How to Switch from Clopidogrel to Prasugrel or







- Slides: 38

How to Switch from Clopidogrel to Prasugrel or Ticagrelor Jorge F. Saucedo M. D, MBA Professor of Medicine Director, Cardiac Catheterization Laboratories OU Medical Center Vice Chief, Section Of Cardiology OU Health Science Center

Jorge F. Saucedo, MD Honoraria: Merck and Company, Inc. Eli Lilly and Company Stocks, Stock Options, other ownership interest: Vascular Solutions

How to Switch from Clopidogrel to Prasugrel or Ticagrelor Clinical Data ¨ No studies published with clinical endpoints evaluating switching from clopidogrel to prasugrel or ticagrelor

Trials Comparing IPA Following the Switch from Clopidogrel to Prasugrel or to Ticagrelor ¨ TABF (healthy volunteers) ¨ PRINCIPLE-TIMI 44 (elective PCI) ¨ ACAPULCO (NSTE-ACS patients undergoing PCI) ¨ SWAP (recent ACS history) ¨ TRIPLET (ACS patients undergoing PCI) ¨ RESPOND ¨ PLATO (pre specified subgroup analysis)★

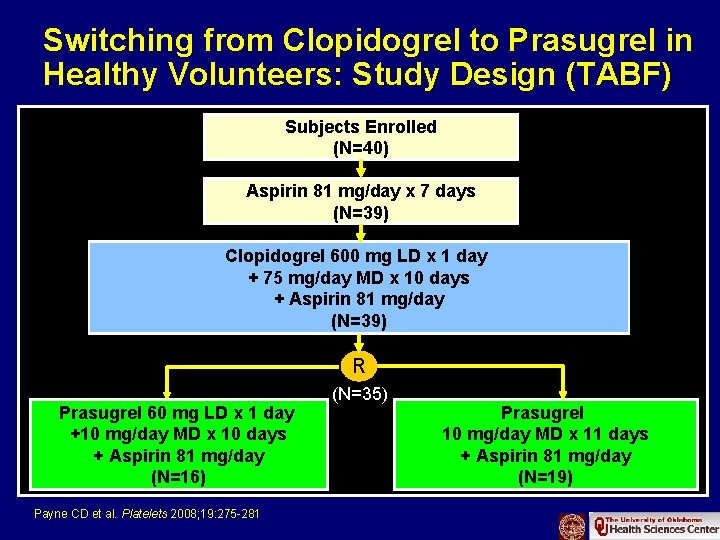
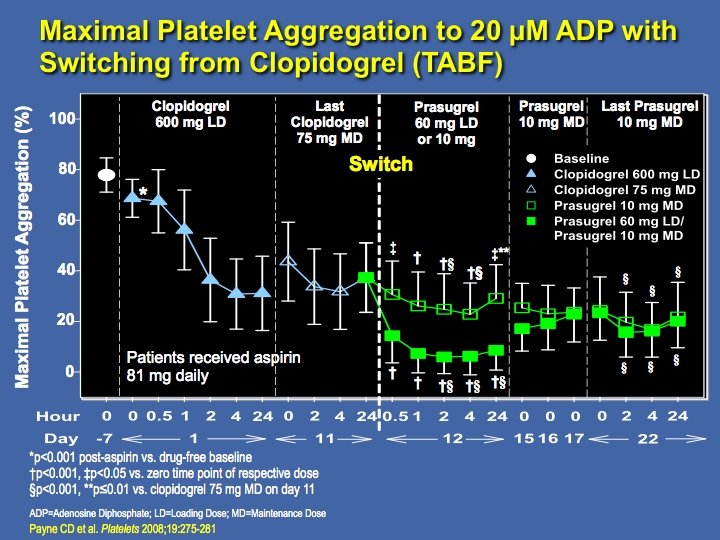
Switching from Clopidogrel to Prasugrel in Healthy Volunteers: Study Design (TABF) Subjects Enrolled (N=40) Aspirin 81 mg/day x 7 days (N=39) Clopidogrel 600 mg LD x 1 day + 75 mg/day MD x 10 days + Aspirin 81 mg/day (N=39) R (N=35) Prasugrel 60 mg LD x 1 day Prasugrel +10 mg/day MD x 10 days 10 mg/day MD x 11 days + Aspirin 81 mg/day (N=16) ADP=Adenosine Diphosphate; LD=Loading Dose; MD=Maintenance Dose (N=19) Payne CD et al. Platelets 2008; 19: 275 -281


