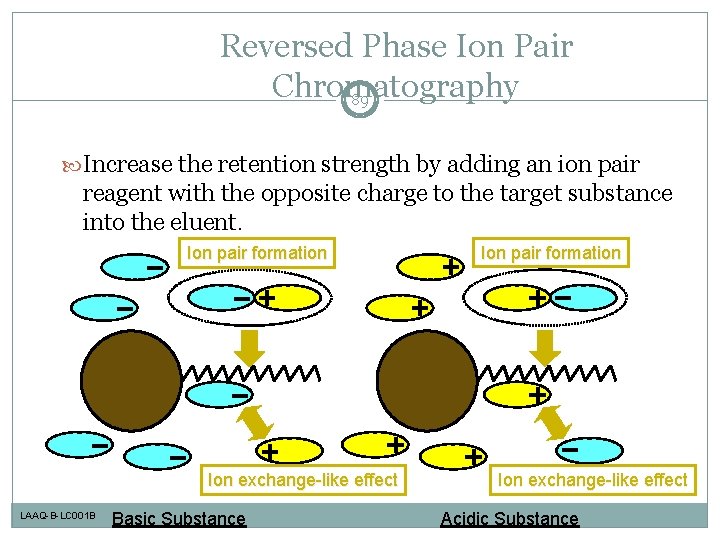
Reversed Phase Ion Pair Chromatography 89 Increase the retention strength by adding an ion pair reagent with the opposite charge to the target substance into the eluent. Ion pair formation Ion exchange-like effect LAAQ-B-LC 001 B Basic Substance Ion pair formation Ion exchange-like effect Acidic Substance
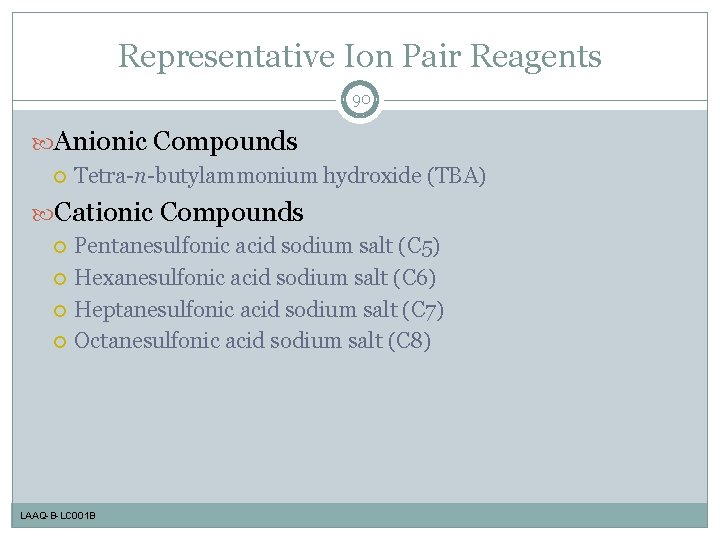
Representative Ion Pair Reagents 90 Anionic Compounds Tetra-n-butylammonium hydroxide (TBA) Cationic Compounds Pentanesulfonic acid sodium salt (C 5) Hexanesulfonic acid sodium salt (C 6) Heptanesulfonic acid sodium salt (C 7) Octanesulfonic acid sodium salt (C 8) LAAQ-B-LC 001 B
Points to Note Concerning the Use of Ion Pairs 91 Selection of Ion Pair Reagent In general, the retention strength increases with the length of the alkyl chain. p. H of Eluent The retention strength changes according to whether or not ionization takes place. Concentration of Ion Pair Reagent In general, the retention strength increases with the ion pair concentration, but there is an upper limit. Proportion of Organic Solvent in Eluent LAAQ-B-LC 001 B Optimize the separation conditions by considering the type and concentration of the ion pair reagent.
HPLC Separation Modes 92 SEPARATION MODES OTHER THAN REVERSED PHASE CHROMATOGRAPHY LAAQ-B-LC 001 B
HPLC Separation Modes 93 Adsorption (liquid-solid) chromatography Partition (liquid-liquid) chromatography Normal phase partition chromatography Reversed phase partition chromatography Ion exchange chromatography Size exclusion chromatography LAAQ-B-LC 001 B
Adsorption Chromatography 94 A solid such as silica gel is used as the stationary phase, and differences, mainly in the degree of adsorption to its surface, are used to separate the solutes. Liquid-solid chromatography The retention strength increases with the hydrophilicity of the solute. LAAQ-B-LC 001 B

Partition Chromatography 95 A liquid (or a substance regarded as a liquid) is used as the stationary phase, and the solute is separated according to whether it dissolves more readily in the stationary or mobile phase. Liquid-liquid chromatography LAAQ-B-LC 001 B
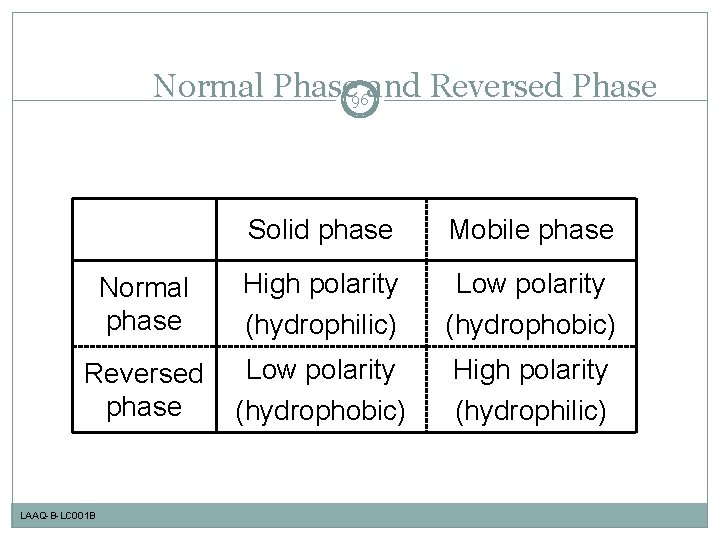
Normal Phase 96 and Reversed Phase Solid phase Mobile phase Normal phase High polarity (hydrophilic) Low polarity (hydrophobic) Reversed phase Low polarity (hydrophobic) High polarity (hydrophilic) LAAQ-B-LC 001 B
Normal Phase (Partition) Chromatography 97 Partition chromatography in which the stationary phase has a high polarity (hydrophilic) and the mobile phase has a low polarity (hydrophobic) Essentially based on the same separation mechanism as adsorption chromatography in which the stationary phase has a hydrophilic base, such as silica gel LAAQ-B-LC 001 B
Invention of Chromatography by M. Tswett 98 Ether Chlorophyll Ca. CO 3 LAAQ-B-LC 001 B Chromatography Chromato Colors
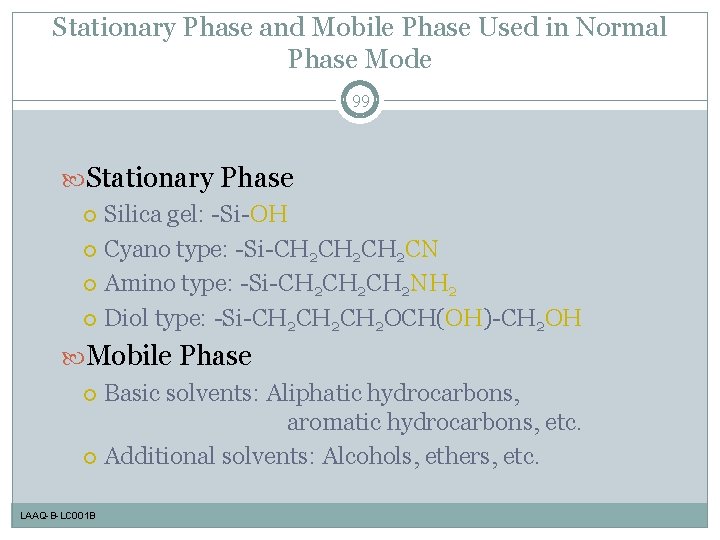
Stationary Phase and Mobile Phase Used in Normal Phase Mode 99 Stationary Phase Silica gel: -Si-OH Cyano type: -Si-CH 2 CH 2 CN Amino type: -Si-CH 2 CH 2 NH 2 Diol type: -Si-CH 2 CH 2 OCH(OH)-CH 2 OH Mobile Phase Basic solvents: Aliphatic hydrocarbons, aromatic hydrocarbons, etc. Additional solvents: Alcohols, ethers, etc. LAAQ-B-LC 001 B
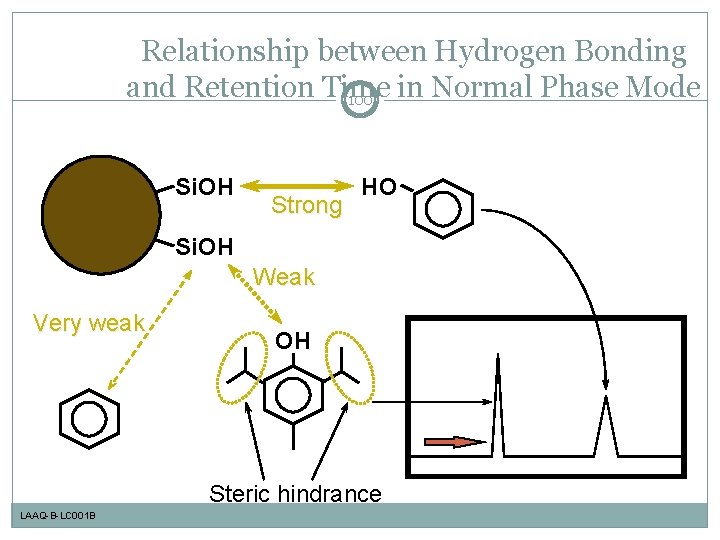
Relationship between Hydrogen Bonding and Retention Time in Normal Phase Mode 100 Si. OH Strong HO Si. OH Weak Very weak OH Steric hindrance LAAQ-B-LC 001 B
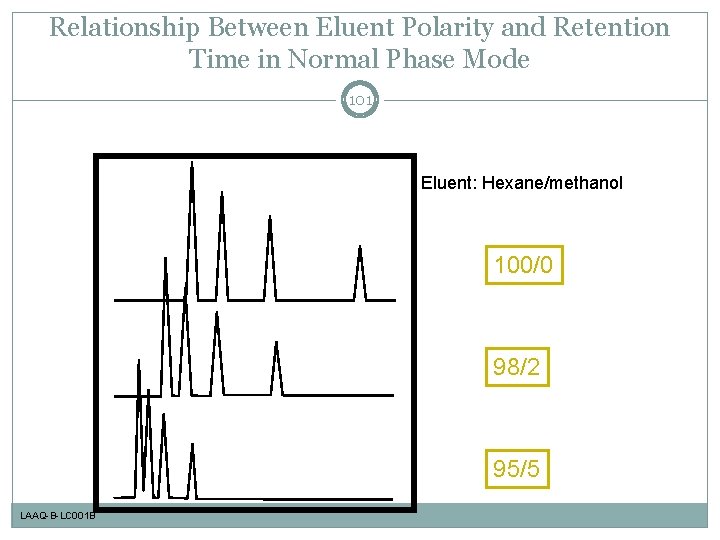
Relationship Between Eluent Polarity and Retention Time in Normal Phase Mode 101 Eluent: Hexane/methanol 100/0 98/2 95/5 LAAQ-B-LC 001 B
