How to talk to patients about Vasomotor Symptoms

How to talk to patients about Vasomotor Symptoms of Menopause and Hormonal Treatment Options Karen J. Antell, MD, MPH, FAAFP, NCMP Christiana Care Department of Family and Community Medicine

DISCLOSURE I have nothing to disclose. Regarding “off-label use” statements: I will discuss off-label use of levonorgestrel IUD and dosing of SSRIs. These will be labeled and noted as such. Some of the slide information comes from position statements of the North American Menopause Society (NAMS), and from presentations given at the NAMS Annual Meeting in September 2020.
LEARNING OBJECTIVES § Understand vasomotor symptoms of menopause and their effects on sleep, mood and physical health § Understand risks and benefits of using hormonal medications to treat vasomotor symptoms § Overview of oral and transdermal hormonal medications § Overview of FDA-approved non-hormonal medications § Overview of OTC and off-label hormonal and non-hormonal medications

PRE-TEST QUESTIONS Answer these Polling Questions


Menopause § Menopause is permanent cessation of menstruation • Surgical • Natural § Diagnosed in retrospect 12 months after final menstrual period (FMP) § Median age in US is 51 years, range 40 -58 § Woman are menopausal more than one third of their lives – median life expectancy is > 80 years § Most sociocultural studies (ideas of self-image, sexuality, femininity) have been conducted on middle-class Caucasian women

Vasomotor Symptoms – hot flashes, flushing, night sweats § Hot flash - sudden wave of heat begins upper body/face, sweating in upper body for 1 -5 minutes. Associated with increase in HR. § 80% of women experience hot flashes, more than 80% of those at least daily and 1/3 more than 10/day § Highest frequency during first 2 years post-menopause • Last 6 months – 14 years • 10% of women continue after 10 years § The majority of menopausal women with vasomotor symptoms don’t receive treatment for their symptoms

Genitourinary Symptoms of Menopause (GSM) § Not just vulvovaginal atrophy § Most common symptoms: • Vulvar irritation, vaginal burning • Insufficient vaginal lubrication • Dysuria, urinary urgency, mild incontinence • Dyspareunia from lack of lubrication and shortening/narrowing of vagina • Vaginal discharge § Affect at least half of postmenopausal women, increasing with years after FMP § Chronic and progressive, doesn’t improve without treatment § Significantly underdiagnosed and undertreated

Case L. O. is a 54 year old patient (she/hers) who previously had monthly menses for 5 days. Two years ago she noted that she went 2 -3 months between cycles, and her last menses was 14 months ago. She tells you that she is having “hot flashes” 5 -8 times per day, and that they are very disruptive. She is having difficulty sleeping because of them. She is periodically sexually active with one partner, and describes intercourse as sometimes painful, with noted vaginal dryness. She has a history of hypercholesterolemia and was recently started on a statin. She is normotensive and otherwise has no other chronic medical conditions. She is self-employed and has no health insurance.
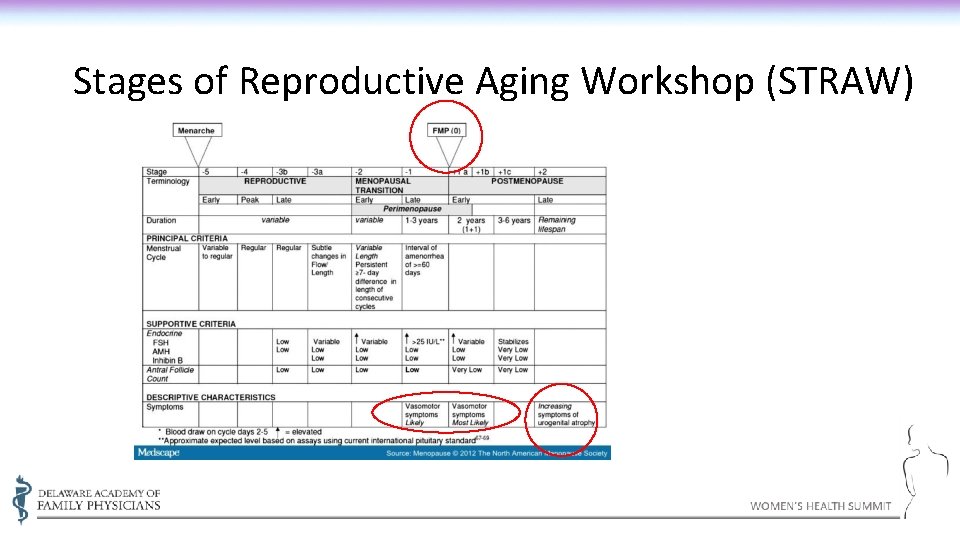
Stages of Reproductive Aging Workshop (STRAW)
Phases of Transition § Climacteric = perimenopause = menopausal transition § Menopause transition begins with variation in cycle length caused by rise in FSH and ends with FMP (although FMP recognized retrospectively after 1 year). § Menopause transition – menstrual cycle and endocrine changes, divided into stage • -2 (early) and -1 (late) § Noticeable physiologic changes begin several years before menopause § Premature menopause – age 40 often used, arbitrary cutoff. Occurs in about 1% of women naturally

Risk Factors for (more) Vasomotor Symptoms – The Study of Women’s Health Across the Nation (SWAN) § SWAN (~3000 multiethnic/multiracial women, 1996 -2013) – median total duration 7. 4 years, 10. 1 in AA women, approx. 5 in Chinese- and Japanese. American women § Best predictor of duration was beginning symptoms at earlier MT stage – the earlier they started, the longer they lasted § Higher perceived stress, depressed symptoms at first report of symptoms – correlated with longer duration, as did lower education level § From other studies: • • • High BMI Low levels of physical activity Cigarette smoking Surgical menopause Menopause induced by chemotherapy or pelvic radiation therapy Avis NE JAMA Intern Med. 2015; 175(4): 531 -539.

Sleep Disturbance § About half of women aged 40 -64 report sleep problems § Hot flushes and night sweats may interrupt sleep • Sleep time, latency, efficiency not affected

Cognitive Function and Mental Health § Some verbal fluency and memory loss may be related to vasomotor symptoms and sleep disturbance § No direct effect of menopause and hormonal changes on dementia has been determined § Increased vulnerability to depression during menopause transition may be secondary to hormonal effects and sleep disturbance

Body Changes § Weight gain (average 5 lbs) • No hormonal cause • Lean body mass decreases with age • Decrease in physical activity • Sleep deprivation • Best managed by increase in exercise and diet modification (low-fat, increased fruit/veg/grain)

Treatment options for vasomotor symptoms: Menopausal Hormone Therapy (MHT) and more
Therapeutic options - Hormones § Systemic hormone therapy (MHT) – estrogen +- progesterone § Effectiveness – 75% reduction in frequency, significant reduction in severity (Cochrane review, 2004) § Also, may improve depressive symptoms, sleep (especially if related to vasomotor symptoms), and some non-specific joint pain § Risks • Endometrial hyperplasia/CA – need progesterone • Breast CA • VTE/CHD/Stroke § Side effects – • Estrogen –breast pain, abdominal pain, nausea • Progestins –headache, breast pain, fatigue, dysphoria, fluid retention, nausea