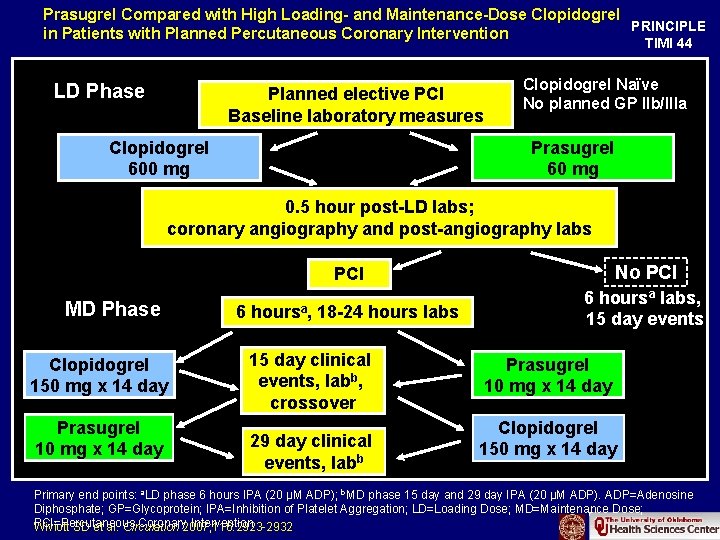
Prasugrel Compared with High Loading- and Maintenance-Dose Clopidogrel PRINCIPLE in Patients with Planned Percutaneous Coronary Intervention TIMI 44 LD Phase Planned elective PCI Baseline laboratory measures Clopidogrel 600 mg Clopidogrel Naïve No planned GP IIb/IIIa Prasugrel 60 mg 0. 5 hour post-LD labs; coronary angiography and post-angiography labs MD Phase Clopidogrel 150 mg x 14 day Prasugrel 10 mg x 14 day PCI No PCI 6 hoursa, 18 -24 hours labs 6 hoursa labs, 15 day events 15 day clinical events, labb, crossover 29 day clinical events, labb Prasugrel 10 mg x 14 day Clopidogrel 150 mg x 14 day Primary end points: a. LD phase 6 hours IPA (20 µM ADP); b. MD phase 15 day and 29 day IPA (20 µM ADP). ADP=Adenosine Diphosphate; GP=Glycoprotein; IPA=Inhibition of Platelet Aggregation; LD=Loading Dose; MD=Maintenance Dose; PCI=Percutaneous Coronary 2007; 116: 2923 -2932 Intervention Wiviott SD et al. Circulation

PRINCIPLE-TIMI 44 Crossover Trial: Loading & Maintenance Dose Phases IPA (20 µM ADP) Primary efficacy end point was IPA at 6 hours. Any difference in the pharmacokinetics of prasugrel compared with other antiplatelet agents has not been correlated to clinical outcomes. ADP=Adenosine Diphosphate; IPA=Inhibition of Platelet Aggregation; PRINCIPLE=The Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation; TIMI=Thrombolysis In Myocardial Infarction Wiviott SD et al. Circulation 2007; 116: 2923 -2932

Primary End Point: MD Phase IPA (20 µM ADP) PRINCIPLE TIMI 44 ADP=Adenosine Diphosphate; CV=Cardiovascular; IPA=Inhibition of Platelet Aggregation; MD=Maintenance Dose; MI=Myocardial Infarction; NF=Nonfatal l. Wiviott SD et al. Circulation 2007; 116: 2923 -2932

