How to Write Chemical Formulas of Compounds In




- Slides: 32

How to Write Chemical Formulas of Compounds In order to write the chemical formula of a compound, the type of compound must be identified based upon its chemical name. The name of a compound references, in many cases, a specific compound. The chemical name may provide information necessary to write the compound’s chemical formula.

Formulas of Ionic Compounds Ionic compounds tend to be composed of the following: Metallic cation bonded to nonmetallic anion Metallic cation bonded to polyatomic anion Polyatomic cation bonded to nonmetallic anion Polyatomic cation bonded to polyatomic anion

Formulas of Ionic Compounds All ionic compounds and their formulas are neutral The total number of positive charges must EQUAL the total number of negative charges In addition, ionic compounds are represented by an empirical formula, meaning, the simplest combining ratio between the ions

Formulas of Ionic Compounds In order to write the formula for an ionic compound, the charges of the combining ions (positive and negative) must be known. These charges may be known through use of the periodic table, use of the chemical name of the compound, or by general knowledge (memorization)

Formulas of Ionic Compounds In some compounds, the metallic ion is identified by the placement of the element on the periodic table. e. g. Alkali metals (Gp 1 or IA) form +1 ions e. g. Alkaline earth metals (Gp 2 or IIA) form +2 ions e. g. Gp 13 (IIIA) (first three elements) form +3 ions

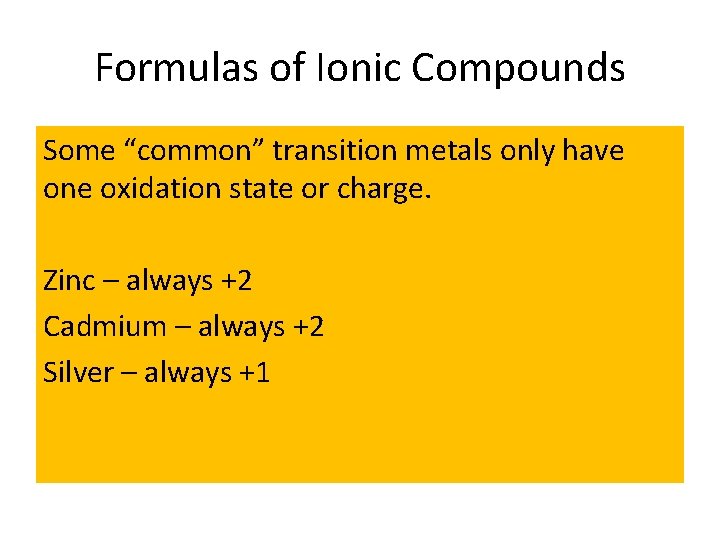
Formulas of Ionic Compounds Some “common” transition metals only have one oxidation state or charge. Zinc – always +2 Cadmium – always +2 Silver – always +1

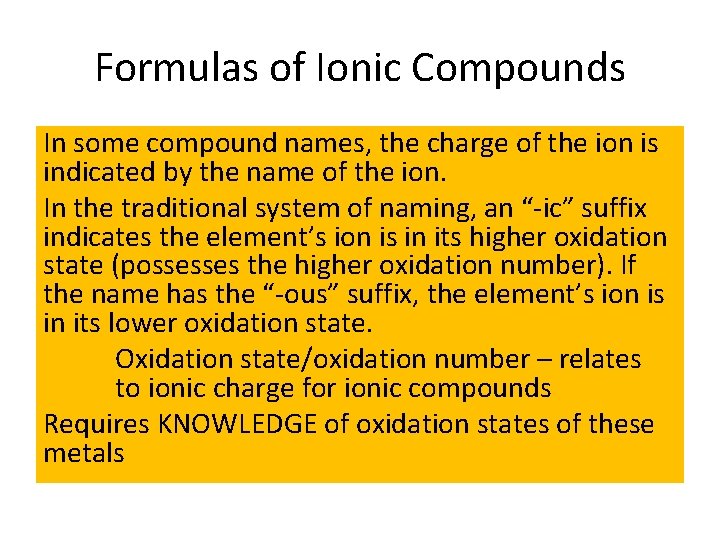
Formulas of Ionic Compounds In some compound names, the charge of the ion is indicated by the name of the ion. In the traditional system of naming, an “-ic” suffix indicates the element’s ion is in its higher oxidation state (possesses the higher oxidation number). If the name has the “-ous” suffix, the element’s ion is in its lower oxidation state. Oxidation state/oxidation number – relates to ionic charge for ionic compounds Requires KNOWLEDGE of oxidation states of these metals