FDA-approved Indications for Hormone Therapy § First-line therapy for relief of vasomotor symptoms in appropriate candidates § To prevent bone loss and reduce fractures in postmenopausal women at elevated risk of osteoporosis or fractures § For women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause without contraindication, hormone therapy is recommended for health benefits until the average of menopause § Low-dose vaginal estrogen therapy is recommended first line for isolated genitourinary syndrome of menopause to treat symptoms of vulvovaginal atrophy The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017; 24(7): 728 -753.
Hormone Therapy and Vasomotor Symptoms § Hormone therapy remains the gold standard for relief of vasomotor symptoms (VMS) • Estrogen-alone therapy is used for symptomatic women after hysterectomy • For symptomatic women with a uterus, combination therapy protects against endometrial neoplasia, either with a progestogen or a combination of conjugated equine estrogen and bazedoxifene § The lowest dose that offers relief should be used and assessed periodically § Progestogens relieve VMS • Micronized progesterone 300 mg nightly decreases VMS • Synthetic progestins have shown benefit • No long-term study results are available The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017; 24(7): 728 -753.

Safety of Hormone Therapy § Women’s Health Initiative largest trial – (68, 000 post-menopausal women) – HRT should NOT be used to prevent CHD • Overall illness and death decreased in women on HRT in their 50 s, increased in their 70 s § Unclear what exact duration is safest, but shortest duration, closest to menopause, and lowest dose is overall safest § American Geriatric Society/Beers criteria places estrogen +- progestin on “potentially inappropriate” list because of risk of breast/endometrial cancer and lack of protective effect against CHD or dementia –recommend avoid oral or patch, but low-dose vaginal estrogen for GSM acceptable. § ACOG recommends against routine discontinuation of MHT at age 65 § General recommendation: lowest effective dose for shortest duration acceptable to patient
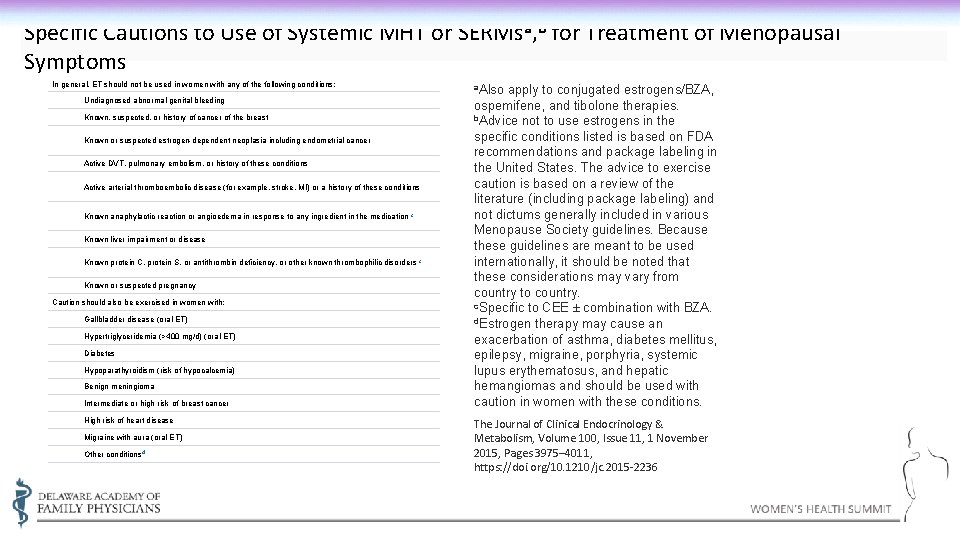
Specific Cautions to Use of Systemic MHT or SERMsa, b for Treatment of Menopausal Symptoms In general, ET should not be used in women with any of the following conditions: a. Also Undiagnosed abnormal genital bleeding Known, suspected, or history of cancer of the breast Known or suspected estrogen-dependent neoplasia including endometrial cancer Active DVT, pulmonary embolism, or history of these conditions Active arterial thromboembolic disease (for example, stroke, MI) or a history of these conditions Known anaphylactic reaction or angioedema in response to any ingredient in the medication c Known liver impairment or disease Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders c Known or suspected pregnancy Caution should also be exercised in women with: Gallbladder disease (oral ET) Hypertriglyceridemia (>400 mg/d) (oral ET) Diabetes Hypoparathyroidism (risk of hypocalcemia) Benign meningioma Intermediate or high risk of breast cancer High risk of heart disease Migraine with aura (oral ET) Other conditions d apply to conjugated estrogens/BZA, ospemifene, and tibolone therapies. b. Advice not to use estrogens in the specific conditions listed is based on FDA recommendations and package labeling in the United States. The advice to exercise caution is based on a review of the literature (including package labeling) and not dictums generally included in various Menopause Society guidelines. Because these guidelines are meant to be used internationally, it should be noted that these considerations may vary from country to country. c. Specific to CEE ± combination with BZA. d. Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions. The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 11, 1 November 2015, Pages 3975– 4011, https: //doi. org/10. 1210/jc. 2015 -2236
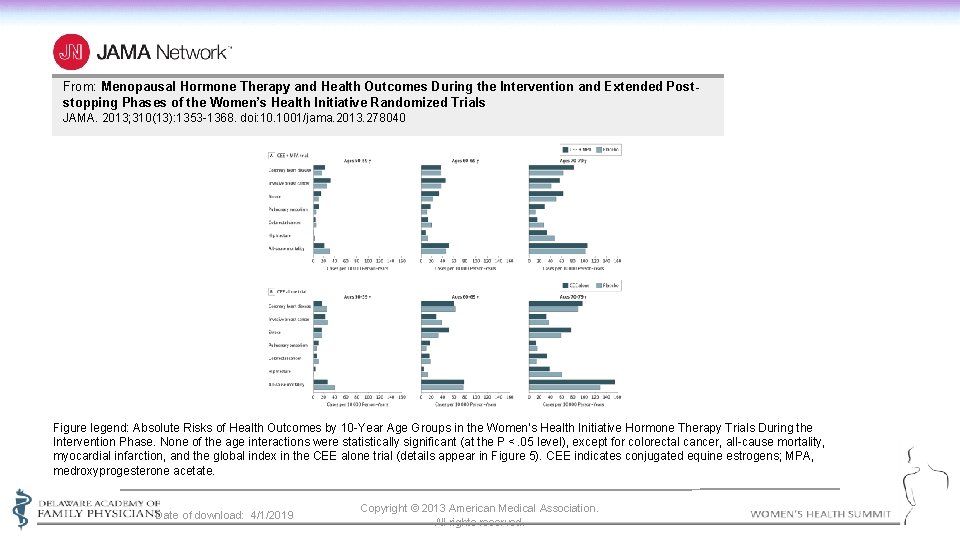
From: Menopausal Hormone Therapy and Health Outcomes During the Intervention and Extended Poststopping Phases of the Women’s Health Initiative Randomized Trials JAMA. 2013; 310(13): 1353 -1368. doi: 10. 1001/jama. 2013. 278040 Figure legend: Absolute Risks of Health Outcomes by 10 -Year Age Groups in the Women’s Health Initiative Hormone Therapy Trials During the Intervention Phase. None of the age interactions were statistically significant (at the P <. 05 level), except for colorectal cancer, all-cause mortality, myocardial infarction, and the global index in the CEE alone trial (details appear in Figure 5). CEE indicates conjugated equine estrogens; MPA, medroxyprogesterone acetate. Date of download: 4/1/2019 Copyright © 2013 American Medical Association. All rights reserved.
Age and time since menopause onset: timing hypothesis § The effects of hormone therapy (HT) on coronary heart disease (CHD) may vary depending on a woman’s age and time since menopause onset § Data show reduced CHD in women who initiate HT aged younger than 60 years and/or within 10 years of menopause onset § There is concern of increased risk of CHD in women who initiate HT more than 10 or 20 years from menopause onset The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017; 24(7): 728 -753.
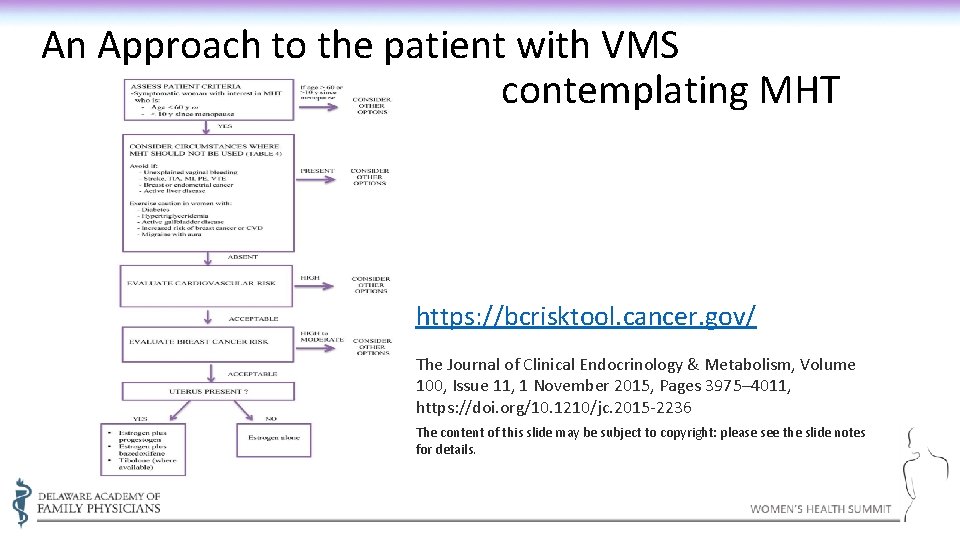
An Approach to the patient with VMS contemplating MHT https: //bcrisktool. cancer. gov/ The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 11, 1 November 2015, Pages 3975– 4011, https: //doi. org/10. 1210/jc. 2015 -2236 The content of this slide may be subject to copyright: please see the slide notes for details.
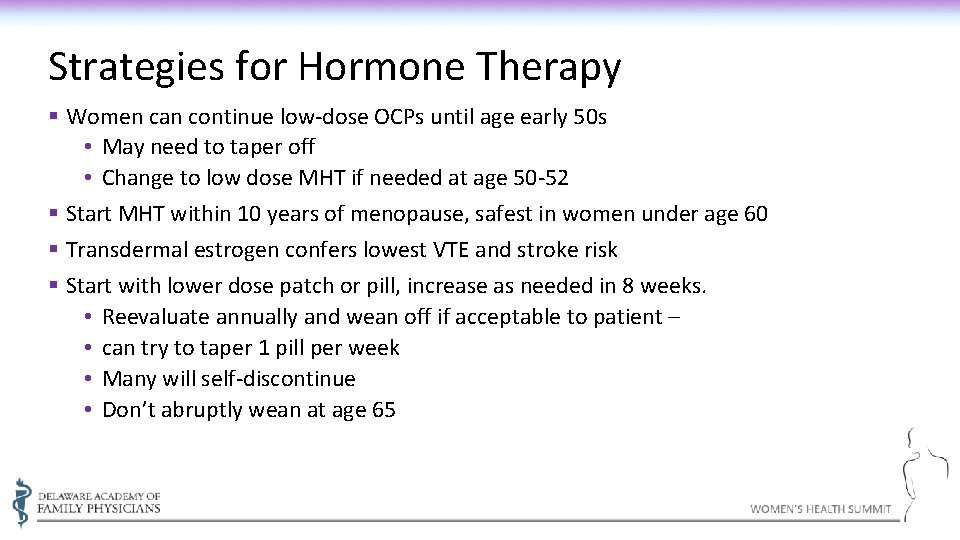
Strategies for Hormone Therapy § Women can continue low-dose OCPs until age early 50 s • May need to taper off • Change to low dose MHT if needed at age 50 -52 § Start MHT within 10 years of menopause, safest in women under age 60 § Transdermal estrogen confers lowest VTE and stroke risk § Start with lower dose patch or pill, increase as needed in 8 weeks. • Reevaluate annually and wean off if acceptable to patient – • can try to taper 1 pill per week • Many will self-discontinue • Don’t abruptly wean at age 65
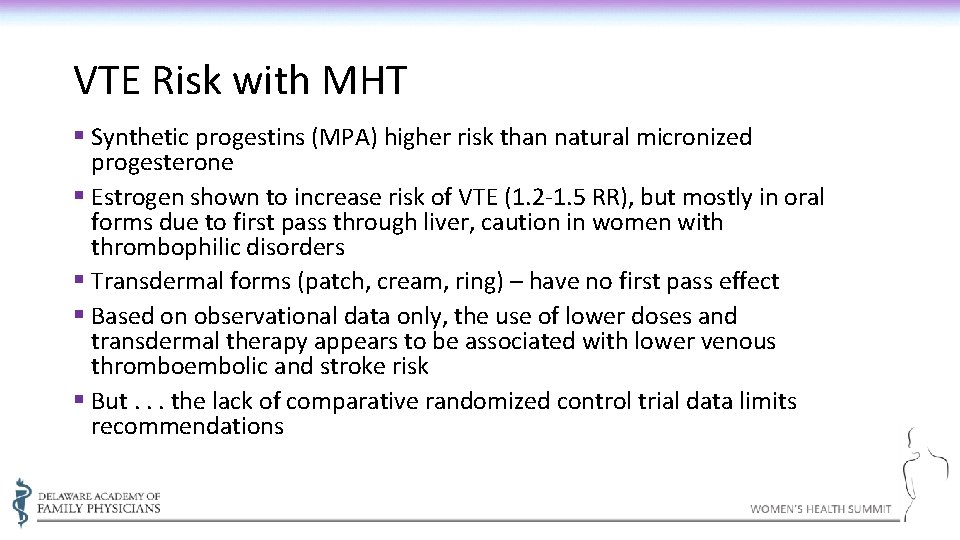
VTE Risk with MHT § Synthetic progestins (MPA) higher risk than natural micronized progesterone § Estrogen shown to increase risk of VTE (1. 2 -1. 5 RR), but mostly in oral forms due to first pass through liver, caution in women with thrombophilic disorders § Transdermal forms (patch, cream, ring) – have no first pass effect § Based on observational data only, the use of lower doses and transdermal therapy appears to be associated with lower venous thromboembolic and stroke risk § But. . . the lack of comparative randomized control trial data limits recommendations