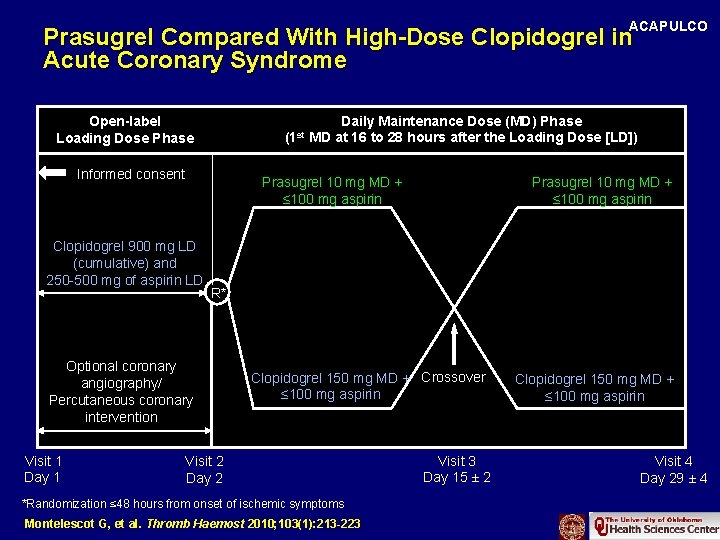
ACAPULCO Prasugrel Compared With High-Dose Clopidogrel in Acute Coronary Syndrome Open-label Loading Dose Phase (1 st Informed consent Prasugrel 10 mg MD + ≤ 100 mg aspirin Clopidogrel 900 mg LD (cumulative) andand 250250 -500 of aspirin 500 mgmg of aspirin LD LD Prasugrel 10 mg MD + ≤ 100 mg aspirin R* Optional coronary angiography/ Percutaneous coronary intervention Visit 1 Day 1 Daily Maintenance Dose (MD) Phase MD at 16 to 28 hours after the Loading Dose [LD]) Clopidogrel 150 mg MD + Crossover ≤ 100 mg aspirin Visit 2 Day 2 *Randomization ≤ 48 hours from onset of ischemic symptoms Montelescot G, et al. Thromb Haemost 2010; 103(1): 213 -223 Visit 3 Day 15 ± 2 Clopidogrel 150 mg MD + ≤ 100 mg aspirin Visit 4 Day 29 ± 4

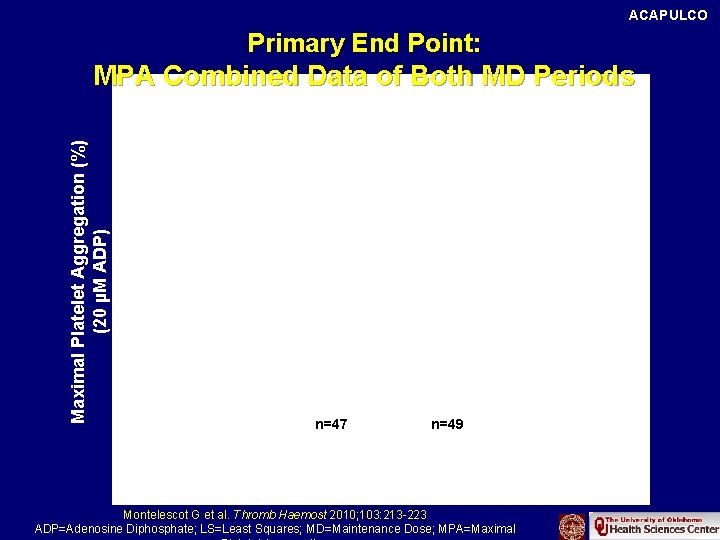
ACAPULCO Primary End Point: Maximal Platelet Aggregation (%) (20 µM ADP) MPA Combined Data of Both MD Periods LS Means p<0. 001 39. 1 26. 2 n=47 n=49 Clopidogrel 150 mg Prasugrel 10 mg Montelescot G et al. Thromb Haemost 2010; 103: 213 -223 ADP=Adenosine Diphosphate; LS=Least Squares; MD=Maintenance Dose; MPA=Maximal