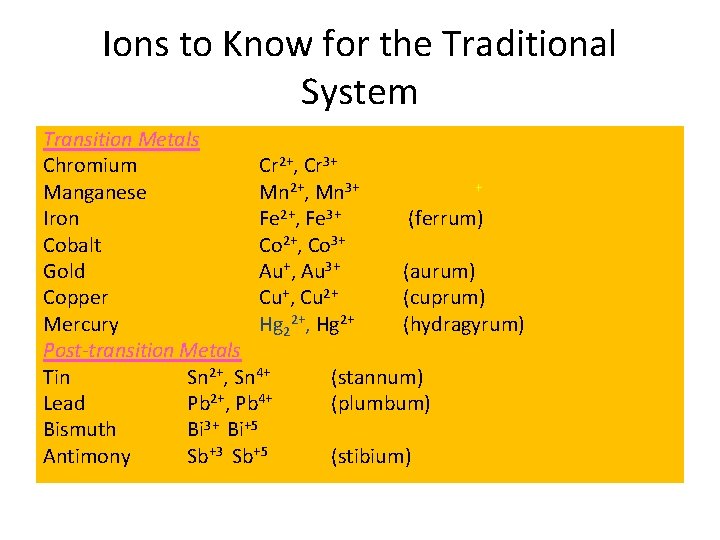
Ions to Know for the Traditional System Transition Metals Chromium Cr 2+, Cr 3+ + Manganese Mn 2+, Mn 3+ Iron Fe 2+, Fe 3+ (ferrum) Cobalt Co 2+, Co 3+ Gold Au+, Au 3+ (aurum) Copper Cu+, Cu 2+ (cuprum) Mercury Hg 22+, Hg 2+ (hydragyrum) Post-transition Metals Tin Sn 2+, Sn 4+ (stannum) Lead Pb 2+, Pb 4+ (plumbum) Bismuth Bi 3+ Bi+5 Antimony Sb+3 Sb+5 (stibium)

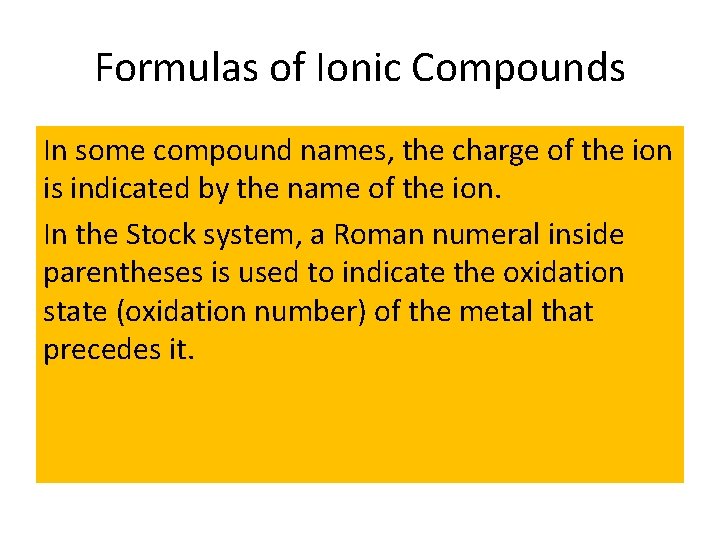
Formulas of Ionic Compounds In some compound names, the charge of the ion is indicated by the name of the ion. In the Stock system, a Roman numeral inside parentheses is used to indicate the oxidation state (oxidation number) of the metal that precedes it.

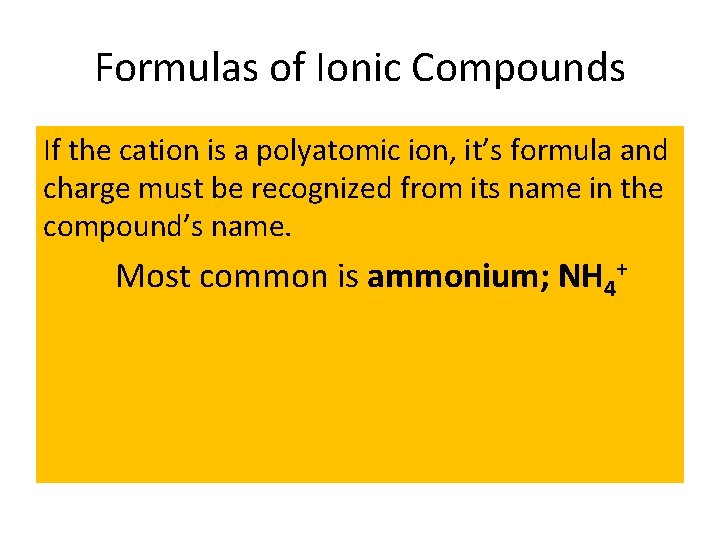
Formulas of Ionic Compounds If the cation is a polyatomic ion, it’s formula and charge must be recognized from its name in the compound’s name. Most common is ammonium; NH 4+