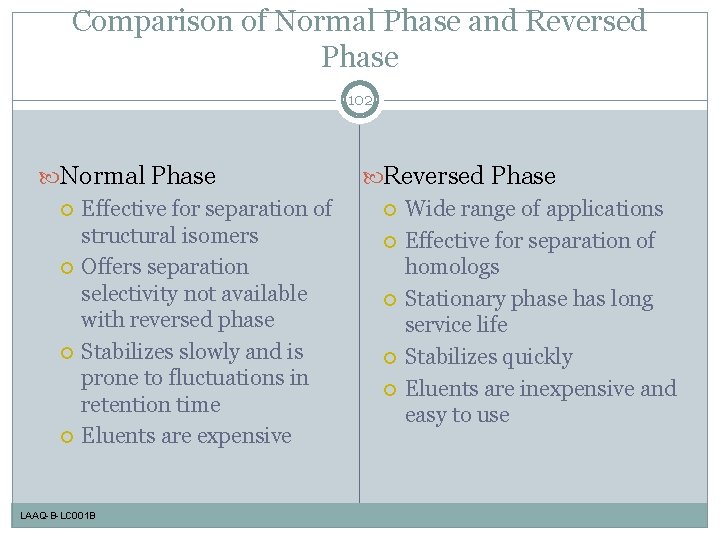
Comparison of Normal Phase and Reversed Phase 102 Normal Phase Effective for separation of structural isomers Offers separation selectivity not available with reversed phase Stabilizes slowly and is prone to fluctuations in retention time Eluents are expensive LAAQ-B-LC 001 B Reversed Phase Wide range of applications Effective for separation of homologs Stationary phase has long service life Stabilizes quickly Eluents are inexpensive and easy to use
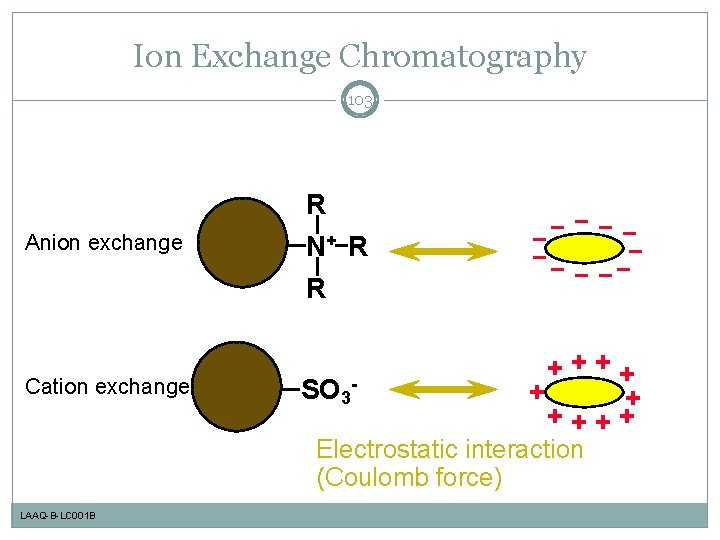
Ion Exchange Chromatography 103 Anion exchange Cation exchange R N+ R R SO 3 - ++++ + + ++++ Electrostatic interaction (Coulomb force) LAAQ-B-LC 001 B

Stationary Phase Used in Ion Exchange Mode 104 Base Material Resin is often used. Silica gel is also used. Cation Exchange Column Strong cation exchange (SCX) Week cation exchange (WCX) Anion Exchange Column Strong anion exchange (SAX) Week anion exchange (WAX) LAAQ-B-LC 001 B -SO 3 -COO-NR 3+ -NHR 2+
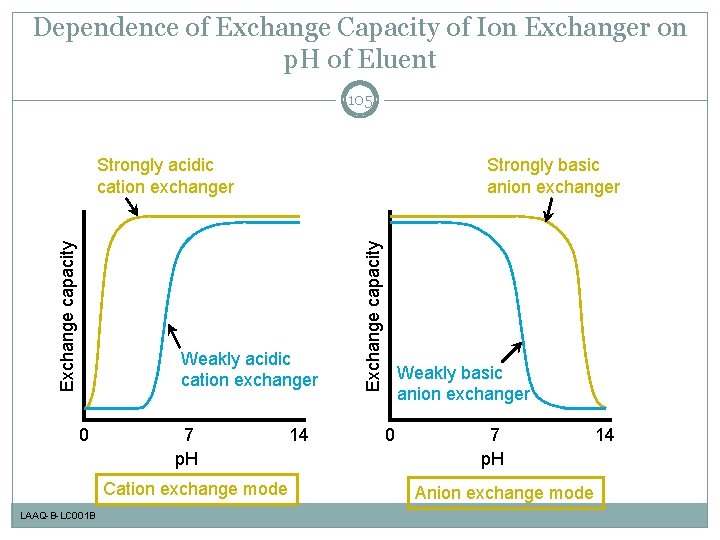
Dependence of Exchange Capacity of Ion Exchanger on p. H of Eluent 105 Strongly basic anion exchanger Weakly acidic cation exchanger 0 7 p. H Cation exchange mode LAAQ-B-LC 001 B 14 Exchange capacity Strongly acidic cation exchanger Weakly basic anion exchanger 0 7 p. H Anion exchange mode 14
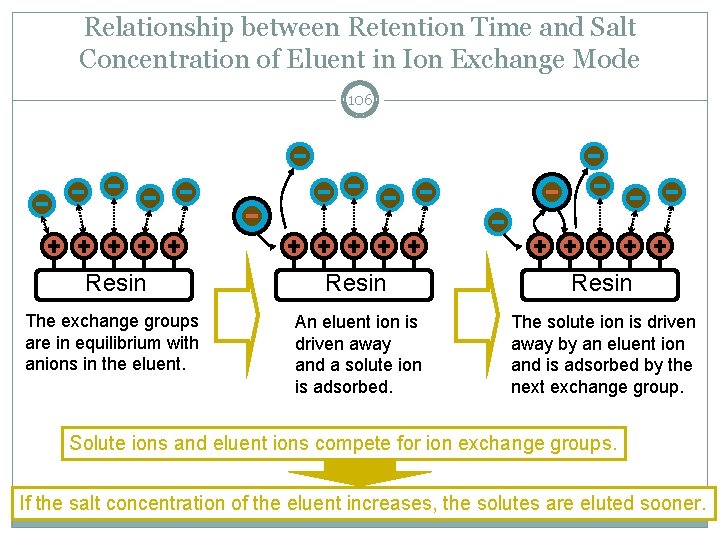
Relationship between Retention Time and Salt Concentration of Eluent in Ion Exchange Mode 106 Resin The exchange groups are in equilibrium with anions in the eluent. An eluent ion is driven away and a solute ion is adsorbed. The solute ion is driven away by an eluent ion and is adsorbed by the next exchange group. Solute ions and eluent ions compete for ion exchange groups. If the salt concentration of the eluent increases, the solutes are eluted sooner. LAAQ-B-LC 001 B
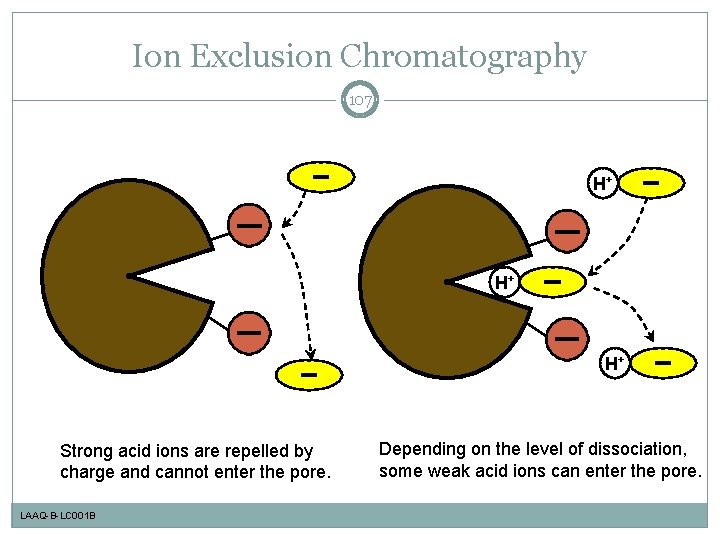
Ion Exclusion Chromatography 107 H+ H+ H+ Strong acid ions are repelled by charge and cannot enter the pore. LAAQ-B-LC 001 B Depending on the level of dissociation, some weak acid ions can enter the pore.
Size Exclusion Chromatography 108 Separation is based on the size (bulkiness) of molecules. The name varies with the application field! Size Exclusion Chromatography (SEC) Gel Permeation Chromatography (GPC) Chemical systems Gel industry fields, synthetic polymers, nonaqueous Filtration Chromatography (GFC) Biochemical systems LAAQ-B-LC 001 B fields, biological macromolecules, aqueous
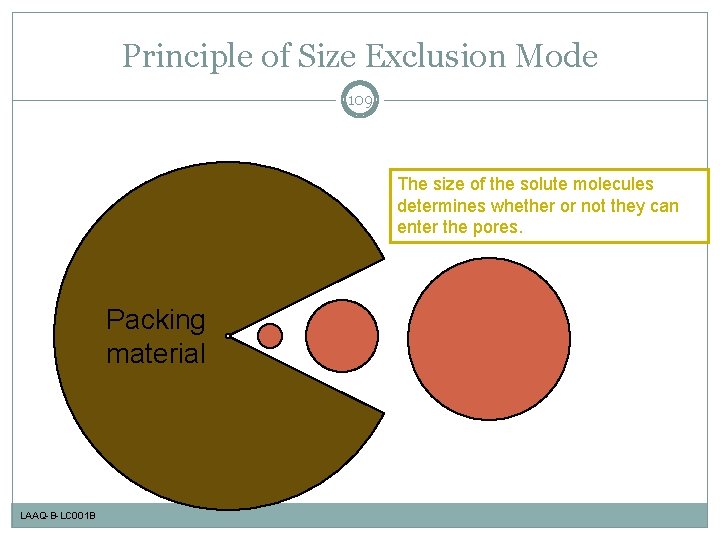
Principle of Size Exclusion Mode 109 The size of the solute molecules determines whether or not they can enter the pores. Packing material LAAQ-B-LC 001 B
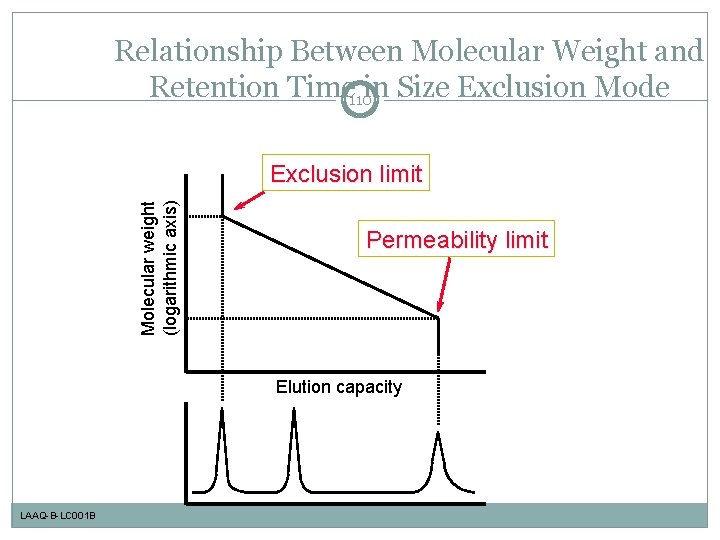
Relationship Between Molecular Weight and Retention Time 110 in Size Exclusion Mode Molecular weight (logarithmic axis) Exclusion limit Permeability limit Elution capacity LAAQ-B-LC 001 B
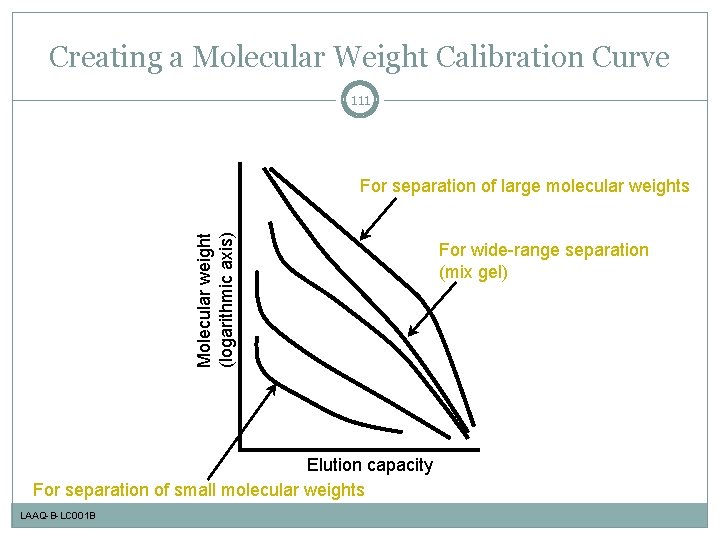
Creating a Molecular Weight Calibration Curve 111 Molecular weight (logarithmic axis) For separation of large molecular weights Elution capacity For separation of small molecular weights LAAQ-B-LC 001 B For wide-range separation (mix gel)
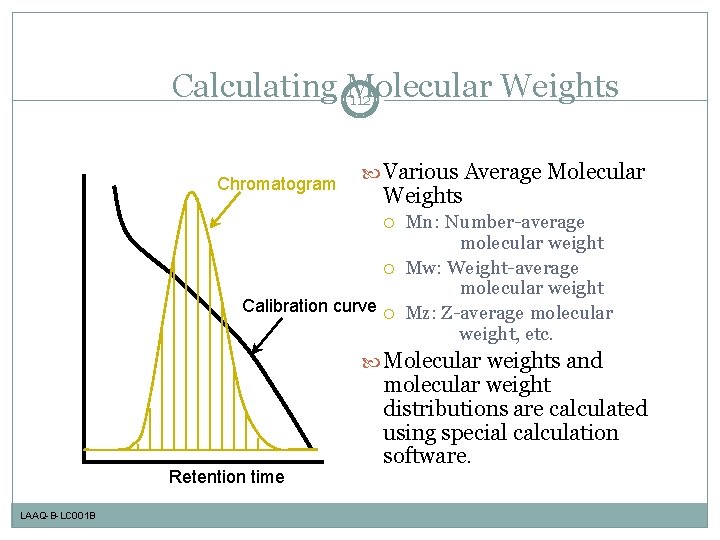
Calculating Molecular Weights 112 Chromatogram Various Average Molecular Weights Calibration curve Mn: Number-average molecular weight Mw: Weight-average molecular weight Mz: Z-average molecular weight, etc. Molecular weights and Retention time LAAQ-B-LC 001 B molecular weight distributions are calculated using special calculation software.