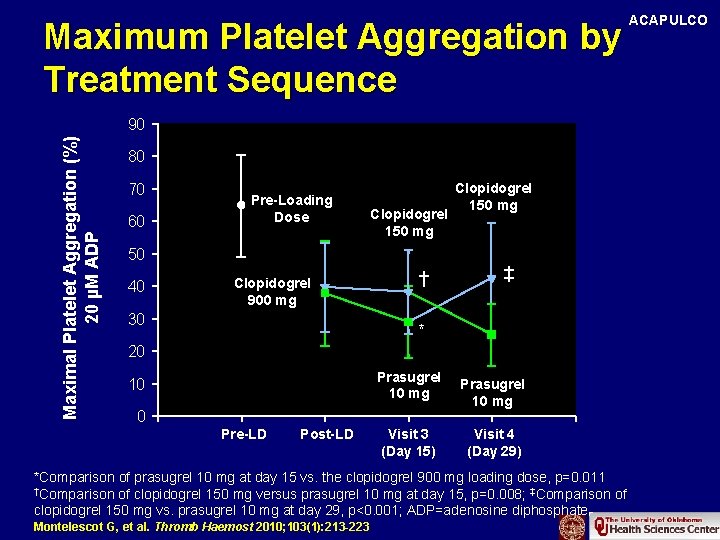
Maximum Platelet Aggregation by Treatment Sequence Maximal Platelet Aggregation (%) 20 µM ADP 90 80 70 60 Pre-Loading Dose Clopidogrel 150 mg 50 40 Clopidogrel 900 mg 30 † Clopidogrel 150 mg ‡ * 20 Prasugrel 10 mg 10 0 Pre-LD Post-LD Visit 3 (Day 15) Prasugrel 10 mg Visit 4 (Day 29) *Comparison of prasugrel 10 mg at day 15 vs. the clopidogrel 900 mg loading dose, p=0. 011 †Comparison of clopidogrel 150 mg versus prasugrel 10 mg at day 15, p=0. 008; ‡Comparison of clopidogrel 150 mg vs. prasugrel 10 mg at day 29, p<0. 001; ADP=adenosine diphosphate Montelescot G, et al. Thromb Haemost 2010; 103(1): 213 -223 ACAPULCO

Increased Platelet Inhibition After Switching From Maintenance Clopidogrel to Prasugrel in Patients with Acute Coronary Syndromes: Results of the SWAP (SWitching Anti Platelet) Study The SWAP Study

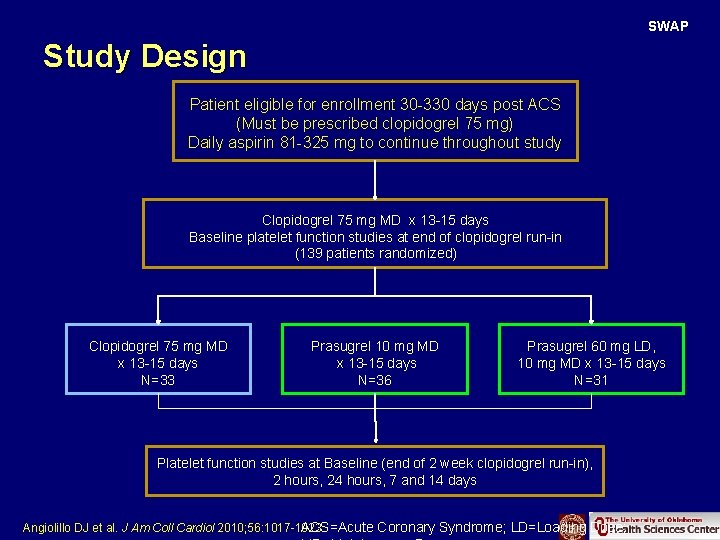
SWAP Study Design Patient eligible for enrollment 30 -330 days post ACS (Must be prescribed clopidogrel 75 mg) Daily aspirin 81 -325 mg to continue throughout study Clopidogrel 75 mg MD x 13 -15 days Baseline platelet function studies at end of clopidogrel run-in (139 patients randomized) Clopidogrel 75 mg MD x 13 -15 days N=33 Prasugrel 10 mg MD x 13 -15 days N=36 Prasugrel 60 mg LD, 10 mg MD x 13 -15 days N=31 Platelet function studies at Baseline (end of 2 week clopidogrel run-in), 2 hours, 24 hours, 7 and 14 days ACS=Acute Coronary Syndrome; LD=Loading Dose; Angiolillo DJ et al. J Am Coll Cardiol 2010; 56: 1017 -1023

SWAP Results: MPA at Various Time Points Following Loading Dose of Study Drug 20 µM ADP Mean ± SD ADP=Adenosine Diphosphate; LD=Loading Dose; MD=Maintenance Dose; MPA=Maximal Platelet Aggregation; SD=Standard Deviation *p < 0. 0001 vs clopidogrel 75 mg MD; †p < 0. 0001 vs prasugrel 10 mg MD Angiolillo DJ et al. JACC 2010; 56: 1017 -1023

Verify. Now® SWAP Results: P 2 Y 12 Assay Following Loading Dose of Study Drug Mean ± SD LD=Loading Dose; MD=Maintenance Dose; PRU=P 2 Y 12 Reaction Units *P<0. 0001 vs clopidogrel 75 mg MD; †P<0. 0001 vs prasugrel 10 mg MD Angiolillo DJ et al. JACC 2010; 56: 1017 -1023