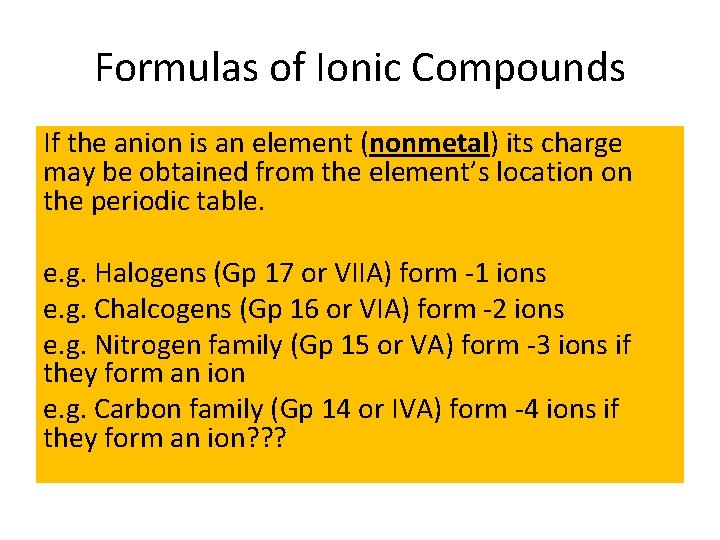
Formulas of Ionic Compounds If the anion is an element (nonmetal) its charge may be obtained from the element’s location on the periodic table. e. g. Halogens (Gp 17 or VIIA) form -1 ions e. g. Chalcogens (Gp 16 or VIA) form -2 ions e. g. Nitrogen family (Gp 15 or VA) form -3 ions if they form an ion e. g. Carbon family (Gp 14 or IVA) form -4 ions if they form an ion? ? ?

Formulas of Ionic Compounds If the anion is a polyatomic anion, its formula and charge must be KNOWN when it is recognized by its name in the name of the compound.

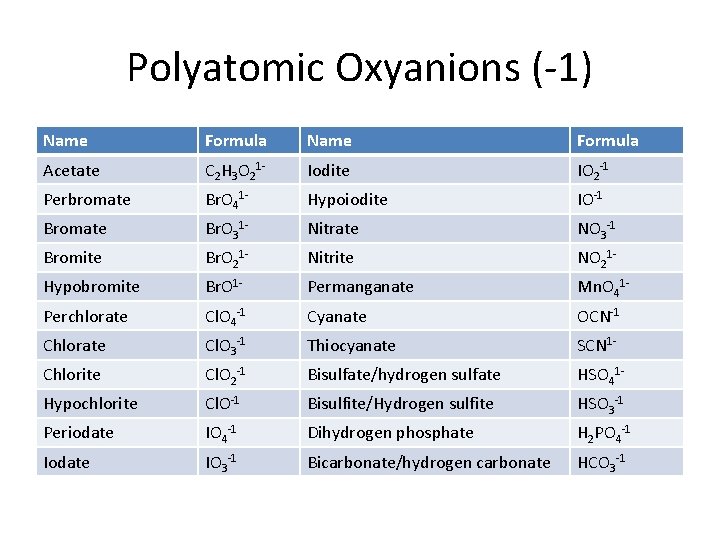
Polyatomic Oxyanions (-1) Name Formula Acetate C 2 H 3 O 21 - Iodite IO 2 -1 Perbromate Br. O 41 - Hypoiodite IO-1 Bromate Br. O 31 - Nitrate NO 3 -1 Bromite Br. O 21 - Nitrite NO 21 - Hypobromite Br. O 1 - Permanganate Mn. O 41 - Perchlorate Cl. O 4 -1 Cyanate OCN-1 Chlorate Cl. O 3 -1 Thiocyanate SCN 1 - Chlorite Cl. O 2 -1 Bisulfate/hydrogen sulfate HSO 41 - Hypochlorite Cl. O-1 Bisulfite/Hydrogen sulfite HSO 3 -1 Periodate IO 4 -1 Dihydrogen phosphate H 2 PO 4 -1 Iodate IO 3 -1 Bicarbonate/hydrogen carbonate HCO 3 -1

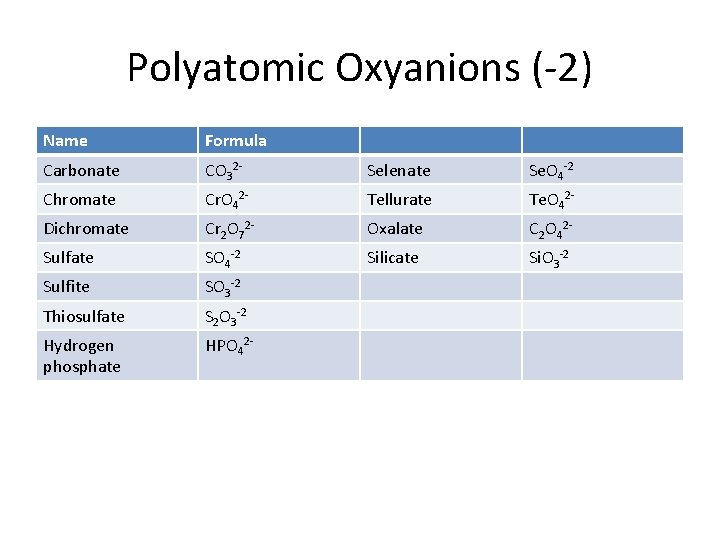
Polyatomic Oxyanions (-2) Name Formula Carbonate CO 32 - Selenate Se. O 4 -2 Chromate Cr. O 42 - Tellurate Te. O 42 - Dichromate Cr 2 O 72 - Oxalate C 2 O 42 - Sulfate SO 4 -2 Silicate Si. O 3 -2 Sulfite SO 3 -2 Thiosulfate S 2 O 3 -2 Hydrogen phosphate HPO 42 -