Guidelines for Selecting Separation Mode (1) Required 113 Information Soluble solvent Molecular weight Structural formula and chemical properties Do the substances ionize? Is there UV absorption or fluorescence? Is derivatization possible? etc. LAAQ-B-LC 001 B

Guidelines for Selecting Separation Mode (2) Basic Policy 114 Reversed phase mode using an ODS column is the first choice! Exceptions Large molecular weight (> 2, 000) Size exclusion Optical isomers Chiral column Stereoisomers, positional isomers Normal phase / adsorption Inorganic ions Ion chromatography Sugars, amino acids, short-chain fatty acids Special column LAAQ-B-LC 001 B

HPLC Hardware: Part 2 115 DETECTORS AND THEIR RANGES OF APPLICATION LAAQ-B-LC 001 B

Detection Condition Requirements 116 Sensitivity The detector must have the appropriate level of sensitivity. Selectivity The detector must be able to detect the target substance without, if possible, detecting other substances. Adaptability to separation conditions Operability, etc. LAAQ-B-LC 001 B
Representative HPLC Detectors 117 UV-VIS absorbance detector Photodiode array-type UV-VIS absorbance detector Fluorescence detector Refractive index detector Evaporative light scattering detector Electrical conductivity detector Electrochemical detector Mass spectrometer LAAQ-B-LC 001 B
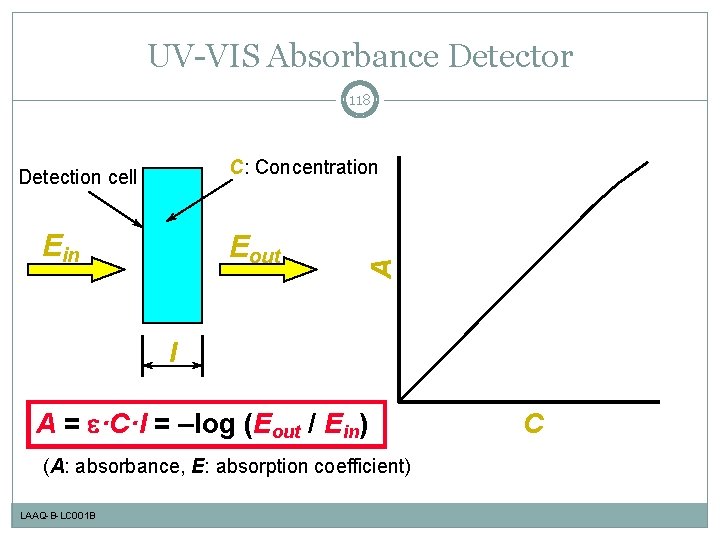
UV-VIS Absorbance Detector 118 Ein Eout A C: Concentration Detection cell l A = e·C·l = –log (Eout / Ein) (A: absorbance, E: absorption coefficient) LAAQ-B-LC 001 B C
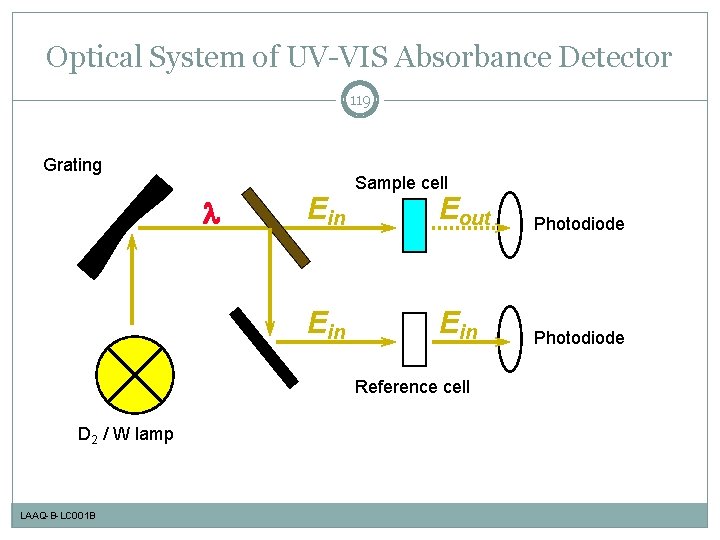
Optical System of UV-VIS Absorbance Detector 119 Grating l Ein Sample cell Eout Photodiode Ein Photodiode Reference cell D 2 / W lamp LAAQ-B-LC 001 B
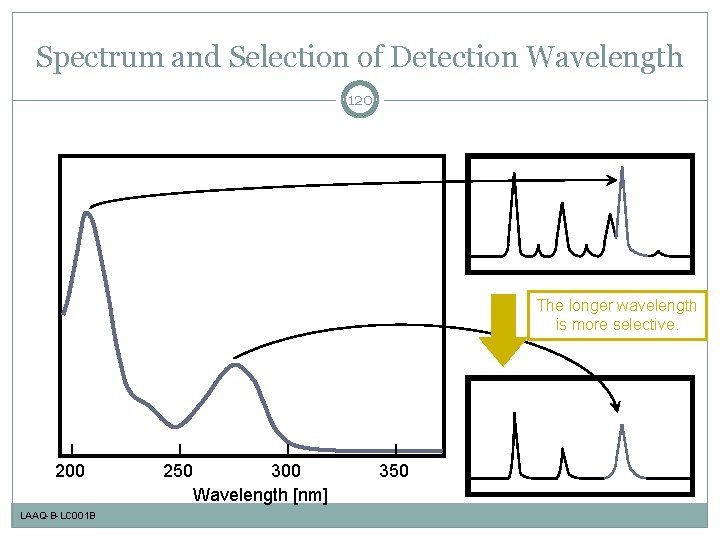
Spectrum and Selection of Detection Wavelength 120 The longer wavelength is more selective. 200 LAAQ-B-LC 001 B 250 300 Wavelength [nm] 350
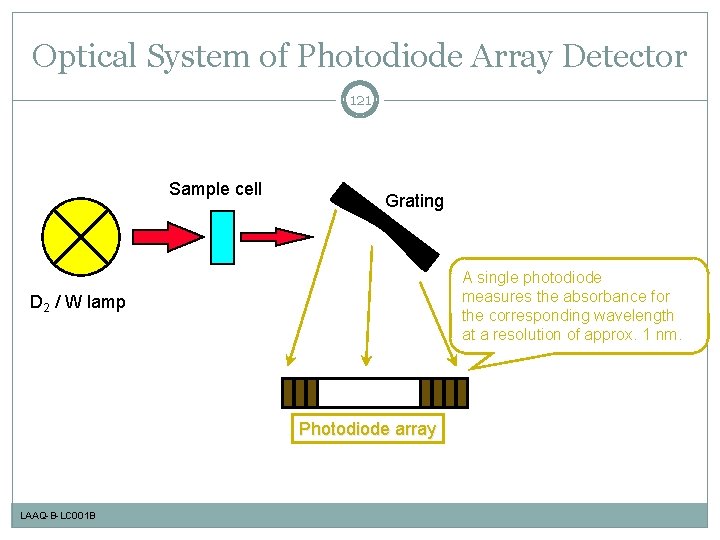
Optical System of Photodiode Array Detector 121 Sample cell Grating A single photodiode measures the absorbance for the corresponding wavelength at a resolution of approx. 1 nm. D 2 / W lamp Photodiode array LAAQ-B-LC 001 B