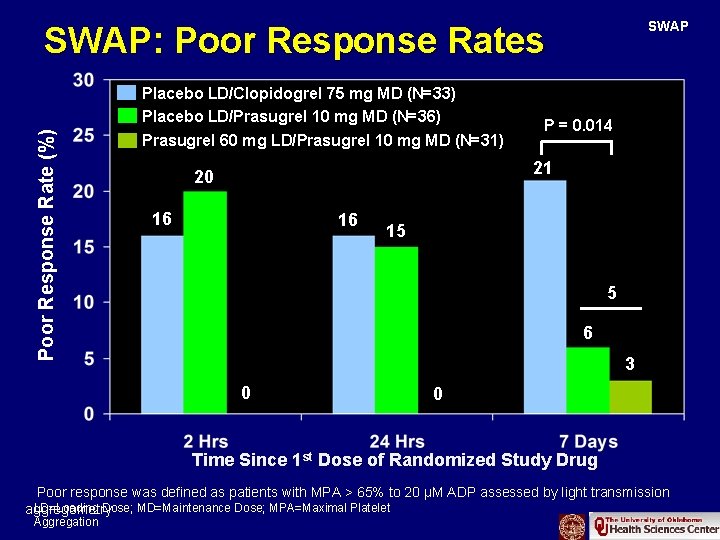
SWAP Poor Response Rate (%) SWAP: Poor Response Rates Placebo LD/Clopidogrel 75 mg MD (N=33) Placebo LD/Prasugrel 10 mg MD (N=36) Prasugrel 60 mg LD/Prasugrel 10 mg MD (N=31) P = 0. 014 21 20 16 16 15 5 6 3 0 0 Time Since 1 st Dose of Randomized Study Drug Poor response was defined as patients with MPA > 65% to 20 µM ADP assessed by light transmission LD=Loading Dose; MD=Maintenance Dose; MPA=Maximal Platelet aggregometry Aggregation

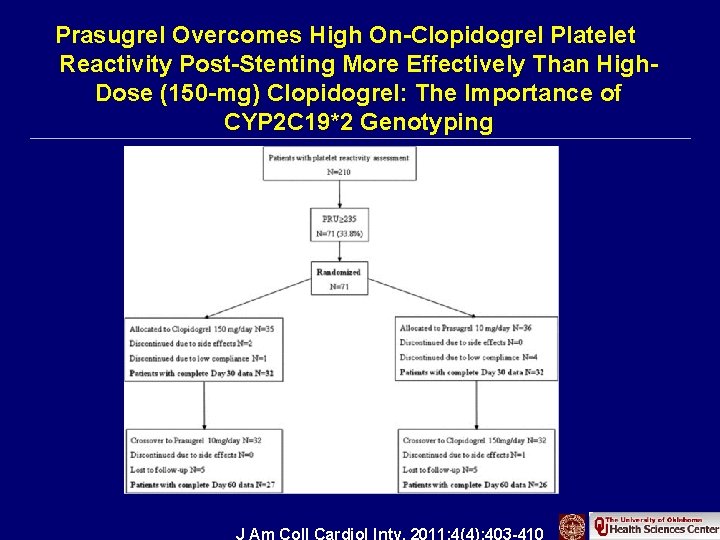
Prasugrel Overcomes High On-Clopidogrel Platelet Reactivity Post-Stenting More Effectively Than High. Dose (150 -mg) Clopidogrel: The Importance of CYP 2 C 19*2 Genotyping J Am Coll Cardiol Intv. 2011; 4(4): 403 -410. doi: 10. 1016/j. jcin. 2010. 12. 011 J Am Coll Cardiol Intv. 2011; 4(4): 403 -410