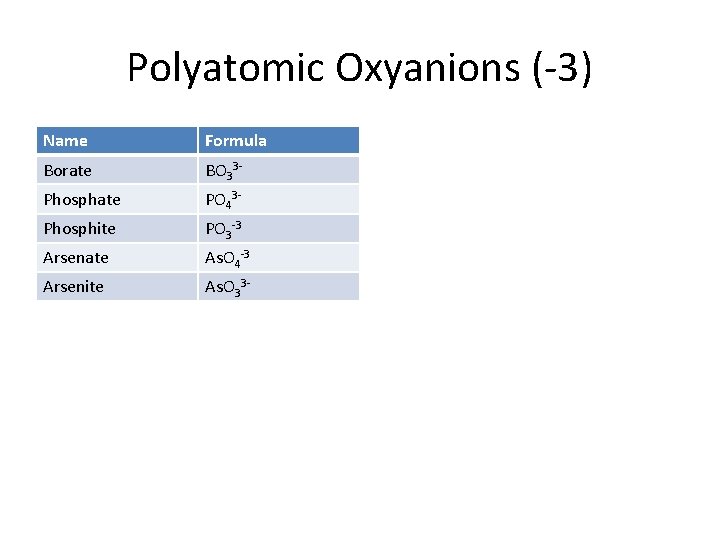
Polyatomic Oxyanions (-3) Name Formula Borate BO 33 - Phosphate PO 43 - Phosphite PO 3 -3 Arsenate As. O 4 -3 Arsenite As. O 33 -

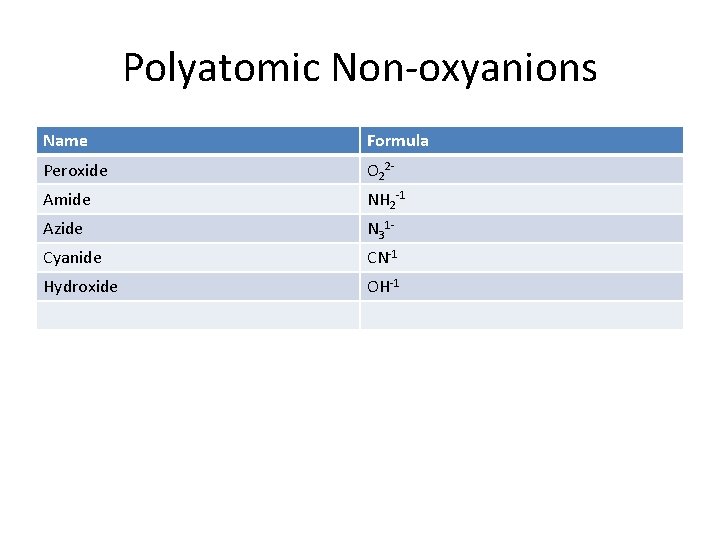
Polyatomic Non-oxyanions Name Formula Peroxide O 22 - Amide NH 2 -1 Azide N 31 - Cyanide CN-1 Hydroxide OH-1

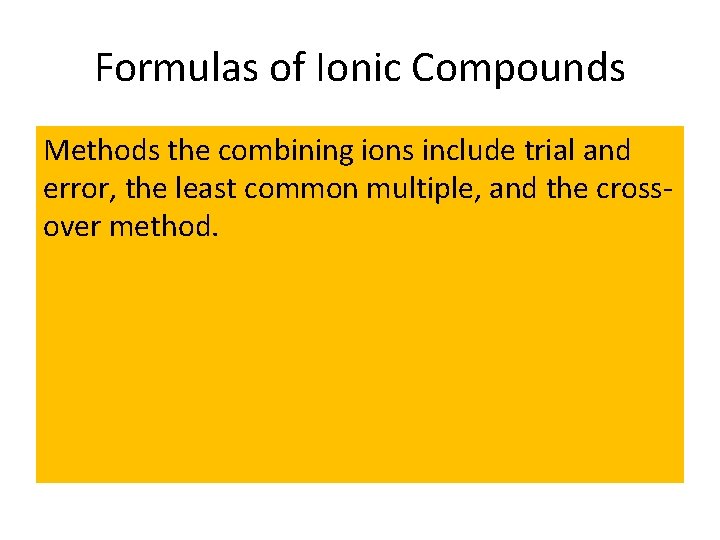
Formulas of Ionic Compounds Methods the combining ions include trial and error, the least common multiple, and the crossover method.