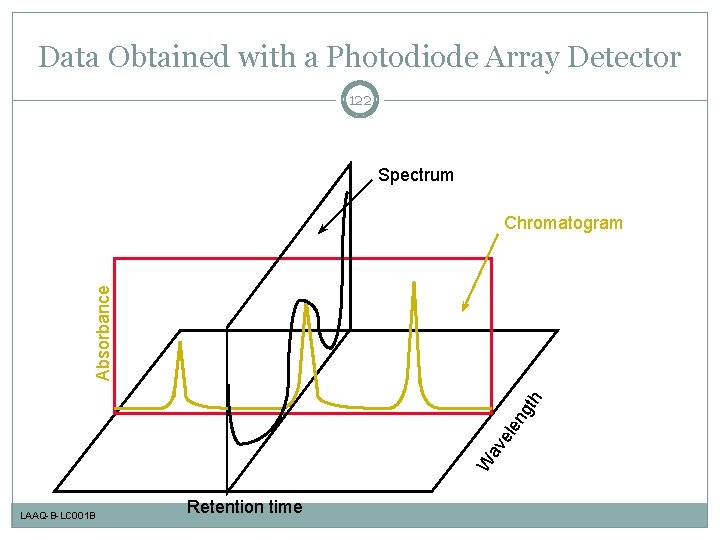
Data Obtained with a Photodiode Array Detector 122 Spectrum W av ele ng th Absorbance Chromatogram LAAQ-B-LC 001 B Retention time
Advantages of Photodiode Array Detectors 123 Peak Identification Using Spectra Complementation of identification based on retention time Library searches Evaluation of Peak Purity evaluation performed by comparison of the shape of spectra from the peak detection start point to the peak detection end point LAAQ-B-LC 001 B
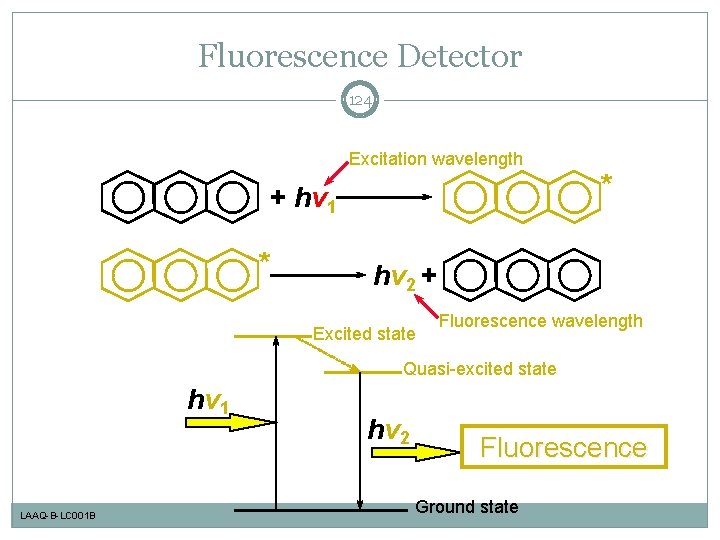
Fluorescence Detector 124 Excitation wavelength + hv 1 * * hv 2 + Excited state Fluorescence wavelength Quasi-excited state hv 1 LAAQ-B-LC 001 B hv 2 Fluorescence Ground state
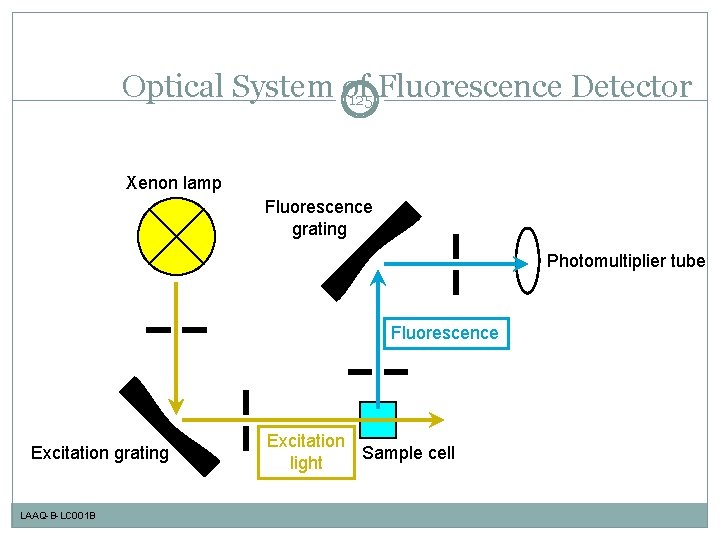
Optical System of 125 Fluorescence Detector Xenon lamp Fluorescence grating Photomultiplier tube Fluorescence Excitation grating LAAQ-B-LC 001 B Excitation Sample cell light
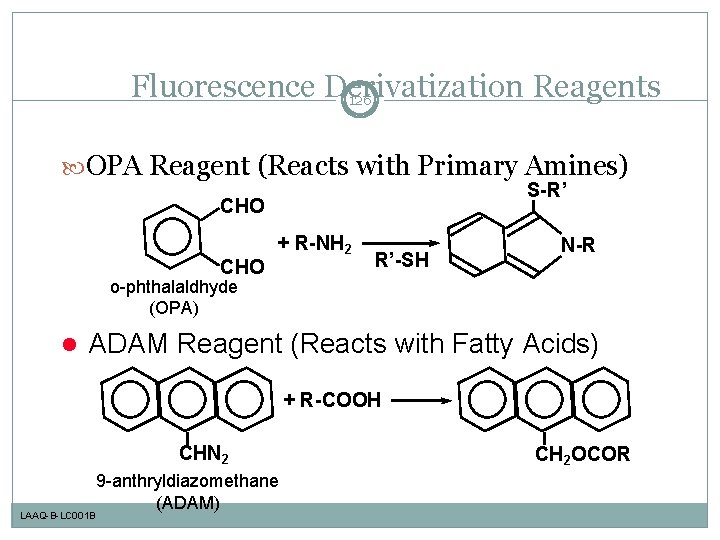
Fluorescence Derivatization Reagents 126 OPA Reagent (Reacts with Primary Amines) S-R’ CHO + R-NH 2 R’-SH N-R o-phthalaldhyde (OPA) l ADAM Reagent (Reacts with Fatty Acids) + R-COOH CHN 2 LAAQ-B-LC 001 B 9 -anthryldiazomethane (ADAM) CH 2 OCOR
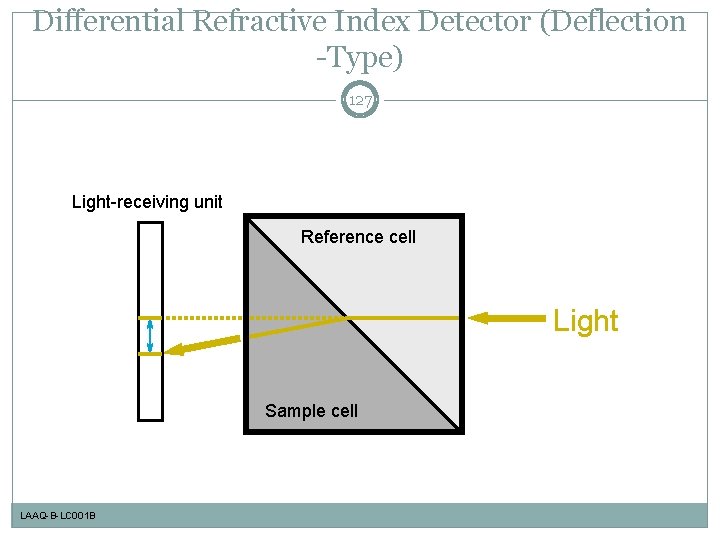
Differential Refractive Index Detector (Deflection -Type) 127 Light-receiving unit Reference cell Light Sample cell LAAQ-B-LC 001 B
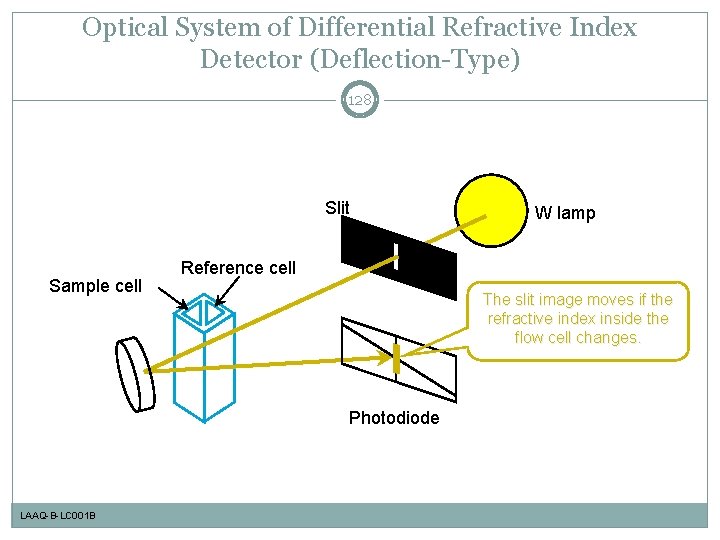
Optical System of Differential Refractive Index Detector (Deflection-Type) 128 Slit Sample cell Reference cell The slit image moves if the refractive index inside the flow cell changes. Photodiode LAAQ-B-LC 001 B W lamp
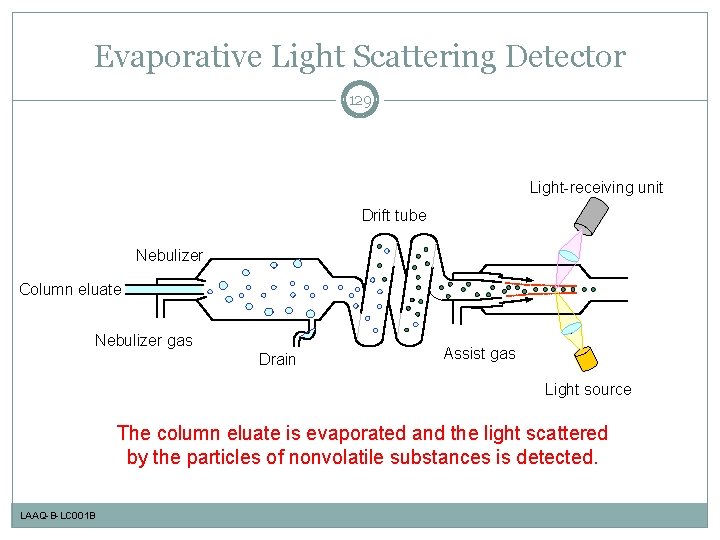
Evaporative Light Scattering Detector 129 Light-receiving unit Drift tube Nebulizer Column eluate Nebulizer gas Drain Assist gas Light source The column eluate is evaporated and the light scattered by the particles of nonvolatile substances is detected. LAAQ-B-LC 001 B
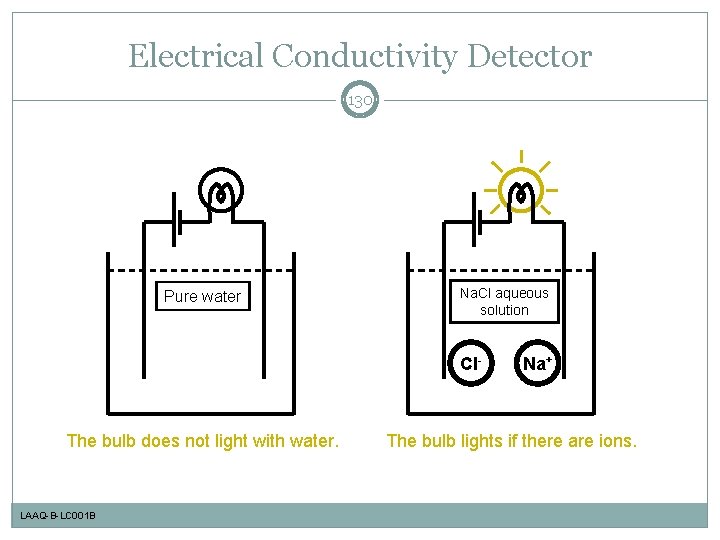
Electrical Conductivity Detector 130 Pure water Na. Cl aqueous solution Cl- The bulb does not light with water. LAAQ-B-LC 001 B Na+ The bulb lights if there are ions.