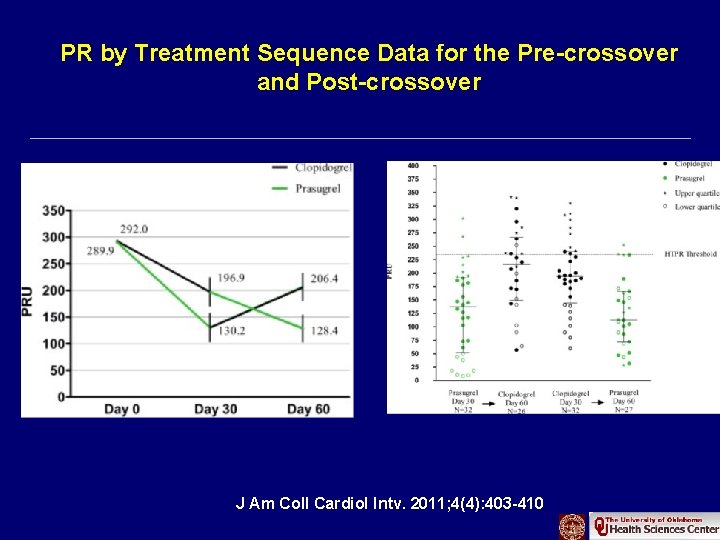
PR by Treatment Sequence Data for the Pre-crossover and Post-crossover J Am Coll Cardiol Intv. 2011; 4(4): 403 -410

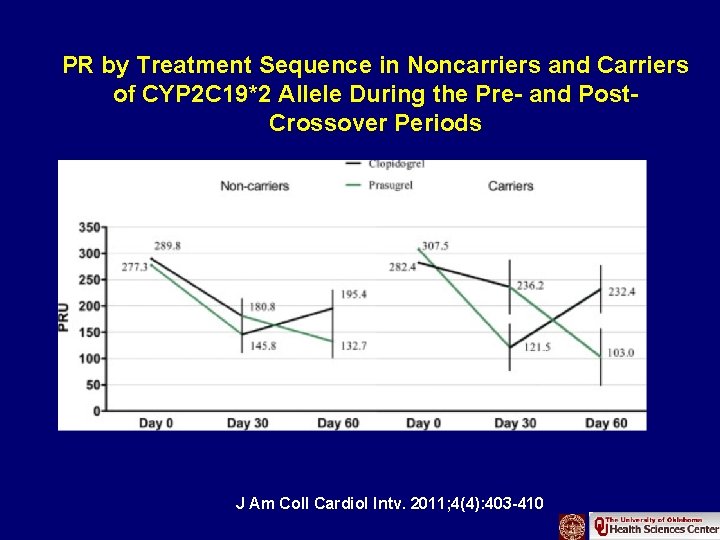
PR by Treatment Sequence in Noncarriers and Carriers of CYP 2 C 19*2 Allele During the Pre- and Post. Crossover Periods J Am Coll Cardiol Intv. 2011; 4(4): 403 -410

Comparison of Platelet Inhibition Following a Prasugrel 60 mg or Prasugrel 30 mg LD with or without Pretreatment with a Clopidogrel LD in ACS Subjects undergoing PCI TRIPLET (H 7 T-CR-TAEH) Study

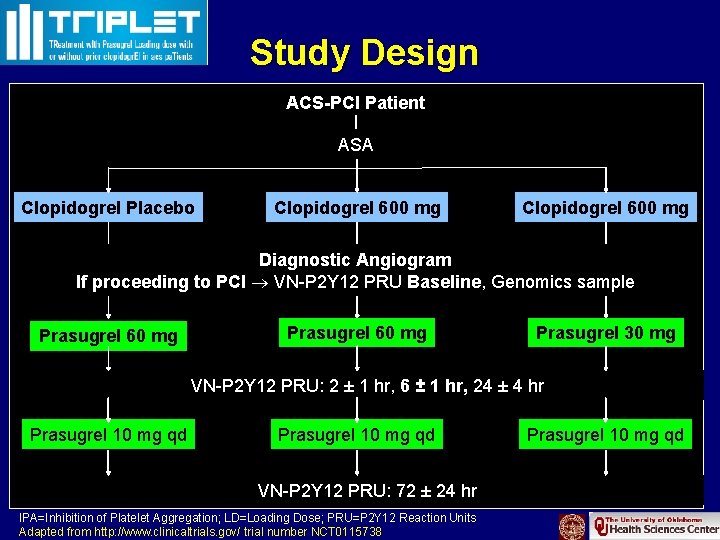
Study Design ACS-PCI Patient ASA Clopidogrel Placebo Clopidogrel 600 mg Diagnostic Angiogram If proceeding to PCI VN-P 2 Y 12 PRU Baseline, Genomics sample Prasugrel 60 mg Prasugrel 30 mg VN-P 2 Y 12 PRU: 2 ± 1 hr, 6 ± 1 hr, 24 ± 4 hr Prasugrel 10 mg qd 26 Prasugrel 10 mg qd VN-P 2 Y 12 PRU: 72 ± 24 hr IPA=Inhibition of Platelet Aggregation; LD=Loading Dose; PRU=P 2 Y 12 Reaction Units Adapted from http: //www. clinicaltrials. gov/ trial number NCT 0115738 Prasugrel 10 mg qd

