How to Write a Clinical Protocol Michael A


















- Slides: 22

How to Write a Clinical Protocol Michael A. Gaglia, Jr. , MD, MSc, FACC, FSCAI Scientific Lead for Population Research Medstar Cardiovascular Research Network Medstar Heart and Vascular Institute

Michael A. Gaglia, Jr. I have no relevant financial relationships

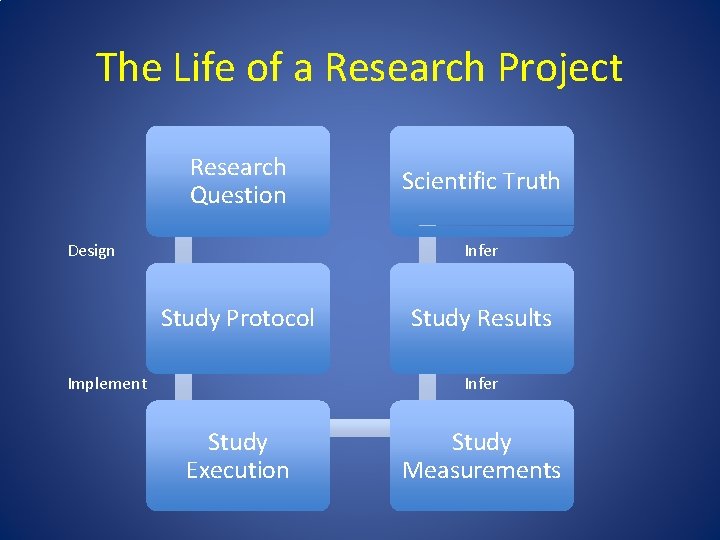
The Life of a Research Project Research Question Design Scientific Truth Infer Study Protocol Implement Study Results Infer Study Execution Study Measurements

What is a Research Protocol? • It is a detailed, written plan for a scientific study. • It is a formal document that specifies what a study intends to accomplish, and how this will be achieved.

Why Should I Write a Research Protocol? • It refines and organizes a research project’s components, thereby improving its scientific rigor. – Therefore, any research project, small or large, should have a protocol. • It is required for funding at almost any level. • It provides a reference and tool for the research team to execute the study.

How Do I Start? • Clearly formulate your hypothesis and specific aims • Perform thorough literature review to verify your project has not been done before • If applying for funding, review application instructions in detail – Use completed protocols and applications as template – Secure assistance for budget • Solicit feedback from mentors and colleagues regarding your specific aims

What Are the Components of a Research Protocol? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Descriptive Title Specific Aims / Study Objectives and Hypothesis Study Background and Scientific Rationale Study Outcome Measures/Endpoints Methodology and Population Studied Statistical Plan for Analysis Study Schedule/Timeline Ethical Considerations Bibliography Budget Appendices: CVs, CRFs, ICFs, measurement tools, questionnaires

Hypothesis Generation • A good research question should pass the “FINER” test: – Feasible • Adequate expertise, funding, number of subjects – Interesting • To you and your mentors – Novel • Need not be entirely original, e. g. progress is often incremental – Ethical • Consult with IRB early in process – Relevant • How will study advance the field, guide clinical management, or prompt further research?

NIH Peer Review Criteria • Significance – Does project address important problem or critical barrier to progress? • Investigator – Do PIs have appropriate experience, training, support? • Innovation – Is research novel? Does it seek to shift current paradigms? • Approach – Are overall strategy and methods appropriate and considered? • Environment – Do resources and scientific environment contribute to probability of success?