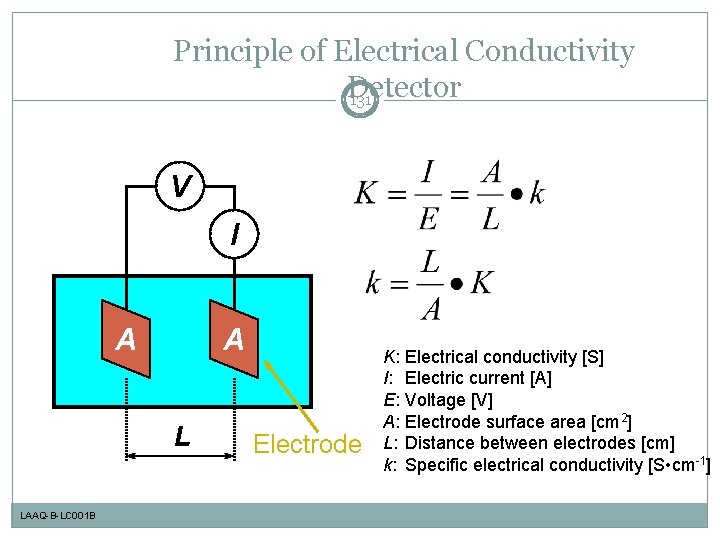
Principle of Electrical Conductivity Detector 131 V I A A L LAAQ-B-LC 001 B Electrode K: I: E: A: L: k: Electrical conductivity [S] Electric current [A] Voltage [V] Electrode surface area [cm 2] Distance between electrodes [cm] Specific electrical conductivity [S • cm-1]
![Limiting Equivalent Ion Conductance, l [S • cm 2/mol], in Aqueous Solution (25ºC) 132 Limiting Equivalent Ion Conductance, l [S • cm 2/mol], in Aqueous Solution (25ºC) 132](http://slidetodoc.com/presentation_image_h2/87ceac6ffa54aebc509f194d707d157b/image-132.jpg)
Limiting Equivalent Ion Conductance, l [S • cm 2/mol], in Aqueous Solution (25ºC) 132 LAAQ-B-LC 001 B
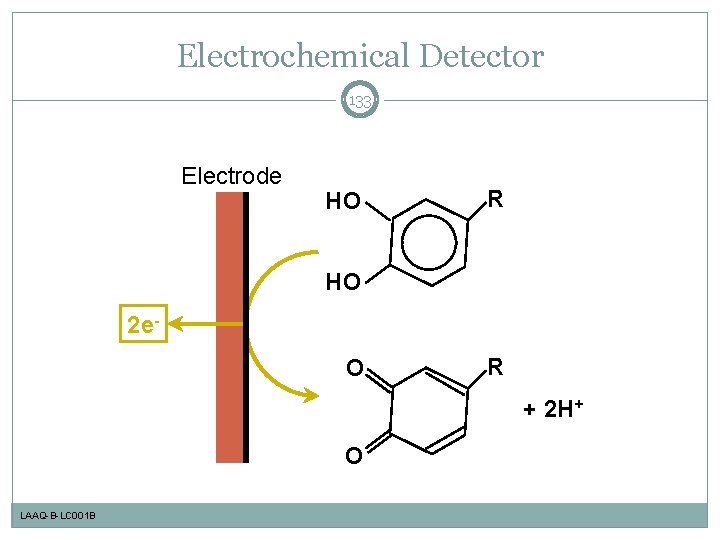
Electrochemical Detector 133 Electrode HO R HO 2 e. O R + 2 H+ O LAAQ-B-LC 001 B
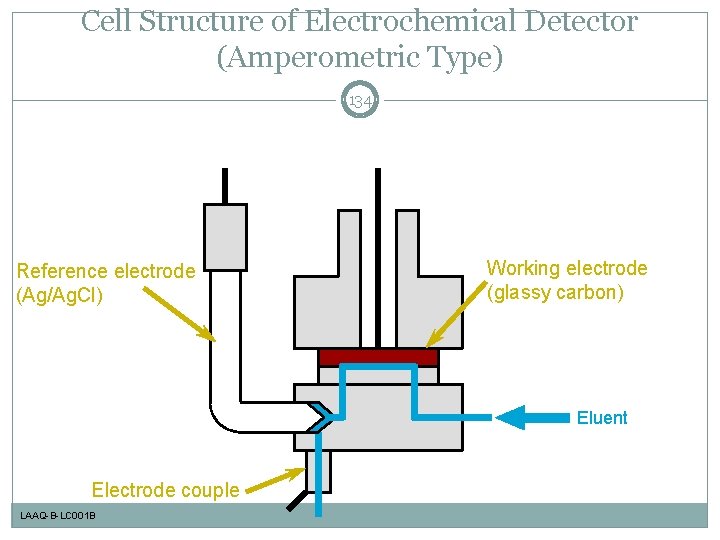
Cell Structure of Electrochemical Detector (Amperometric Type) 134 Reference electrode (Ag/Ag. Cl) Working electrode (glassy carbon) Eluent Electrode couple LAAQ-B-LC 001 B
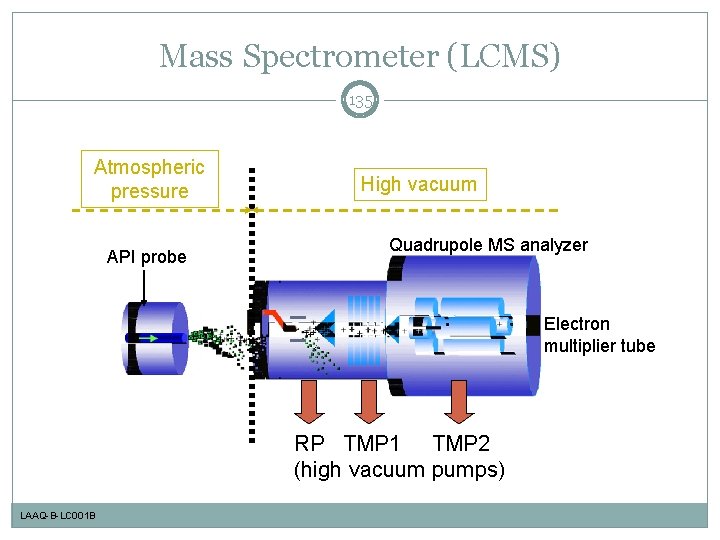
Mass Spectrometer (LCMS) 135 Atmospheric pressure API probe High vacuum Quadrupole MS analyzer Electron multiplier tube RP TMP 1 TMP 2 (high vacuum pumps) LAAQ-B-LC 001 B
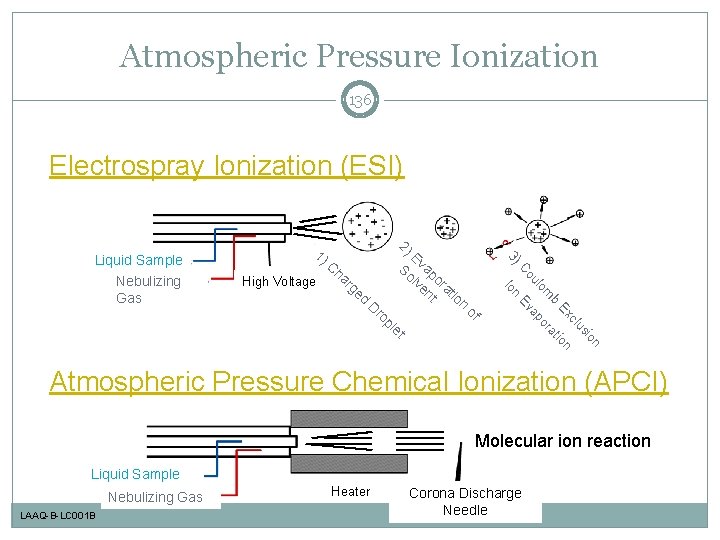
Atmospheric Pressure Ionization 136 Electrospray Ionization (ESI) pl e of n io us cl Ex tion b a m or lo ap ro Ev D n ge d ou ha r C High Voltage E So vap lv ora en t t ion Io Nebulizing Gas C 2) 3) Liquid Sample 1) t Atmospheric Pressure Chemical Ionization (APCI) Molecular ion reaction Liquid Sample Nebulizing Gas LAAQ-B-LC 001 B Heater Corona Discharge Needle
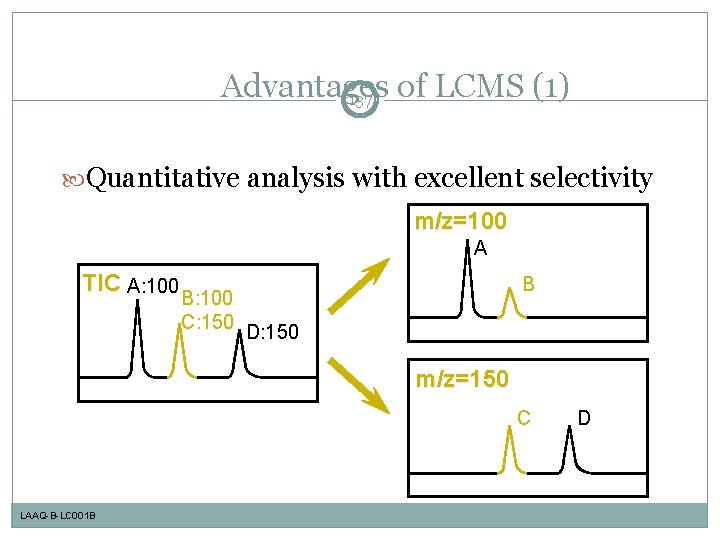
Advantages of LCMS (1) 137 Quantitative analysis with excellent selectivity m/z=100 A TIC A: 100 B B: 100 C: 150 D: 150 m/z=150 C LAAQ-B-LC 001 B D
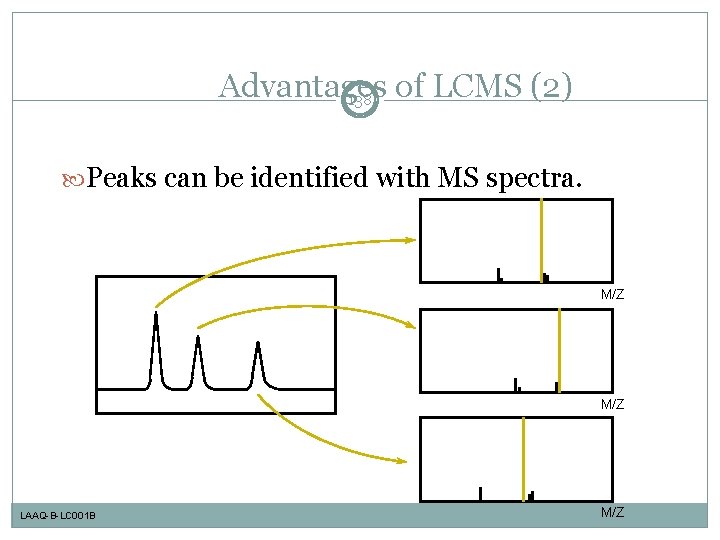
Advantages of LCMS (2) 138 Peaks can be identified with MS spectra. M/Z LAAQ-B-LC 001 B M/Z
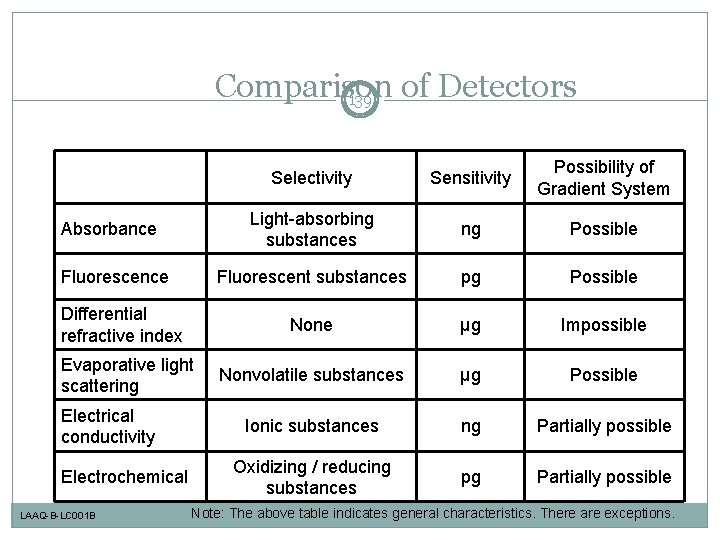
Comparison of Detectors 139 Absorbance Fluorescence Differential refractive index Evaporative light scattering Electrical conductivity Electrochemical LAAQ-B-LC 001 B Selectivity Sensitivity Possibility of Gradient System Light-absorbing substances ng Possible Fluorescent substances pg Possible None µg Impossible Nonvolatile substances µg Possible Ionic substances ng Partially possible Oxidizing / reducing substances pg Partially possible Note: The above table indicates general characteristics. There are exceptions.