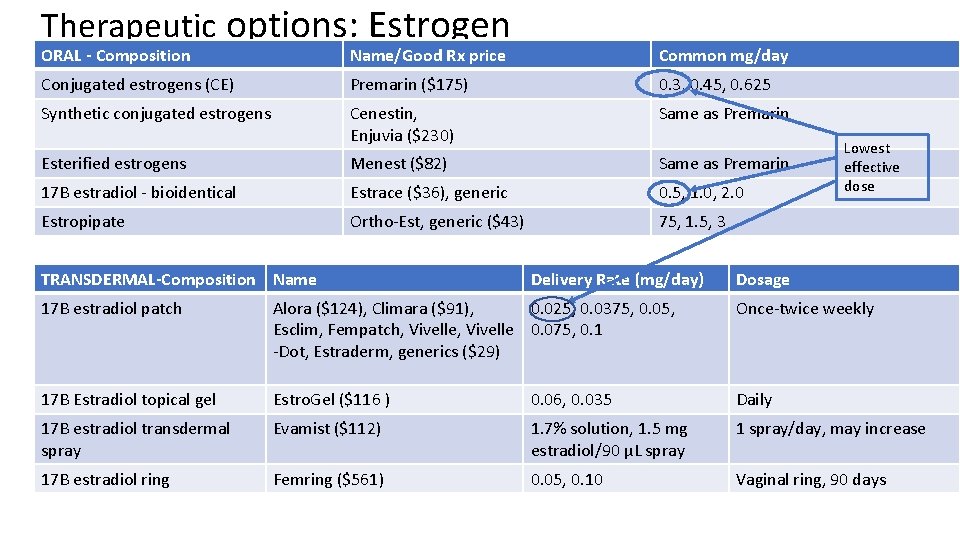
Therapeutic options: Estrogen ORAL - Composition Name/Good Rx price Common mg/day Conjugated estrogens (CE) Premarin ($175) 0. 3, 0. 45, 0. 625 Synthetic conjugated estrogens Cenestin, Enjuvia ($230) Same as Premarin Esterified estrogens Menest ($82) Same as Premarin 17 B estradiol - bioidentical Estrace ($36), generic 0. 5, 1. 0, 2. 0 Estropipate Ortho-Est, generic ($43) 75, 1. 5, 3 TRANSDERMAL-Composition Name Delivery Rate (mg/day) Lowest effective dose Dosage 17 B estradiol patch Alora ($124), Climara ($91), 0. 025, 0. 0375, 0. 05, Esclim, Fempatch, Vivelle 0. 075, 0. 1 -Dot, Estraderm, generics ($29) Once-twice weekly 17 B Estradiol topical gel Estro. Gel ($116 ) 0. 06, 0. 035 Daily 17 B estradiol transdermal spray Evamist ($112) 1. 7% solution, 1. 5 mg estradiol/90 µL spray 1 spray/day, may increase 17 B estradiol ring Femring ($561) 0. 05, 0. 10 Vaginal ring, 90 days
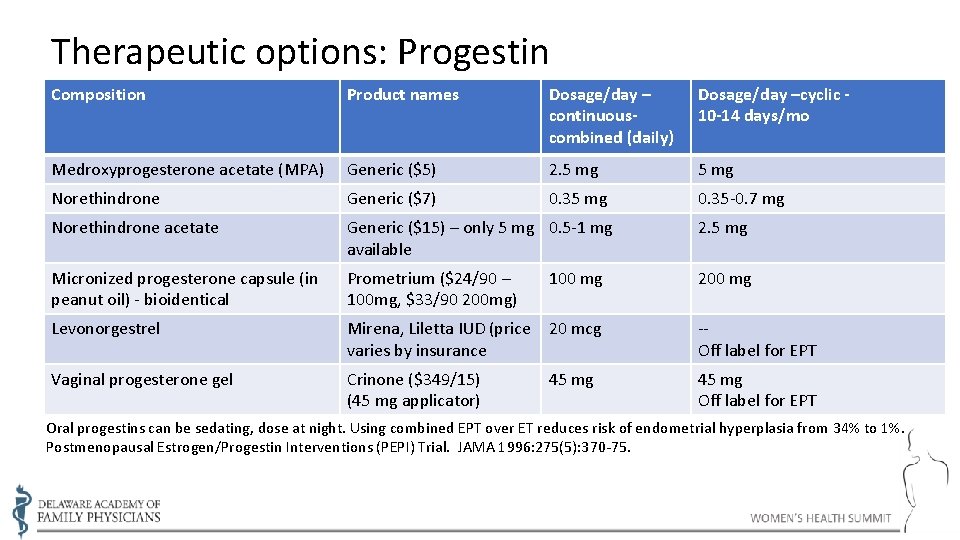
Therapeutic options: Progestin Composition Product names Dosage/day – continuouscombined (daily) Dosage/day –cyclic 10 -14 days/mo Medroxyprogesterone acetate (MPA) Generic ($5) 2. 5 mg Norethindrone Generic ($7) 0. 35 mg 0. 35 -0. 7 mg Norethindrone acetate Generic ($15) – only 5 mg 0. 5 -1 mg available 2. 5 mg Micronized progesterone capsule (in peanut oil) - bioidentical Prometrium ($24/90 – 100 mg, $33/90 200 mg) 200 mg Levonorgestrel Mirena, Liletta IUD (price 20 mcg varies by insurance -Off label for EPT Vaginal progesterone gel Crinone ($349/15) (45 mg applicator) 45 mg Off label for EPT 100 mg 45 mg Oral progestins can be sedating, dose at night. Using combined EPT over ET reduces risk of endometrial hyperplasia from 34% to 1%. Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1996: 275(5): 370 -75.
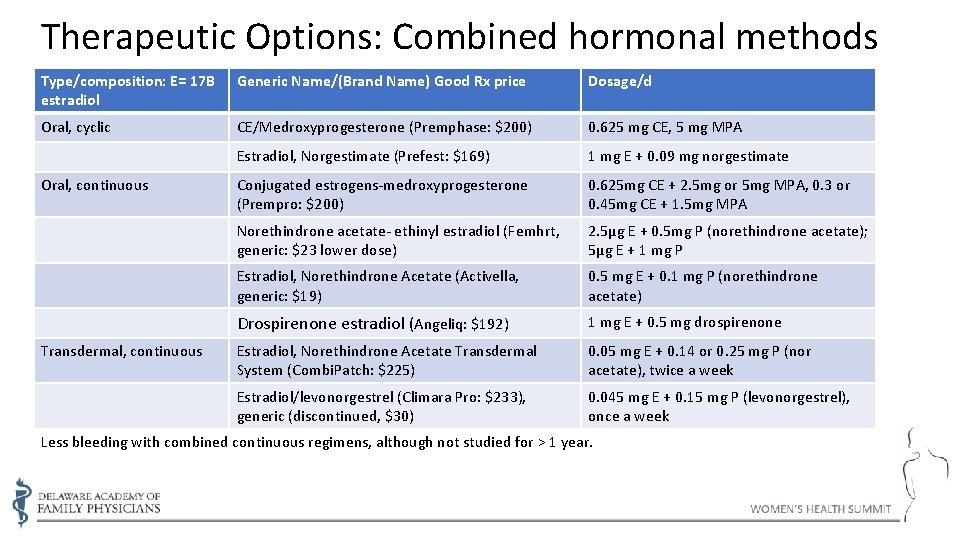
Therapeutic Options: Combined hormonal methods Type/composition: E= 17 B estradiol Generic Name/(Brand Name) Good Rx price Dosage/d Oral, cyclic CE/Medroxyprogesterone (Premphase: $200) 0. 625 mg CE, 5 mg MPA Estradiol, Norgestimate (Prefest: $169) 1 mg E + 0. 09 mg norgestimate Conjugated estrogens-medroxyprogesterone (Prempro: $200) 0. 625 mg CE + 2. 5 mg or 5 mg MPA, 0. 3 or 0. 45 mg CE + 1. 5 mg MPA Norethindrone acetate- ethinyl estradiol (Femhrt, generic: $23 lower dose) 2. 5µg E + 0. 5 mg P (norethindrone acetate); 5µg E + 1 mg P Estradiol, Norethindrone Acetate (Activella, generic: $19) 0. 5 mg E + 0. 1 mg P (norethindrone acetate) Drospirenone estradiol (Angeliq: $192) 1 mg E + 0. 5 mg drospirenone Estradiol, Norethindrone Acetate Transdermal System (Combi. Patch: $225) 0. 05 mg E + 0. 14 or 0. 25 mg P (nor acetate), twice a week Estradiol/levonorgestrel (Climara Pro: $233), generic (discontinued, $30) 0. 045 mg E + 0. 15 mg P (levonorgestrel), once a week Oral, continuous Transdermal, continuous Less bleeding with combined continuous regimens, although not studied for > 1 year.
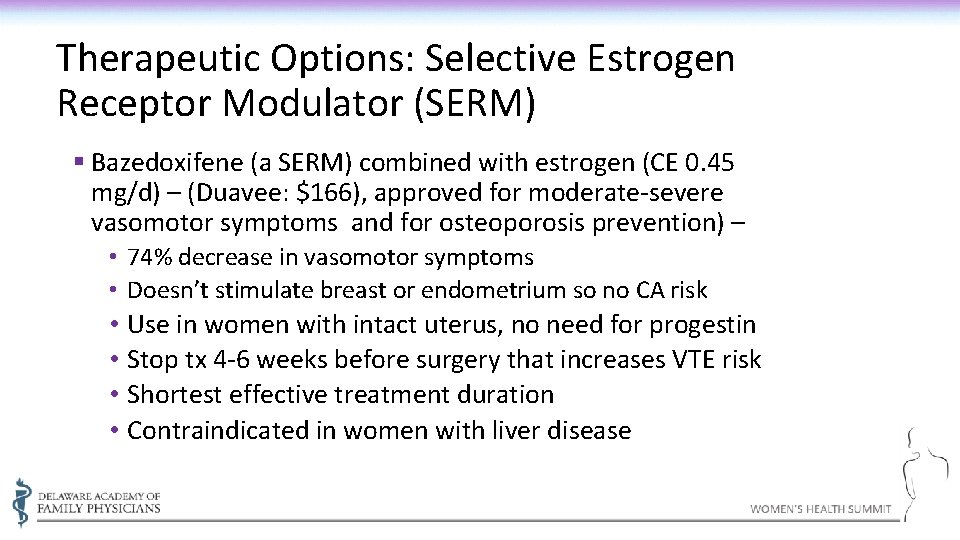
Therapeutic Options: Selective Estrogen Receptor Modulator (SERM) § Bazedoxifene (a SERM) combined with estrogen (CE 0. 45 mg/d) – (Duavee: $166), approved for moderate-severe vasomotor symptoms and for osteoporosis prevention) – • 74% decrease in vasomotor symptoms • Doesn’t stimulate breast or endometrium so no CA risk • Use in women with intact uterus, no need for progestin • Stop tx 4 -6 weeks before surgery that increases VTE risk • Shortest effective treatment duration • Contraindicated in women with liver disease
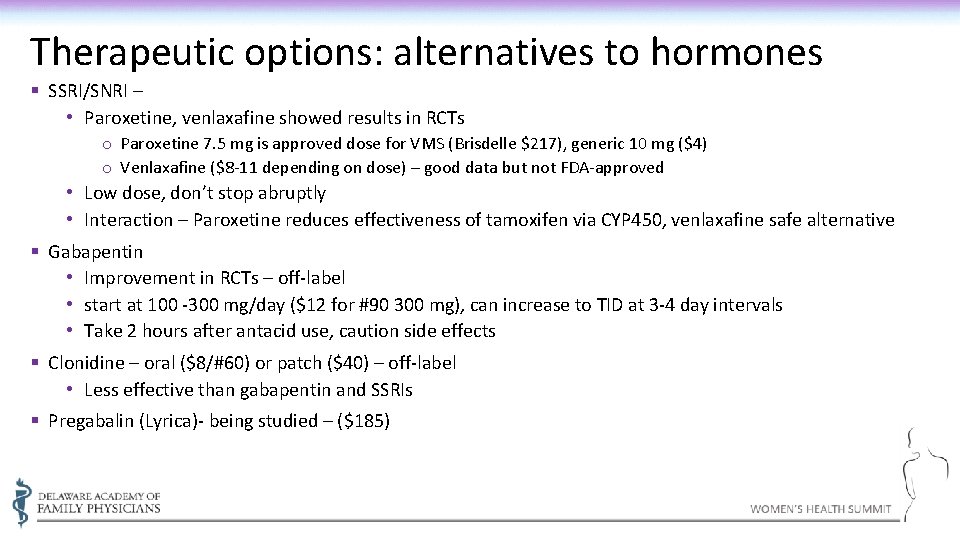
Therapeutic options: alternatives to hormones § SSRI/SNRI – • Paroxetine, venlaxafine showed results in RCTs o Paroxetine 7. 5 mg is approved dose for VMS (Brisdelle $217), generic 10 mg ($4) o Venlaxafine ($8 -11 depending on dose) – good data but not FDA-approved • Low dose, don’t stop abruptly • Interaction – Paroxetine reduces effectiveness of tamoxifen via CYP 450, venlaxafine safe alternative § Gabapentin • Improvement in RCTs – off-label • start at 100 -300 mg/day ($12 for #90 300 mg), can increase to TID at 3 -4 day intervals • Take 2 hours after antacid use, caution side effects § Clonidine – oral ($8/#60) or patch ($40) – off-label • Less effective than gabapentin and SSRIs § Pregabalin (Lyrica)- being studied – ($185)
Compounded bioidentical hormones § Individualized compounded preparation, plant-derived, not regulated by FDA, marketed as natural, closer to endogenous hormones and therefore safer § 20% estradiol, 80% estriol (Biest), 10% estradiol, 10% estrone, 80% estriol (Triest) § Also DHEA, pregnelone, testosterone, progesterone § Progestin from Mexican wild yam is not bioavailable to humans – does not provide endometrial protection § No evidence that hormone levels in saliva are biologically meaningful (ACOG, CO#532) – imprecise, variable, don’t necessarily correlate with systemic concentrations § No evidence that custom mixes are more effective or safer, and run risk of unanticipated effects, also don’t include contraindication/warning labels and not tested for purity, potency, efficacy § Best to titrate to symptom control (since that’s why we prescribe it!), not to serum/urine/salivary levels § Estradiol and micronized progesterone are bioidentical (but not compounded)
Therapeutic options: supplements and herbal products § Evening primrose oil – not effective in RCTs § Dong quai (angelica) – not effective as monotherapy. Widely used in Chinese medicine in combination with other herbs, has not been tested in RCTs § Black cohosh (Remifemin)– effective in several studies, • One RCT showed more effective in women within 1 to 2 years of entering menopause, less so in women with 3 years of symptoms and FSH >40 IU/L. • One RCT found equally effective with topical estradiol • Little or no estrogenic activity, but overall effect on breast cancer is unknown, no demonstrated endometrial effect • Side effects uncommon – GI upset, dizziness, frontal H/A, bradycardia
Therapeutic options: supplements and herbal products (2) § Gingko – studied for treatment of age-related cognitive decline • Cochrane review 2009 – inconsistent evidence of benefit • Risk of bleeding complications in patients on anticoagulants § Ginseng – one RCT • no effect of vasomotor symptoms or endometrial thickness • Some improvement in depression, general health and wellbeing • Uncommon side effects – nervousness, insomnia, dizziness, HTN, mastalgia