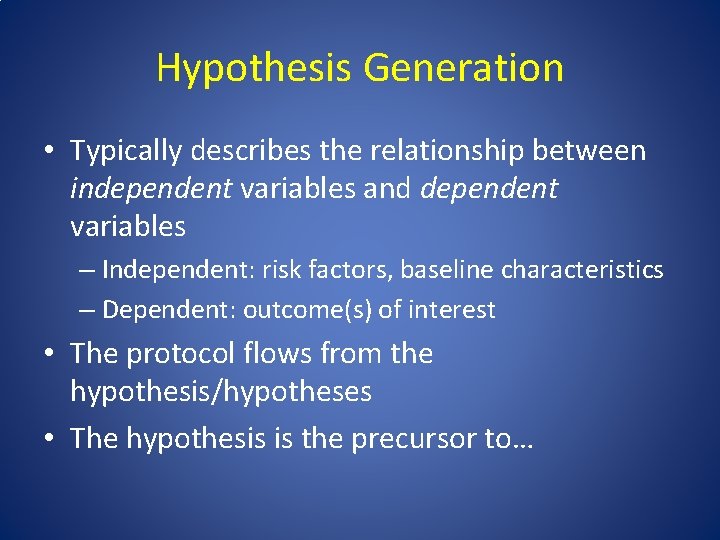
Hypothesis Generation • Typically describes the relationship between independent variables and dependent variables – Independent: risk factors, baseline characteristics – Dependent: outcome(s) of interest • The protocol flows from the hypothesis/hypotheses • The hypothesis is the precursor to…

Specific Aims • Concisely describe goals of the proposed research • Summarize the expected outcomes of research, including the impact of the results upon the field • States specific objectives of the research – Test a stated hypothesis – Challenge existing paradigm – Develop new technology – Address a specific problem or barrier to progress

Structure of Specific Aims • Introductory paragraph – – First sentence or “hook”: convey importance or urgency What is known: brief, 3 -5 sentences Gap in knowledge: one sentence, what is not known Critical need: 1 -2 sentences why should be funded • Second paragraph: introduce the solution – What will you do? – How will you do it? – Why are you doing it? • Aims: describe aims you will use to test hypotheses – 2 -4 aims; 2 -4 sentences each • Final summary: 3 -4 sentences – State what is innovative – State the expected outcomes – State the expected impact

Common Research Designs • Experimental: randomized clinical trial – Treatment – Prevention • Observational – Descriptive: describe distributions of disease, health-related population characteristics, diagnostic test (sensitivity/specificity) • Retrospective, Prospective, Cross-sectional – Analytical • Cohort, Case-Control, Propensity-matched

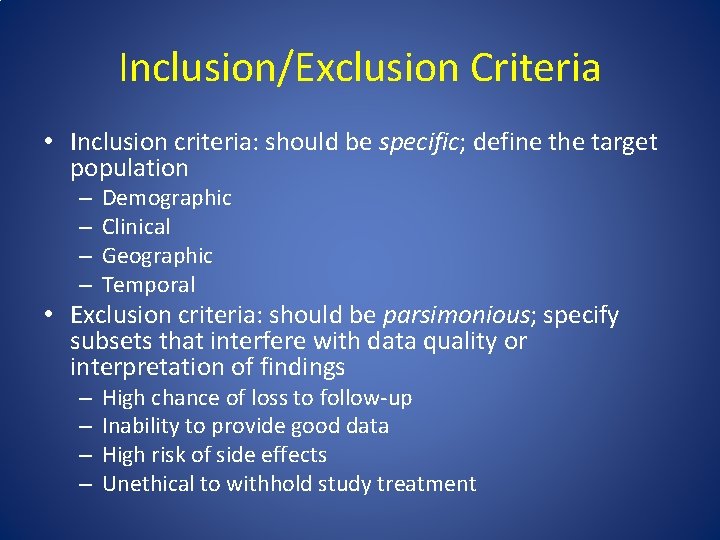
Inclusion/Exclusion Criteria • Inclusion criteria: should be specific; define the target population – – Demographic Clinical Geographic Temporal • Exclusion criteria: should be parsimonious; specify subsets that interfere with data quality or interpretation of findings – – High chance of loss to follow-up Inability to provide good data High risk of side effects Unethical to withhold study treatment