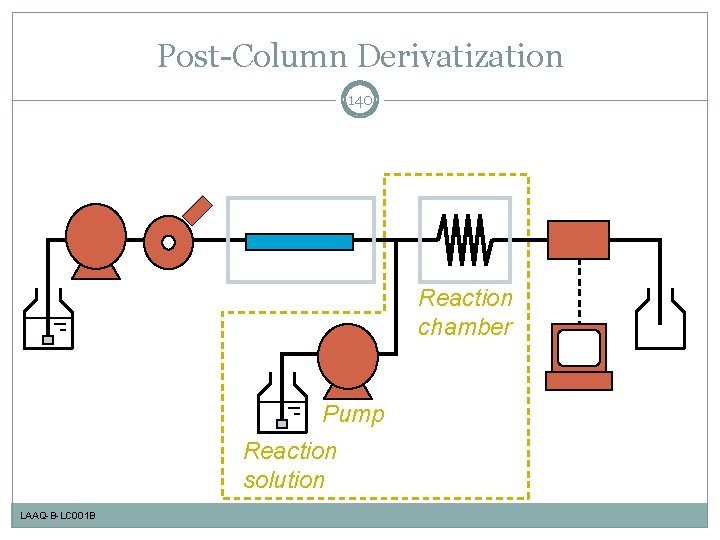
Post-Column Derivatization 140 Reaction chamber Pump Reaction solution LAAQ-B-LC 001 B
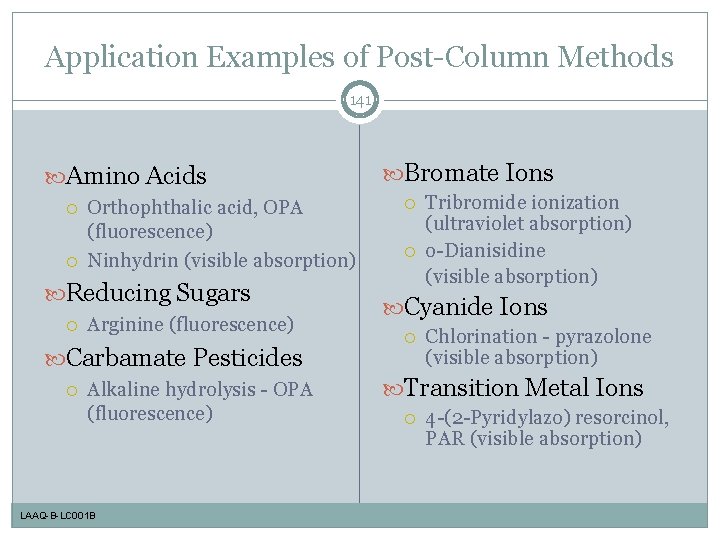
Application Examples of Post-Column Methods 141 Amino Acids Orthophthalic acid, OPA (fluorescence) Ninhydrin (visible absorption) Reducing Sugars Arginine (fluorescence) Carbamate Pesticides Alkaline hydrolysis - OPA (fluorescence) LAAQ-B-LC 001 B Bromate Ions Tribromide ionization (ultraviolet absorption) o-Dianisidine (visible absorption) Cyanide Ions Chlorination - pyrazolone (visible absorption) Transition Metal Ions 4 -(2 -Pyridylazo) resorcinol, PAR (visible absorption)

Quantitative Analysis 142 ABSOLUTE CALIBRATION CURVE METHOD AND INTERNAL STANDARD METHOD LAAQ-B-LC 001 B
Qualitative Analysis 143 Identification based on retention time Acquisition of spectra with detector UV spectra MS spectra Transfer to other analytical instruments after preparative separation LAAQ-B-LC 001 B
Quantitative Analysis 144 Quantitation performed with peak area or height. Calibration curve created beforehand using a standard. Absolute calibration curve method Internal standard method Standard addition method LAAQ-B-LC 001 B
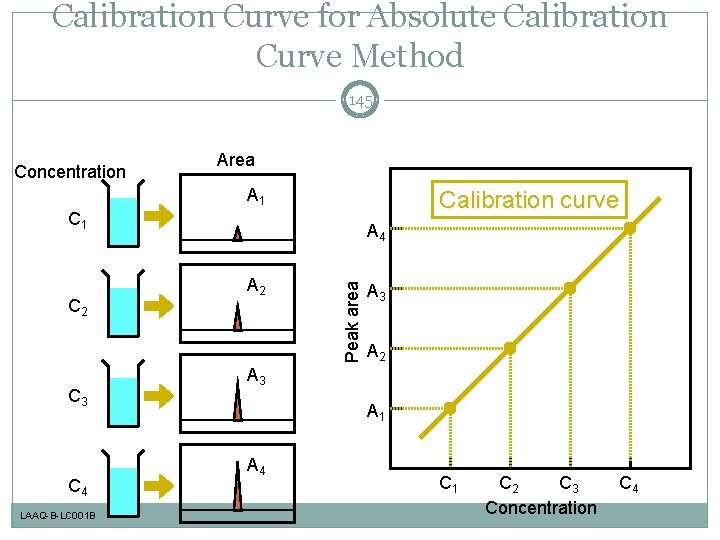
Calibration Curve for Absolute Calibration Curve Method 145 Concentration Area A 1 Calibration curve C 1 C 3 C 4 LAAQ-B-LC 001 B A 2 Peak area C 2 A 4 A 3 A 2 A 3 A 1 A 4 C 1 C 2 C 3 Concentration C 4
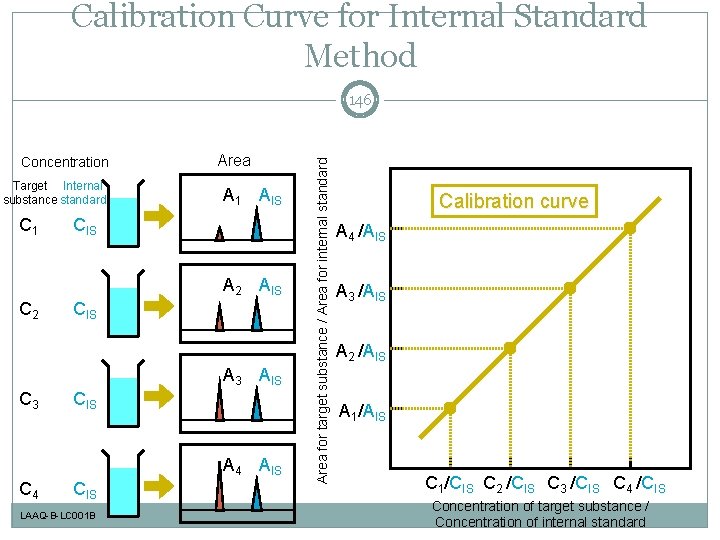
Calibration Curve for Internal Standard Method Concentration Target Internal substance standard C 1 Area A 1 AIS CIS A 2 AIS C 2 CIS A 3 AIS C 3 CIS A 4 AIS C 4 CIS LAAQ-B-LC 001 B Area for target substance / Area for internal standard 146 Calibration curve A 4 /AIS A 3 /AIS A 2 /AIS A 1/AIS C 1/CIS C 2 /CIS C 3 /CIS C 4 /CIS Concentration of target substance / Concentration of internal standard
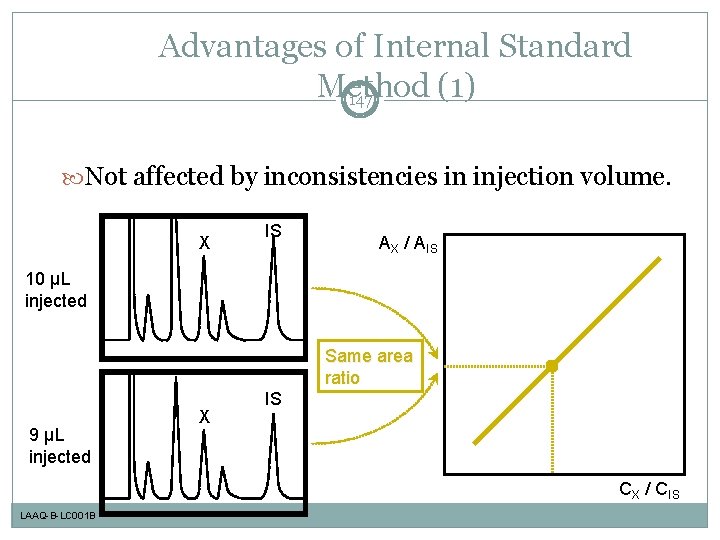
Advantages of Internal Standard Method (1) 147 Not affected by inconsistencies in injection volume. X IS AX / AIS 10 µL injected Same area ratio 9 µL injected X IS CX / CIS LAAQ-B-LC 001 B
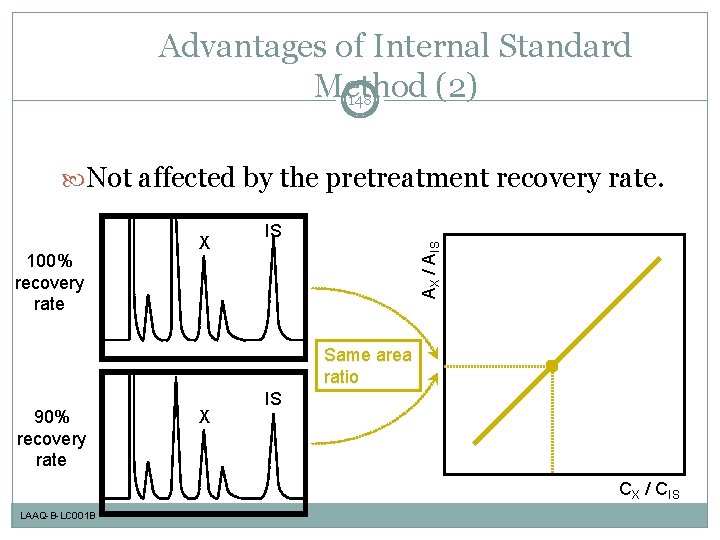
Advantages of Internal Standard Method (2) 148 Not affected by the pretreatment recovery rate. IS AX / AIS 100% recovery rate X Same area ratio 90% recovery rate X IS CX / CIS LAAQ-B-LC 001 B
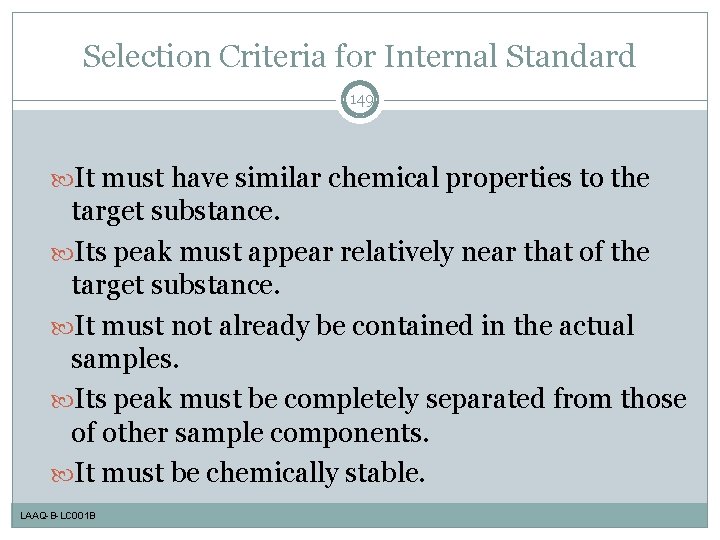
Selection Criteria for Internal Standard 149 It must have similar chemical properties to the target substance. Its peak must appear relatively near that of the target substance. It must not already be contained in the actual samples. Its peak must be completely separated from those of other sample components. It must be chemically stable. LAAQ-B-LC 001 B

Sample Pretreatment 150 TASKS PERFORMED BEFORE INJECTION LAAQ-B-LC 001 B

Objectives of Pretreatment 151 To improve the accuracy of quantitative values To improve sensitivity and selectivity To protect and prevent the deterioration of columns and analytical instruments To simplify measurement operations and procedures To stabilize target substances LAAQ-B-LC 001 B

Substances That Must Not Be Injected into the Column 152 Insoluble substances (e. g. , microscopic particles and precipitation) Substances that are precipitated in the eluent Substances that irreversibly adsorb to the packing material Substances that dissolve, or chemically react, with the packing material LAAQ-B-LC 001 B
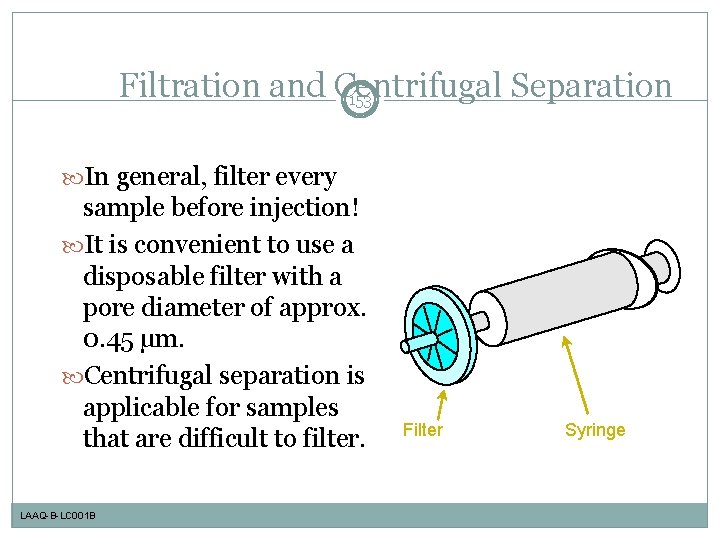
Filtration and Centrifugal Separation 153 In general, filter every sample before injection! It is convenient to use a disposable filter with a pore diameter of approx. 0. 45 µm. Centrifugal separation is applicable for samples that are difficult to filter. LAAQ-B-LC 001 B Filter Syringe
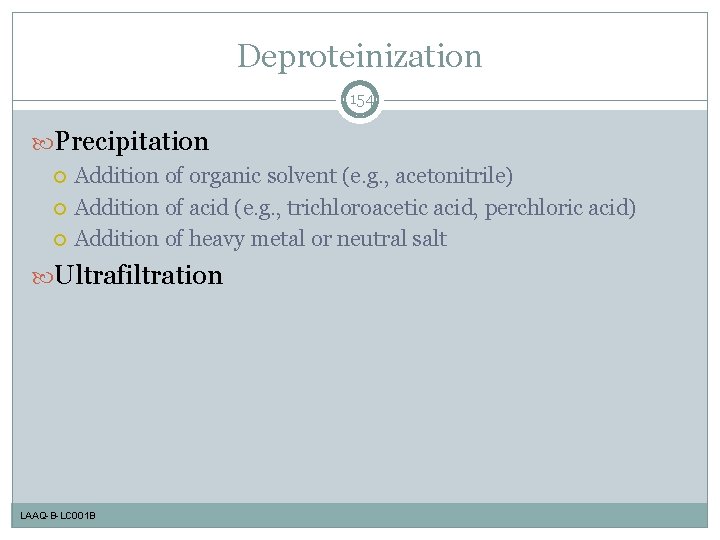
Deproteinization 154 Precipitation Addition of organic solvent (e. g. , acetonitrile) Addition of acid (e. g. , trichloroacetic acid, perchloric acid) Addition of heavy metal or neutral salt Ultrafiltration LAAQ-B-LC 001 B
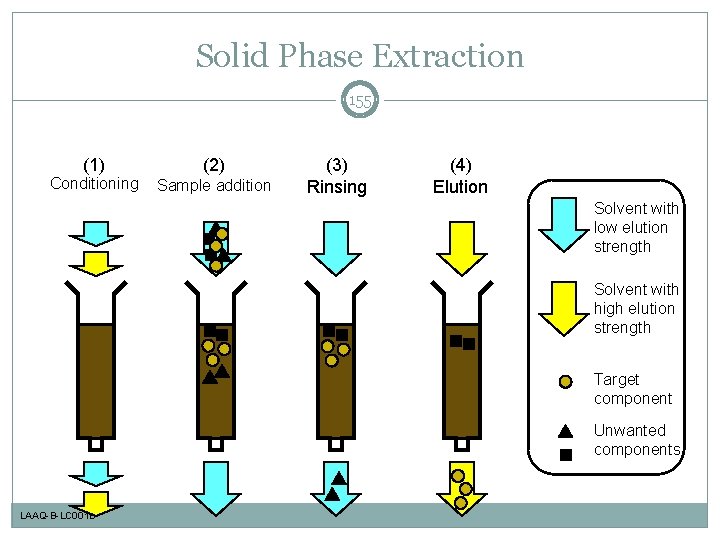