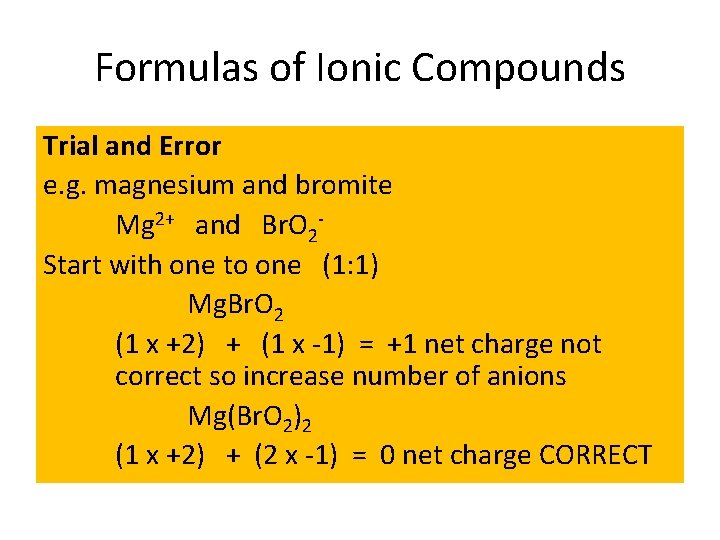
Formulas of Ionic Compounds Trial and Error e. g. magnesium and bromite Mg 2+ and Br. O 2 Start with one to one (1: 1) Mg. Br. O 2 (1 x +2) + (1 x -1) = +1 net charge not correct so increase number of anions Mg(Br. O 2)2 (1 x +2) + (2 x -1) = 0 net charge CORRECT

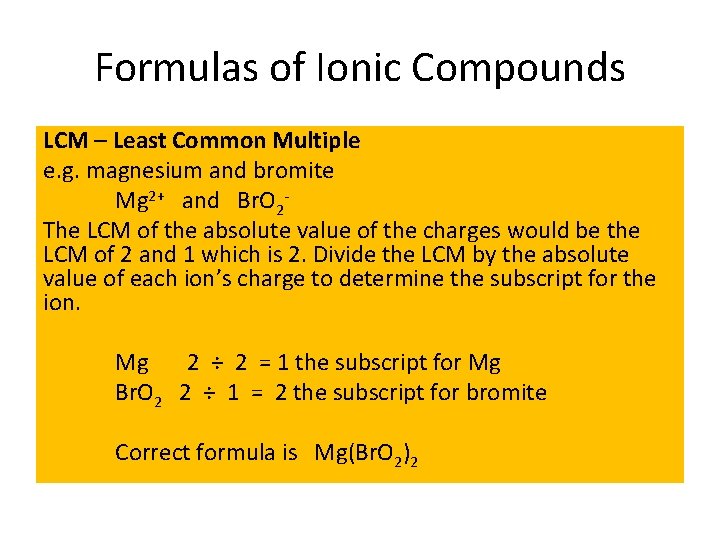
Formulas of Ionic Compounds LCM – Least Common Multiple e. g. magnesium and bromite Mg 2+ and Br. O 2 The LCM of the absolute value of the charges would be the LCM of 2 and 1 which is 2. Divide the LCM by the absolute value of each ion’s charge to determine the subscript for the ion. Mg 2 ÷ 2 = 1 the subscript for Mg Br. O 2 2 ÷ 1 = 2 the subscript for bromite Correct formula is Mg(Br. O 2)2

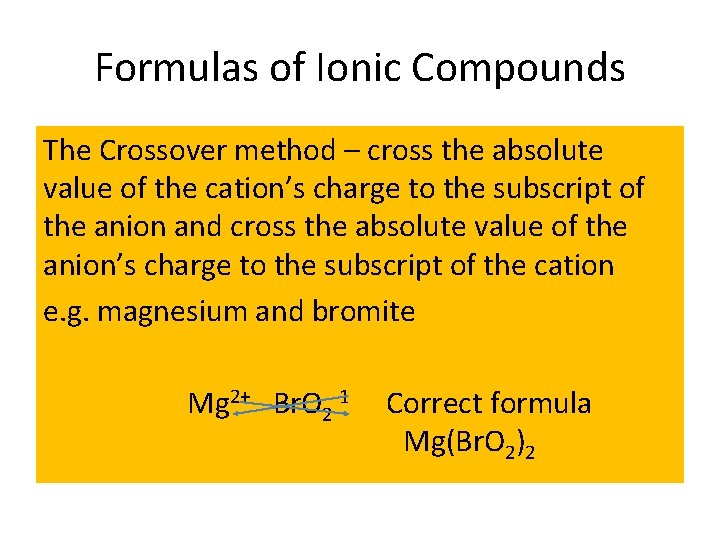
Formulas of Ionic Compounds The Crossover method – cross the absolute value of the cation’s charge to the subscript of the anion and cross the absolute value of the anion’s charge to the subscript of the cation e. g. magnesium and bromite Mg 2+ Br. O 2 -1 Correct formula Mg(Br. O 2)2