Therapeutic options: supplements and herbal products (3) Kava § Used for treating anxiety, hot flashes, sleep disruption § Cochrane review in 2003 – effective for anxiety § Has been linked with severe hepatotoxicity § FDA issued a warning in 2002 – not to be used before consulting a physician. Banned in UK, Canada, Germany § Should not be used with alcohol, psychotropics, antihistamines, any sedative medications § Side effects (uncommon) – minor GI upset, H/A, sedation § Kava dermopathy – ichthyosiform eruption, yellowing – in heavy, chronic users
Therapeutic options: supplements and herbal products (4) § Sage • Not well-studied • Teas are safe, but extracts may contain toxic volatile oil thujone, associated with vomiting, vertigo, seizures and renal damage § St. John’s Wort • Used singly or combined with black cohosh • Cochrane review 2008 – similarly effective as antidepressants, fewer side effects • 300 -600 mg TID • Side effects – minor GI upset, fatigue, rare photosensitivity, may contribute to cataract formation • May decrease serum levels of warfarin, digoxin, theopylline, indinavir, cyclosporine.
Therapeutic options: supplements and herbal products (5) - Valerian § Used to treat nervousness and insomnia § Shown effective in RCTs § No significant side effects at recommended dosages § Little effect with single use, 5 -7 days needed for effectiveness § Long-term use may be associated with headache, restlessness, insomnia § Does not interact with alcohol § Dose is 50 – 100 mg of extract BID-TID