Solid Phase Extraction 155 (1) Conditioning (2) Sample addition (3) Rinsing (4) Elution Solvent with low elution strength Solvent with high elution strength Target component Unwanted components LAAQ-B-LC 001 B
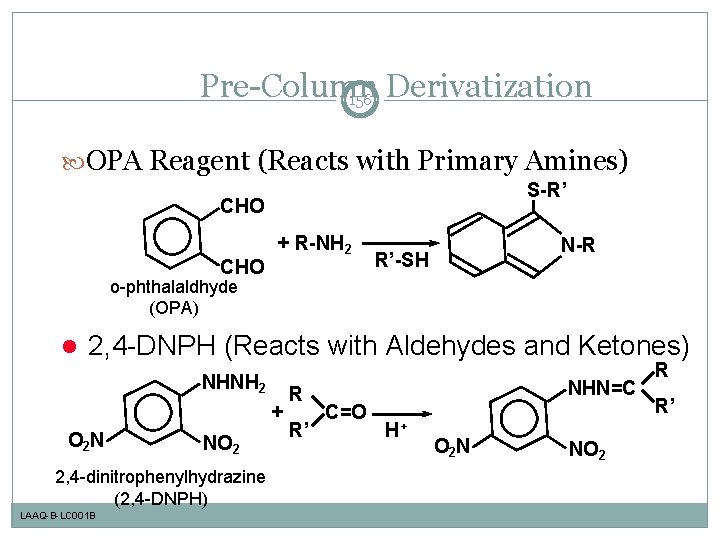
Pre-Column Derivatization 156 OPA Reagent (Reacts with Primary Amines) S-R’ CHO + R-NH 2 N-R R’-SH o-phthalaldhyde (OPA) l 2, 4 -DNPH (Reacts with Aldehydes and Ketones) NHNH 2 + O 2 N NO 2 2, 4 -dinitrophenylhydrazine (2, 4 -DNPH) LAAQ-B-LC 001 B R R’ NHN=C C=O H+ O 2 N NO 2 R R’

Evaluation of the Reliability of Analysis 157 VALIDATION OF ANALYTICAL METHODS LAAQ-B-LC 001 B
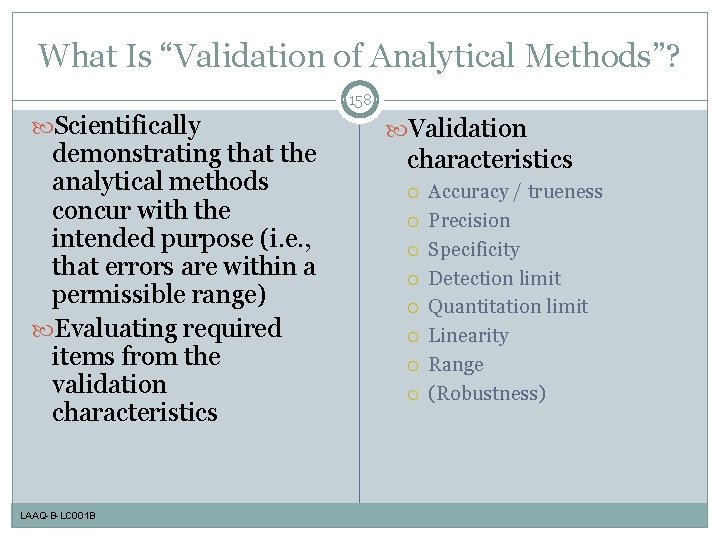
What Is “Validation of Analytical Methods”? 158 Scientifically demonstrating that the analytical methods concur with the intended purpose (i. e. , that errors are within a permissible range) Evaluating required items from the validation characteristics LAAQ-B-LC 001 B Validation characteristics Accuracy / trueness Precision Specificity Detection limit Quantitation limit Linearity Range (Robustness)
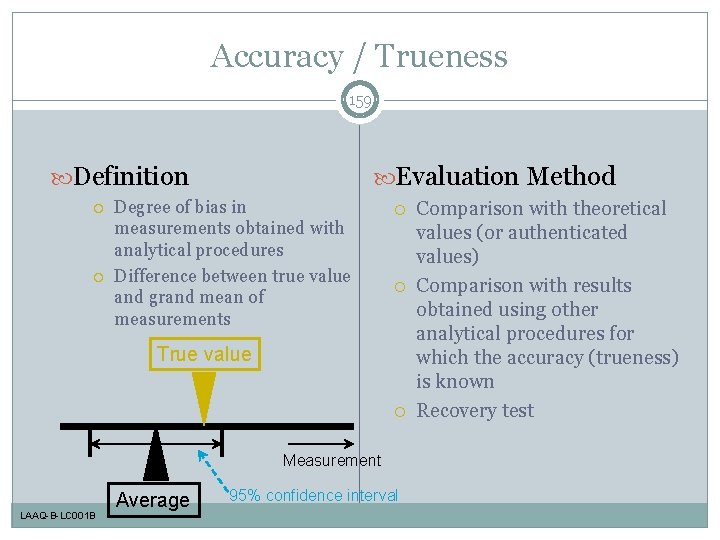
Accuracy / Trueness 159 Definition Evaluation Method Degree of bias in measurements obtained with analytical procedures Difference between true value and grand mean of measurements True value Measurement LAAQ-B-LC 001 B Average 95% confidence interval Comparison with theoretical values (or authenticated values) Comparison with results obtained using other analytical procedures for which the accuracy (trueness) is known Recovery test
Precision 160 Repeatability / Intra- Assay Precision Definition Precision of measurements Degree of coincidence of taken over a short time period under the same series of measurements conditions obtained by repeatedly analyzing multiple samples Intermediate Precision taken from a homogenous Reproducibility test substance Variance, standard deviation, or relative standard deviation of measurements LAAQ-B-LC 001 B
Specificity 161 Definition The ability to accurately analyze the target substance in the presence of other expected substances The discrimination capability of the analytical methods Multiple analytical procedures may be combined in order to attain the required level of discrimination LAAQ-B-LC 001 B Evaluation Method Confirmation that the target substance can be discriminated (separated) from co-existing components, related substances, decomposition products, etc. If reference standards for impurities cannot be obtained, the measurement results for samples thought to contain the impurities are compared.
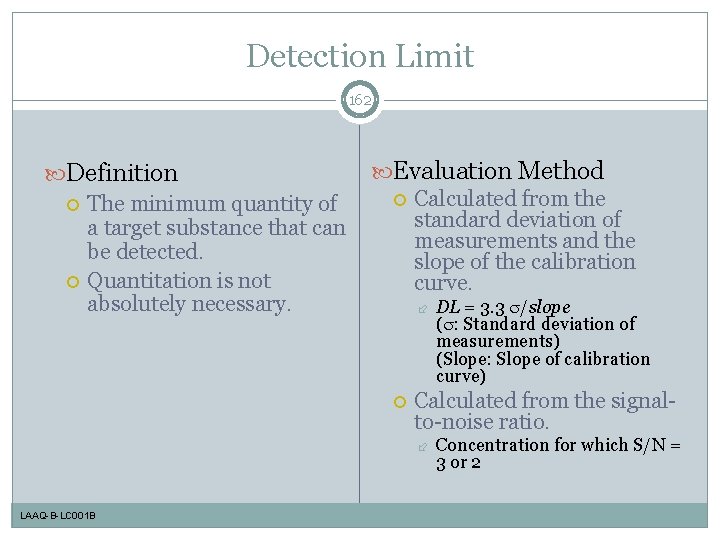
Detection Limit 162 Definition The minimum quantity of a target substance that can be detected. Quantitation is not absolutely necessary. Evaluation Method Calculated from the standard deviation of measurements and the slope of the calibration curve. Calculated from the signalto-noise ratio. LAAQ-B-LC 001 B DL = 3. 3 /slope ( : Standard deviation of measurements) (Slope: Slope of calibration curve) Concentration for which S/N = 3 or 2
Quantitation Limit 163 Definition The minimum quantity of a target substance that can be quantified Quantitation with an appropriate level of accuracy and precision must be possible. (In general, the relative standard deviation must not exceed 10%. ) Evaluation Method Calculated from the standard deviation of measurements and the slope of the calibration curve. Calculated from the signal-tonoise ratio. LAAQ-B-LC 001 B QL = 10 /slope ( : Standard deviation of measurements) (Slope: Slope of calibration curve) Concentration for which S/N = 10
Linearity 164 Definition The ability of the analytical method to produce measurements for the quantity of a target substance that satisfy a linear relationship. Values produced by converting quantities or measurements of the target substance using a precisely defined formula may be used. LAAQ-B-LC 001 B Evaluation Method Samples containing different quantities of the target substance (usually 5 concentrations) are analyzed repeatedly, and regression equations and correlation coefficients are obtained. Residuals obtained from the regression equations of the measurements are plotted, and it is confirmed that there is no specific slope.