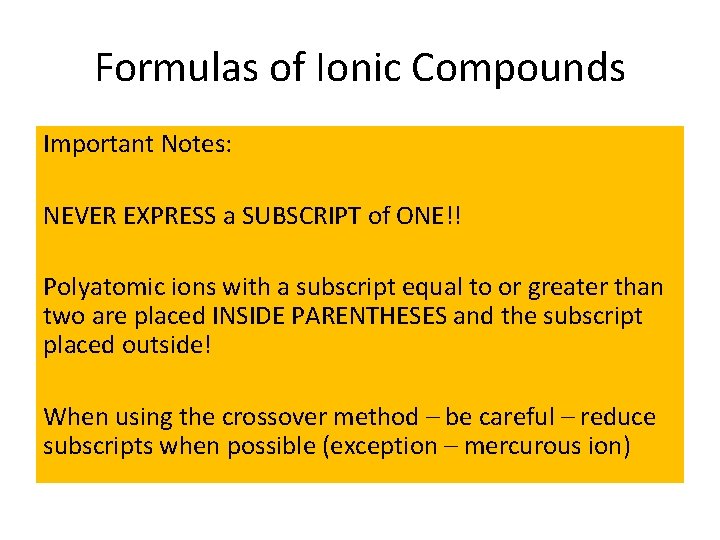
Formulas of Ionic Compounds Important Notes: NEVER EXPRESS a SUBSCRIPT of ONE!! Polyatomic ions with a subscript equal to or greater than two are placed INSIDE PARENTHESES and the subscript placed outside! When using the crossover method – be careful – reduce subscripts when possible (exception – mercurous ion)

Writing Formulas for molecular Compounds A molecular compound is either going to be organic (involve carbon) or be composed of two nonmetals. Organic compounds have their own nomenclature – see later slide. Molecular compounds composed of two nonmetals may be named traditionally or at times with a Stock system name.

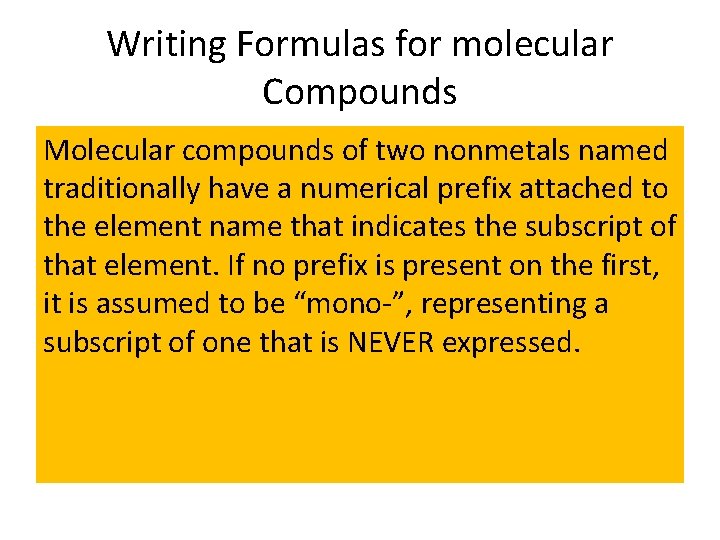
Writing Formulas for molecular Compounds Molecular compounds of two nonmetals named traditionally have a numerical prefix attached to the element name that indicates the subscript of that element. If no prefix is present on the first, it is assumed to be “mono-”, representing a subscript of one that is NEVER expressed.

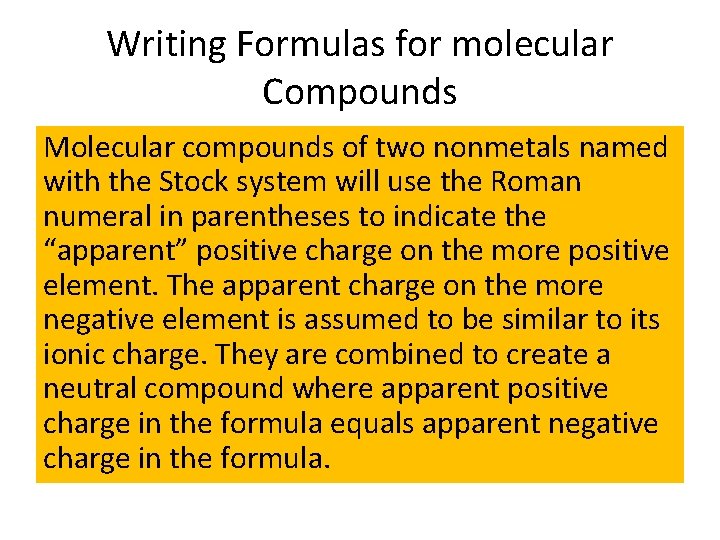
Writing Formulas for molecular Compounds Molecular compounds of two nonmetals named with the Stock system will use the Roman numeral in parentheses to indicate the “apparent” positive charge on the more positive element. The apparent charge on the more negative element is assumed to be similar to its ionic charge. They are combined to create a neutral compound where apparent positive charge in the formula equals apparent negative charge in the formula.