Therapeutic options: supplements and herbal products (6) - Vitex § Used for PMS, irregular cycles, cyclic mastalgia § RCTs have shown improvement in common menopausal symptoms, PMS, and luteal phase irregularities § Has been used to reduce heavy, irregular bleeding in menopause transition § Known as chasteberry and monk’s pepper because of (rare) libido-reducing effect. § Mild side effects – nausea, headache, acne, pruritis, rash § Dose 20 mg/day of extract
Therapeutic options: supplements and herbal products (7) – Soy and Isoflavones § Multiple RCTs for vasomotor symptoms with soy protein § Inconsistent results, but some have shown benefit in reducing hot flashes and night sweats, cognitive function § Red clover also a source of isoflavones – benefit noted in RCTs § Supplements of specific isoflavones, genistein and equol, have shown some reduction in hot flashes in RCTs. Effects on BMD are being studied § Study of 5, 000 breast cancer survivors in China showed soy protein/soy isoflavones inversely associated with recurrence and mortality
Therapeutic options: supplements and herbal products (8) – OTC Hormones § Progesterone creams, some made from wild yam plant • No data to support reduction in hot flashes in RCTs, but widely used § DHEA • Requires a prescription in Canada • Some benefits on cognitive function, results inconsistent • Frequently used to treat low libido and adrenal insufficiency at dose of 50 mg/day – converted to testosterone • Androgenic side effects (acne, facial hair) • Higher doses - jaundice, elevated LFTs, virilization, depression