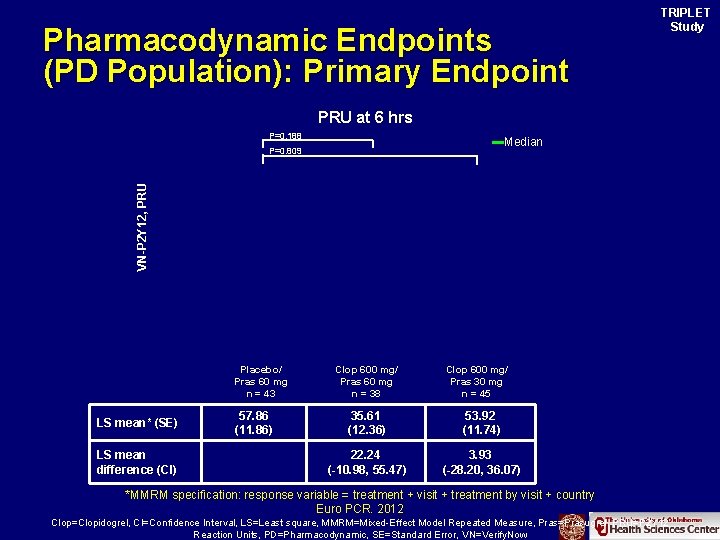
Pharmacodynamic Endpoints (PD Population): Primary Endpoint TRIPLET Study PRU at 6 hrs P=0. 188 Median VN-P 2 Y 12, PRU P=0. 809 Placebo/ Pras 60 mg n = 43 LS mean* (SE) LS mean difference (CI) 57. 86 (11. 86) Clop 600 mg/ Pras 60 mg n = 38 Clop 600 mg/ Pras 30 mg n = 45 35. 61 (12. 36) 53. 92 (11. 74) 22. 24 (-10. 98, 55. 47) 3. 93 (-28. 20, 36. 07) *MMRM specification: response variable = treatment + visit + treatment by visit + country Euro PCR. 2012 Clop=Clopidogrel, CI=Confidence Interval, LS=Least square, MMRM=Mixed-Effect Model Repeated Measure, Pras=Prasugrel, PRU=P 2 Y 12 Reaction Units, PD=Pharmacodynamic, SE=Standard Error, VN=Verify. Now

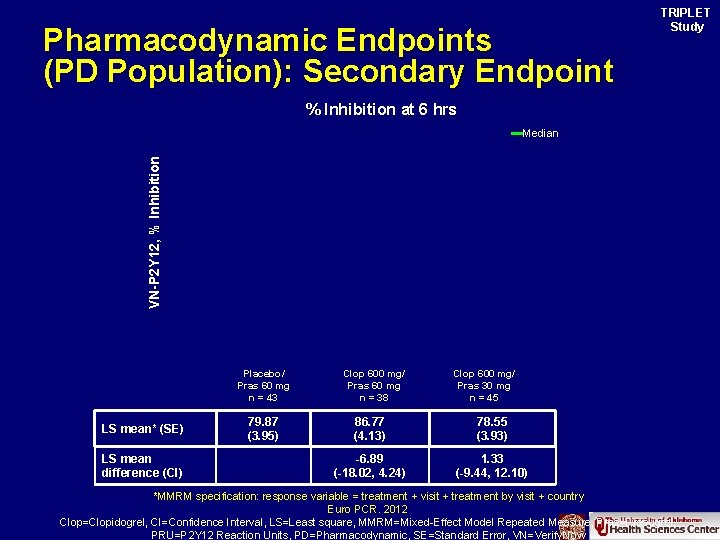
Pharmacodynamic Endpoints (PD Population): Secondary Endpoint TRIPLET Study % Inhibition at 6 hrs VN-P 2 Y 12, % Inhibition Median Placebo/ Pras 60 mg n = 43 LS mean* (SE) LS mean difference (CI) 79. 87 (3. 95) Clop 600 mg/ Pras 60 mg n = 38 Clop 600 mg/ Pras 30 mg n = 45 86. 77 (4. 13) 78. 55 (3. 93) -6. 89 (-18. 02, 4. 24) 1. 33 (-9. 44, 12. 10) *MMRM specification: response variable = treatment + visit + treatment by visit + country Euro PCR. 2012 Clop=Clopidogrel, CI=Confidence Interval, LS=Least square, MMRM=Mixed-Effect Model Repeated Measure, Pras=Prasugrel, PRU=P 2 Y 12 Reaction Units, PD=Pharmacodynamic, SE=Standard Error, VN=Verify. Now