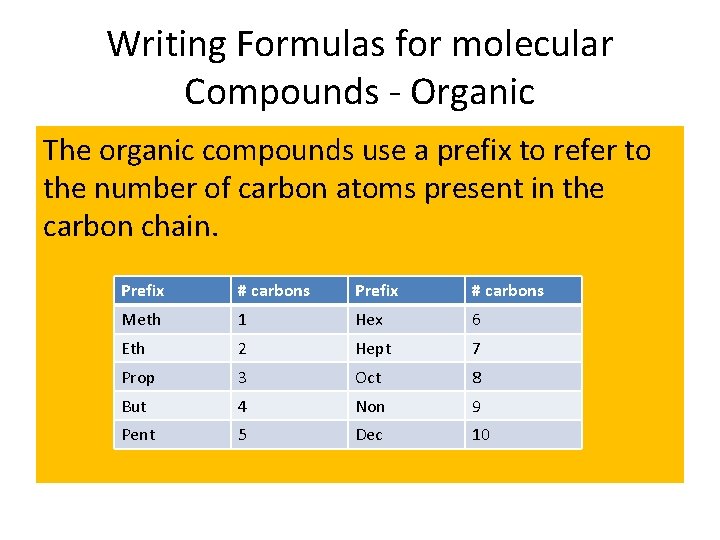
Writing Formulas for molecular Compounds - Organic The organic compounds use a prefix to refer to the number of carbon atoms present in the carbon chain. Prefix # carbons Meth 1 Hex 6 Eth 2 Hept 7 Prop 3 Oct 8 But 4 Non 9 Pent 5 Dec 10

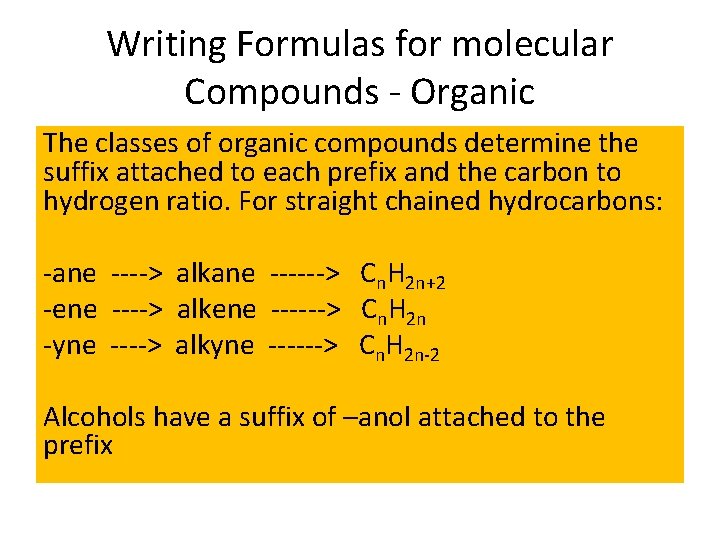
Writing Formulas for molecular Compounds - Organic The classes of organic compounds determine the suffix attached to each prefix and the carbon to hydrogen ratio. For straight chained hydrocarbons: -ane ----> alkane ------> Cn. H 2 n+2 -ene ----> alkene ------> Cn. H 2 n -yne ----> alkyne ------> Cn. H 2 n-2 Alcohols have a suffix of –anol attached to the prefix

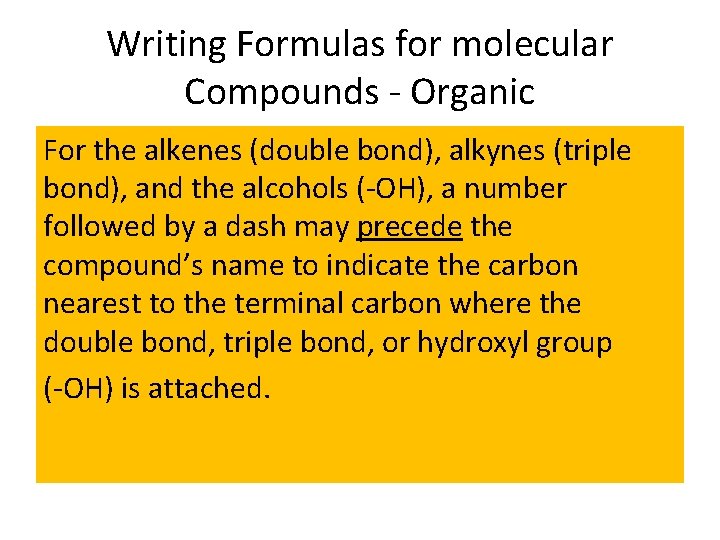
Writing Formulas for molecular Compounds - Organic For the alkenes (double bond), alkynes (triple bond), and the alcohols (-OH), a number followed by a dash may precede the compound’s name to indicate the carbon nearest to the terminal carbon where the double bond, triple bond, or hydroxyl group (-OH) is attached.