Therapeutic options for vasomotor symptoms: lifestyle modifications § Keep body temperature cool • Layers, wicking fabrics • ice pack under pillow, fans § Maintain BMI < 27 § Avoid smoking § Regular exercise § Meditation/yoga/massage/lukewarm baths § Deep slow abdominal breathing (paced respiration, 5 -7/min) at onset of flash – maybe § Clinical hypnosis – one study of 5 45 -min sessions § Avoid personal triggers – hot drinks, caffeine, spicy food, alcohol
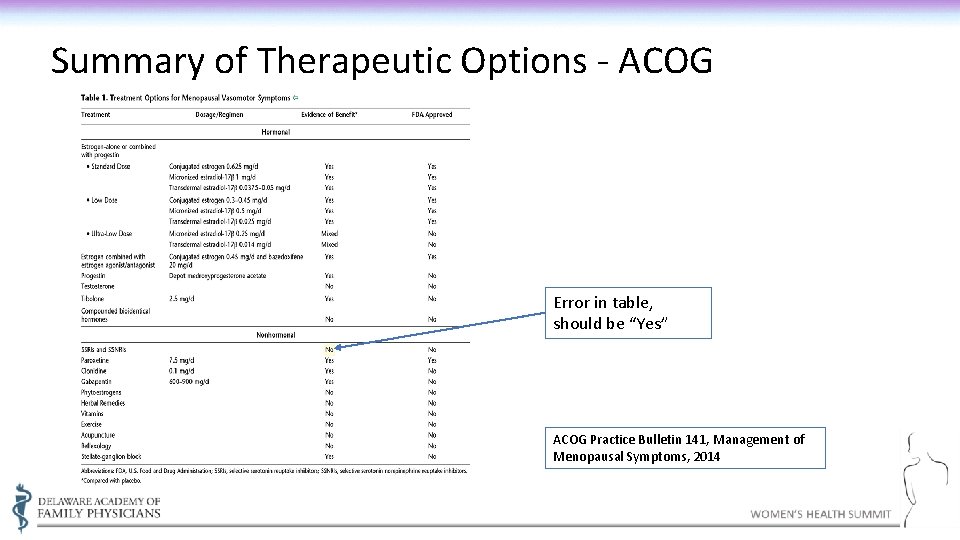
Summary of Therapeutic Options - ACOG Error in table, should be “Yes” ACOG Practice Bulletin 141, Management of Menopausal Symptoms, 2014