Range 165 Definition The region between the lower and upper limits of the quantity of a target substance that gives appropriate levels of accuracy and precision LAAQ-B-LC 001 B Evaluation Method The accuracy, precision, and linearity are investigated for samples containing quantities of a target substance that correspond to the lower limit, upper limit, and approximate center of the range.
Robustness 166 Definition The ability of an analytical procedure to remain unaffected by small changes in analytical conditions. LAAQ-B-LC 001 B Evaluation Method Some or all of the variable factors (i. e. , the analytical conditions) are changed and the effects are evaluated.

Maintenance of Separation Column 167 EXTENDING THE COLUMN’S SERVICE LIFE LAAQ-B-LC 001 B
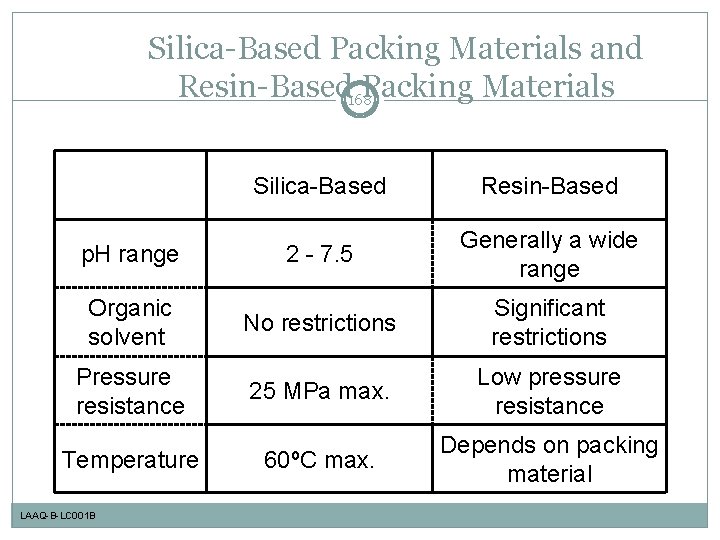
Silica-Based Packing Materials and Resin-Based 168 Packing Materials Silica-Based Resin-Based p. H range 2 - 7. 5 Generally a wide range Organic solvent No restrictions Significant restrictions Pressure resistance 25 MPa max. Low pressure resistance Temperature 60ºC max. Depends on packing material LAAQ-B-LC 001 B

General Handling of Columns 169 Observe restrictions Use as low a load related to solvents and pressure as possible. the p. H range. Do not exceed the upper Never allow the pressure limit. packing material to dry. Do not subject the column to sudden pressure changes. Do not allow solids or microscopic particles to Do not subject the enter the column to intense shocks. LAAQ-B-LC 001 B Filter samples.
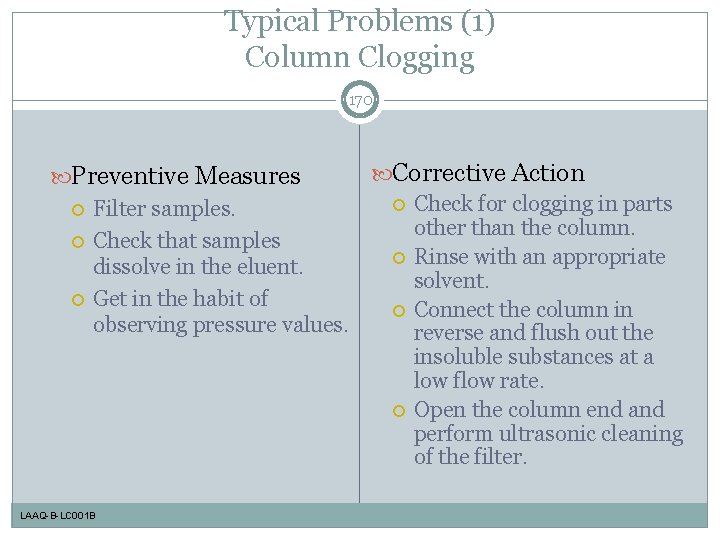
Typical Problems (1) Column Clogging 170 Preventive Measures Filter samples. Check that samples dissolve in the eluent. Get in the habit of observing pressure values. LAAQ-B-LC 001 B Corrective Action Check for clogging in parts other than the column. Rinse with an appropriate solvent. Connect the column in reverse and flush out the insoluble substances at a low flow rate. Open the column end and perform ultrasonic cleaning of the filter.
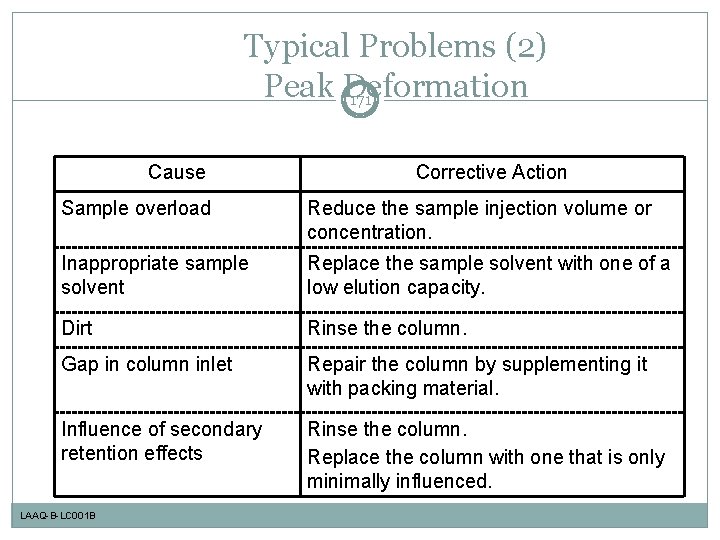
Typical Problems (2) Peak Deformation 171 Cause Corrective Action Sample overload Reduce the sample injection volume or concentration. Inappropriate sample solvent Replace the sample solvent with one of a low elution capacity. Dirt Rinse the column. Gap in column inlet Repair the column by supplementing it with packing material. Influence of secondary retention effects Rinse the column. Replace the column with one that is only minimally influenced. LAAQ-B-LC 001 B
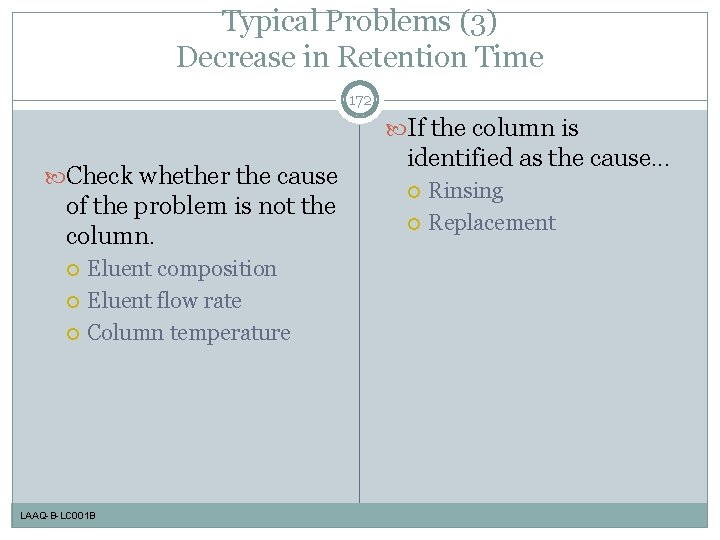
Typical Problems (3) Decrease in Retention Time 172 If the column is Check whether the cause of the problem is not the column. Eluent composition Eluent flow rate Column temperature LAAQ-B-LC 001 B identified as the cause. . . Rinsing Replacement
Typical Problems (4) Baseline Drift 173 If the column is Check whether the cause of the problem is not the column. LAAQ-B-LC 001 B If the problem persists when the column is removed, it is caused by the eluent, the solvent delivery system (pump or degasser), or the detector. identified as the cause. . . Rinsing Review of temperature control Replacement
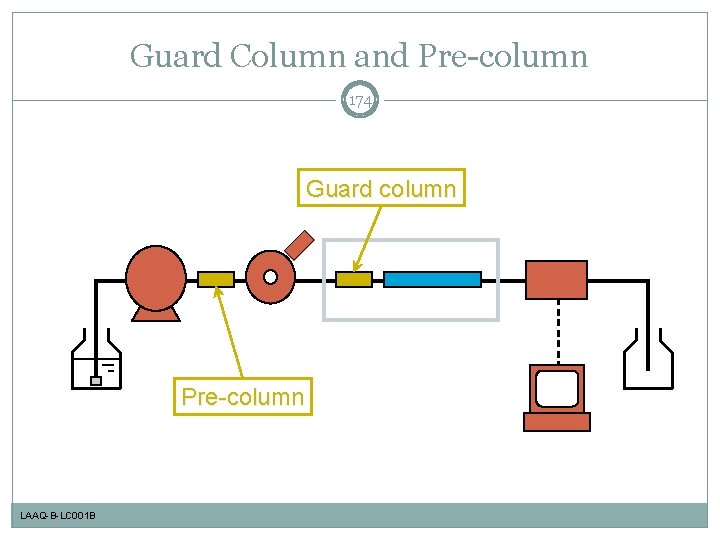
Guard Column and Pre-column 174 Guard column Pre-column LAAQ-B-LC 001 B
Column Rinsing 175 Use an eluent with a high elution capacity Reversed phase mode: Solution with a high proportion of organic solvent Ion exchange mode: Solution with a high salt concentration Consider secondary retention effects To remove basic substances from a reversed phase column Use an acidic solution and add an ion pair reagent. To remove hydrophobic substances from an ion exchange column Add an organic solvent. LAAQ-B-LC 001 B
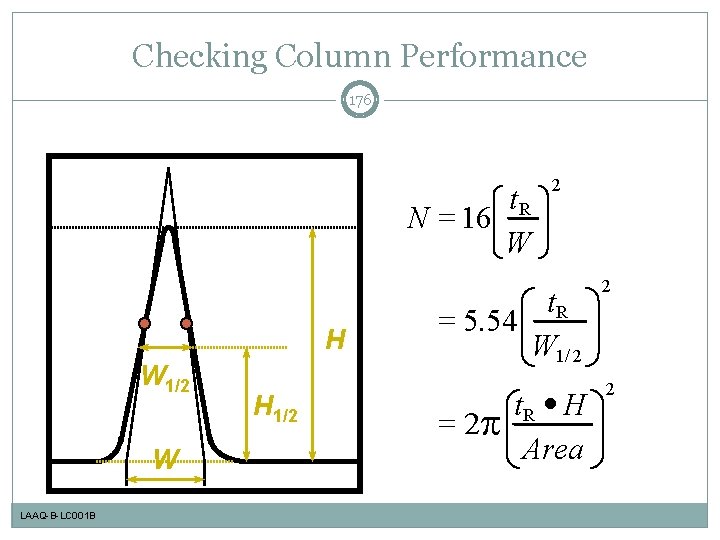
Checking Column Performance 176 t. R N = 16 W H W 1/2 W LAAQ-B-LC 001 B H 1/2 2 t. R = 5. 54 W 1/ 2 2 t. R · H Area 2 = 2
Column Storage 177 Storage Solution It is generally safe to use the same storage solution as used at shipment. In order to prevent putrefaction, alcohol or some other preservative substance may be added. Storage Conditions LAAQ-B-LC 001 B Insert an airtight stopper in the column end. Never allow the packing material to dry. Make a record of the storage solution and final usage conditions and store it together with the column. Store the column in a location not subject to shocks or sudden temperature changes.
- Slides: 177