Statistical Plan • Get help; most of us do not have degrees in biostatistics (but your reviewer might) • Accurate sample size calculation is essential; do it early in the design process – Usually requires some form of preliminary data – Use the literature if you don’t have your own data – Be realistic about effect size; most interventions are not penicillin – Be prepared to abandon study if sample size is not practical or affordable • Take time to specify a plan for analysis of data – This helps to refine measurements and endpoint definitions

Sample Size 1. State the null hypothesis and alternative hypothesis 2. Select an appropriate statistical test 3. Select a reasonable effect size and variability (e. g. mean and standard deviation) 4. Set α and β levels (e. g. 0. 05 and 0. 20) 5. Calculate sample size 6. Don’t forget to account for dropouts

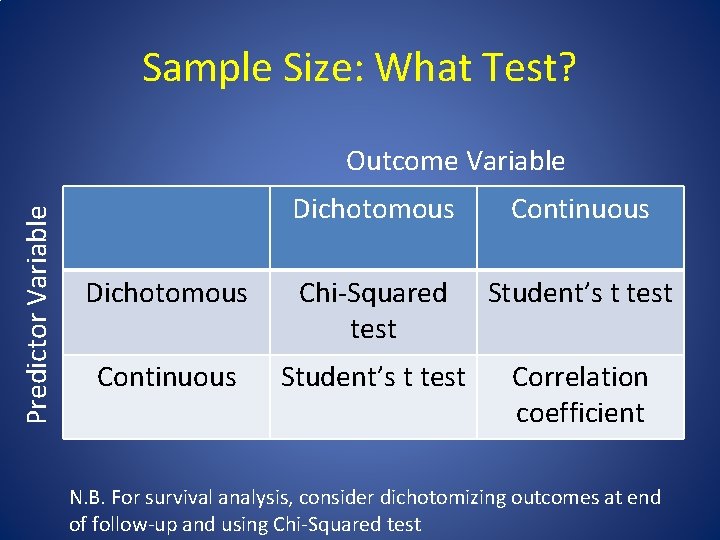
Sample Size: What Test? Predictor Variable Outcome Variable Dichotomous Continuous Dichotomous Chi-Squared test Student’s t test Continuous Student’s t test Correlation coefficient N. B. For survival analysis, consider dichotomizing outcomes at end of follow-up and using Chi-Squared test

Strategies to Minimize Sample Size • Modify effect size, variability, α or β • Use continuous variables • Use paired measurements – outcome is the difference between baseline and follow-up in each subject • Use more precise variables – Decreases standard deviation, e. g. variability • Choose a more common outcome – Increases power (e. g. 1 -β)

Ethical Considerations • A thorough and compliant ethical plan is the responsibility of every primary investigator – Vulnerable populations, genetic research, and research on previously collected specimens require special attention Guidance from local IRB is essential Consider all side effects and potential for harm Be frank about risks vs. benefits Specify that patient may discontinue study at any time For large trials, specify the role and makeup of Data Safety Monitoring Board • Consider Clinical Events Committee to ensure confidentiality and veracity of data • • •

Tips For Writing • Think like a reviewer – Your proposal is one among many, so your chances are best if yours is well-written, organized, and concise. – Proofreading is essential, as grammatical and spelling errors irritate reviewers. – If writing is not your strength, find someone to help – Capture the reviewer’s attention by making persuasive arguments – Do not assume a reviewer has detailed understanding of the background or proposed research methods • Even if you are not seeking funding, the success of your project depends on ability of team to execute the protocol as it is written

Proofreading • Have zero tolerance for spelling, grammar, syntax, and formatting errors • Write in the active voice, not passive • Utilize bullets, numbered lists, and bold print to improve readability • Utilize diagrams, figures and tables to synthesize concepts

Resources • Advice on Writing Grant Applications: http: //grants. nih. gov/grants/writing_applicati on. htm • Advice on Writing Specific Aims: http: //www. biosciencewriters. com/NIH-Grant -Applications-The-Anatomy-of-a-Specific-Aims -Page. aspx • Advice on Study Design: Designing Clinical Research. SB Hulley et al; Lippincott 2013