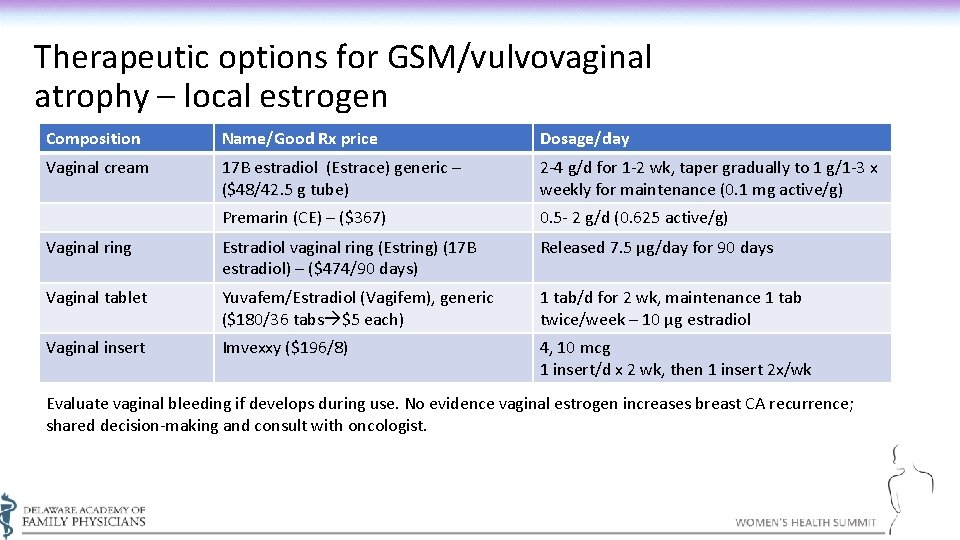
Therapeutic options for GSM/vulvovaginal atrophy – local estrogen Composition Name/Good Rx price Dosage/day Vaginal cream 17 B estradiol (Estrace) generic – ($48/42. 5 g tube) 2 -4 g/d for 1 -2 wk, taper gradually to 1 g/1 -3 x weekly for maintenance (0. 1 mg active/g) Premarin (CE) – ($367) 0. 5 - 2 g/d (0. 625 active/g) Vaginal ring Estradiol vaginal ring (Estring) (17 B estradiol) – ($474/90 days) Released 7. 5 µg/day for 90 days Vaginal tablet Yuvafem/Estradiol (Vagifem), generic ($180/36 tabs $5 each) 1 tab/d for 2 wk, maintenance 1 tab twice/week – 10 µg estradiol Vaginal insert Imvexxy ($196/8) 4, 10 mcg 1 insert/d x 2 wk, then 1 insert 2 x/wk Evaluate vaginal bleeding if develops during use. No evidence vaginal estrogen increases breast CA recurrence; shared decision-making and consult with oncologist.
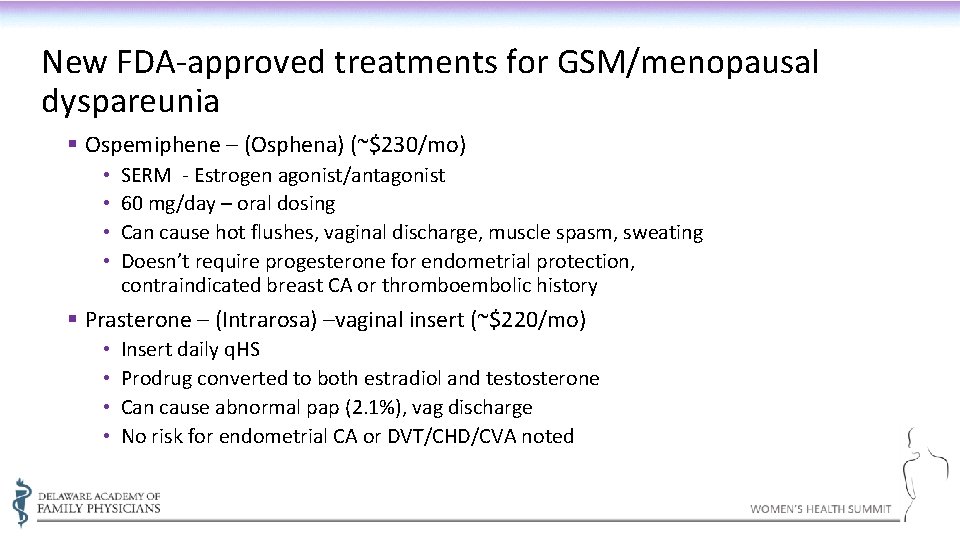
New FDA-approved treatments for GSM/menopausal dyspareunia § Ospemiphene – (Osphena) (~$230/mo) • • SERM - Estrogen agonist/antagonist 60 mg/day – oral dosing Can cause hot flushes, vaginal discharge, muscle spasm, sweating Doesn’t require progesterone for endometrial protection, contraindicated breast CA or thromboembolic history § Prasterone – (Intrarosa) –vaginal insert (~$220/mo) • • Insert daily q. HS Prodrug converted to both estradiol and testosterone Can cause abnormal pap (2. 1%), vag discharge No risk for endometrial CA or DVT/CHD/CVA noted