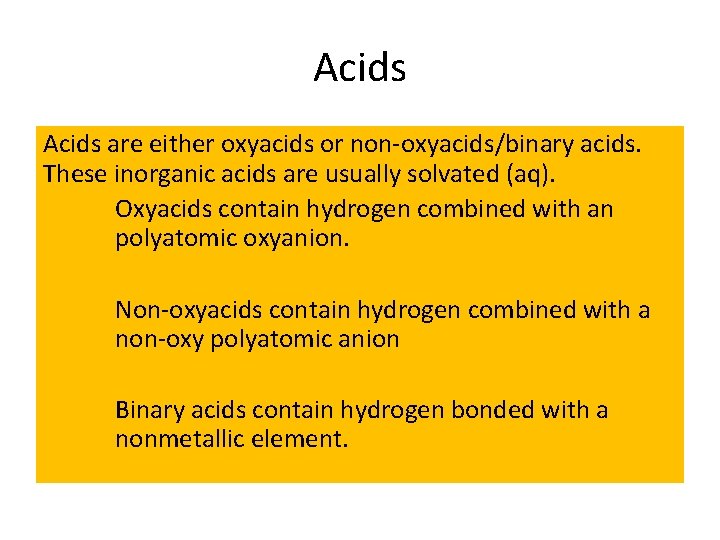
Acids are either oxyacids or non-oxyacids/binary acids. These inorganic acids are usually solvated (aq). Oxyacids contain hydrogen combined with an polyatomic oxyanion. Non-oxyacids contain hydrogen combined with a non-oxy polyatomic anion Binary acids contain hydrogen bonded with a nonmetallic element.

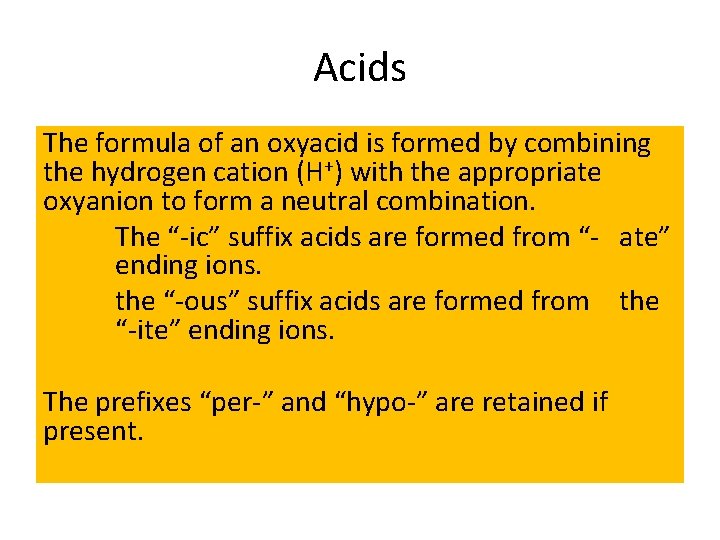
Acids The formula of an oxyacid is formed by combining the hydrogen cation (H+) with the appropriate oxyanion to form a neutral combination. The “-ic” suffix acids are formed from “- ate” ending ions. the “-ous” suffix acids are formed from the “-ite” ending ions. The prefixes “per-” and “hypo-” are retained if present.

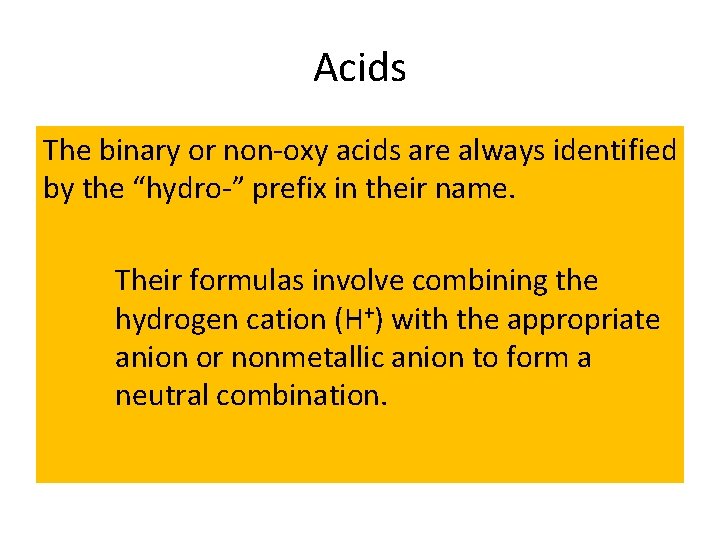
Acids The binary or non-oxy acids are always identified by the “hydro-” prefix in their name. Their formulas involve combining the hydrogen cation (H+) with the appropriate anion or nonmetallic anion to form a neutral combination.

Hydrated Compounds The name of a hydrated compound (hydrate) consists of the name of an anhydrous salt followed by a numerical prefix attached to the word hydrate. Anhydrous formula – this is the formula of a salt or ionic compound. Prefixes: mono-, di-, tri-, tetra-, penta-, hexahepta-, octa-, nona-, deca- …….

Hydrated Compounds To write the formula of a hydrate, follow the instructions for ionic compounds to write the formula of the anhydrous salt. Place a raised dot ( • ), a numerical coefficient (except one; 1) that refers to the numerical prefix attached to the word hydrate, and the formula for water (H 2 O).