Vaginal lubricants and moisturizers for treating vulvovaginal symptoms § Lubricants • Used to treat dyspareunia, applied locally prior to intercourse • Water-based, silicone and oil-based, oil-based not safe with latex condom use • From the North American Menopause Society: Examples include: (Water-Based) - Astroglide, Fem. Glide, Just Like Me, K-Y Jelly, Slippery Stuff, Summer’s Eve (Silicone-based) – ID Millennium, Pink, Pjur, Pure Pleasure (Oil-based - avoid) – Mineral oil, Elegance Woman’s Lubricant § Moisturizers • Used to treat dryness, itching, irritation and dyspareunia, change p. H and elasticity Examples include: K-Y Silk-E, Moist Again, Replens ($15+/mo), Re. Phresh, • Beware Vagisil – benzocaine – irritant, can cause contact dermatitis
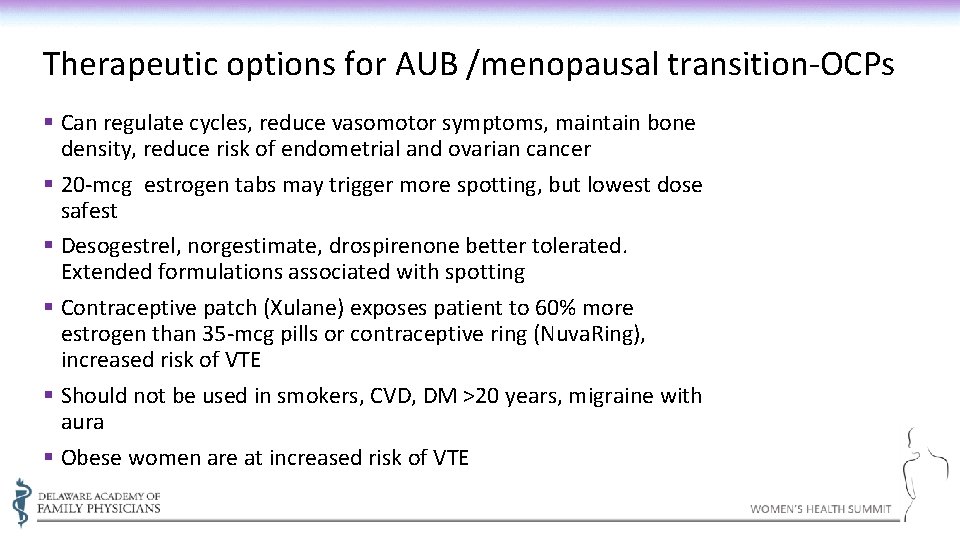
Therapeutic options for AUB /menopausal transition-OCPs § Can regulate cycles, reduce vasomotor symptoms, maintain bone density, reduce risk of endometrial and ovarian cancer § 20 -mcg estrogen tabs may trigger more spotting, but lowest dose safest § Desogestrel, norgestimate, drospirenone better tolerated. Extended formulations associated with spotting § Contraceptive patch (Xulane) exposes patient to 60% more estrogen than 35 -mcg pills or contraceptive ring (Nuva. Ring), increased risk of VTE § Should not be used in smokers, CVD, DM >20 years, migraine with aura § Obese women are at increased risk of VTE
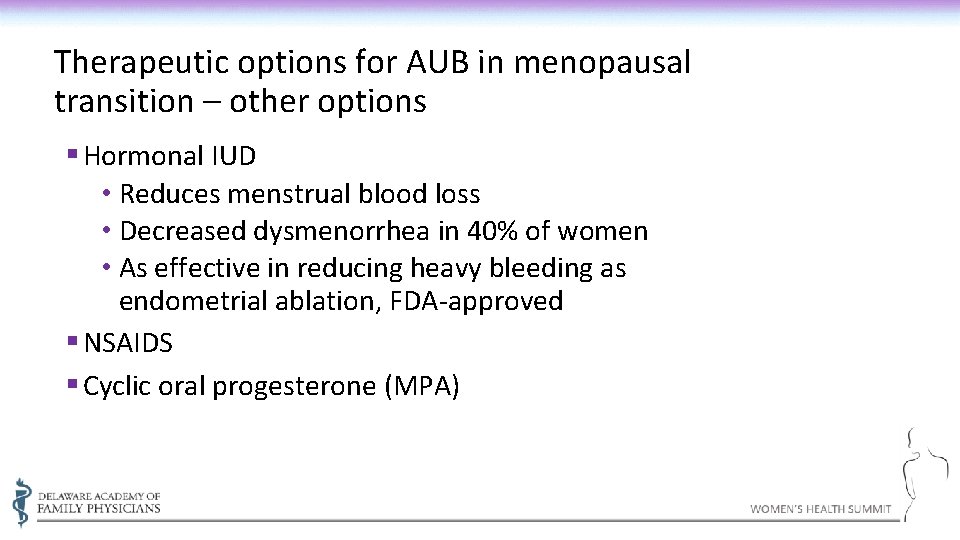
Therapeutic options for AUB in menopausal transition – other options § Hormonal IUD • Reduces menstrual blood loss • Decreased dysmenorrhea in 40% of women • As effective in reducing heavy bleeding as endometrial ablation, FDA-approved § NSAIDS § Cyclic oral progesterone (MPA)
Fertility § Pregnancy is possible until menopause occurs (2 -3% after age 45, 1% after age 50) § 25% cycles longer than 60 days are ovulatory § Overall, 40 -60% of cycles are anovulatory during STRAW -1 § Ovulatory cycles more commonly release multiple follicles (higher incidence of twins in midlife women)

Case L. O. is a 54 year old patient (she/hers) who previously had monthly menses for 5 days. Two years ago she noted that she went 2 -3 months between cycles, and her last menses was 14 months ago. She tells you that she is having “hot flashes” 5 -8 times per day, and that they are very disruptive. She is having difficulty sleeping because of them. She is periodically sexually active with one partner, and describes intercourse as sometimes painful, with noted vaginal dryness. She has a history of hypercholesterolemia and was recently started on a statin. She is normotensive and otherwise has no other chronic medical conditions. She is self-employed and has no health insurance.

POST-TEST QUESTIONS Answer these Polling Questions

References • Patient handouts: • https: //www. aafp. org/afp/2016/1201/p 884 -s 1. pdf, https: //familydoctor. org/condition/menopause/, https: //familydoctor. org/condition/perimenopause/ • North American Menopause Society: http: //www. menopause. org/for-women/expert-answers-to-frequently-asked-questionsabout-menopause • ACOG Practice Bulletin 141 – Management of Menopausal Symptoms, 1/2014, reaffirmed 2018 • ACOG Practice Bulletin 119 – Female Sexual Dysfunction, 4/2011, reaffirmed 2017 • ACOG Committee Opinion 556 – Postmenopausal Estrogen Therapy: Route of Administration and Risk of Venous Thromboembolism, 4/2013, reaffirmed 2017 • ACOG Committee Opinion 565 – Hormone Therapy and Heart Disease, 6/2013, reaffirmed 2018 • ACOG Committee Opinion 698 – Hormone Therapy in Primary Ovarian Insufficiency, 5/2017 • Menopause Practice: A Clinician’s Guide, 4 th Edition. North American Menopause Society, 2010 • Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal Hormone Therapy and Health Outcomes During the Intervention and Extended Poststopping Phases of the Women’s Health Initiative Randomized Trials. JAMA. 2013; 310(13): 1353– 1368. • Women’s Health Initiative reaffirms use of short-term hormone replacement therapy for younger women. National Institutes of Health News Release, Oct. 17, 2013

References (continued) • Cochrane Menopause Day – link to multiple Cochrane analyses of menopause therapies: https: //www. cochrane. org/news/worldmenopause-day • Avis NE, Crawford SL, Greendale G, et al. Duration of Menopausal Vasomotor Symptoms Over the Menopause Transition. JAMA Intern Med. 2015; 175(4): 531– 539. doi: 10. 1001/jamainternmed. 2014. 8063 • Study of Women’s Health Across the Nation: https: //www. swanstudy. org/up-to-14 -years-of-hot-flashes-found-in-menopausestudy/ • Lindahl S. H. (2014). Reviewing the options for local estrogen treatment of vaginal atrophy. International journal of women's health, 6, 307– 312. doi: 10. 2147/IJWH. S 52555 • The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 11, 1 November 2015, Pages 3975– 4011, https: //doi. org/10. 1210/jc. 2015 -2236 • Hill DA, Crider M. Hormone Therapy and Other Treatments for Symptoms of Menopause. American Family Physician 2016; 94(11): 884 -889. • Elkins GR, Fisher WI, Johnson AK et al; Clinical hypnosis in the treatment of postmenopausal hot flashes. Menopause 2013; 20(3): 291 -298. • Grady D, Sawaya GF SO Am J Med. Discontinuation of postmenopausal hormone therapy. 2005; 118 Suppl 12 B: 163. • American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. 08 October 2015. https: //doi. org/10. 1111/jgs. 13702 • Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1996: 275(5): 370 -75.

& QA Please use the Chat feature located either at the upper or lower portion of your screen.

Thank you Karen Antell, MD, MPH, FAAFP, NCMP kantell@christianacare. org
- Slides: 55