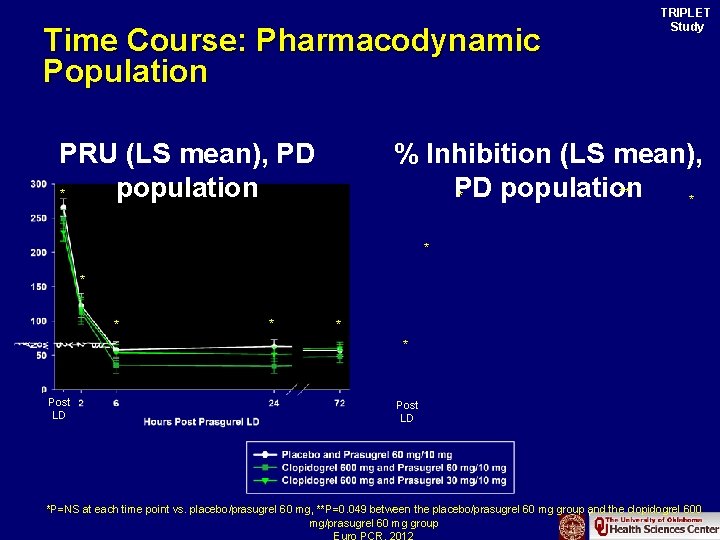
Time Course: Pharmacodynamic Population PRU (LS mean), PD population * TRIPLET Study % Inhibition (LS mean), PD population ** * * * * Post LD *P=NS at each time point vs. placebo/prasugrel 60 mg, **P=0. 049 between the placebo/prasugrel 60 mg group and the clopidogrel 600 mg/prasugrel 60 mg group Euro PCR. 2012

TRIPLET Study Safety: Bleeding ¨ 106 TEAEs in 276 patients • 14 hemorrhagic events in 12 patients were reported - 3: placebo LD/prasugrel 60 mg LD - 3: clopidogrel 600 mg LD/prasugrel 60 mg LD - 6: clopidogrel 600 mg LD/prasugrel 30 mg LD • 2 patients experienced a Hgb change from baseline (decrease of ≥ 3 g/d. L) - Neither of these patients received a prasugrel LD as both were discontinued and referred for CABG surgery ¨ 3 bleeding SAEs in 2 patients, one of which was related to study drug (hematemesis) Euro PCR. 2012 CABG=Coronary Artery Bypass Surgery, TEAES=Treatment Emergent Adverse Events, LD=Loading Dose,

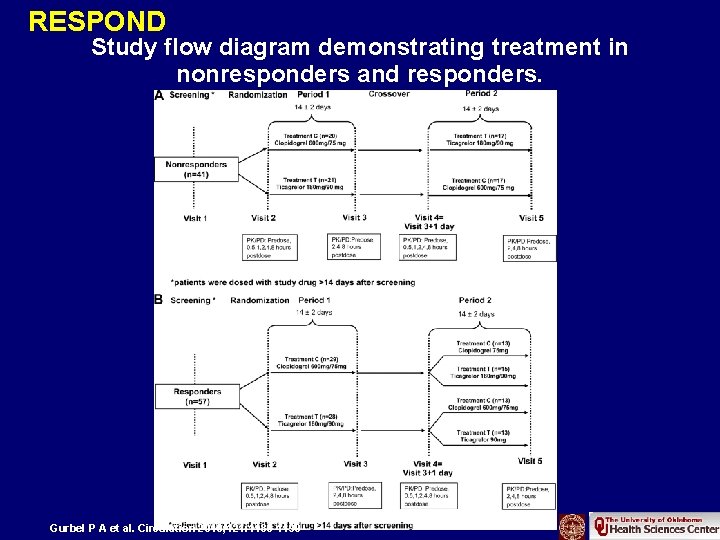
RESPOND Study flow diagram demonstrating treatment in nonresponders and responders. Gurbel P A et al. Circulation 2010; 121: 1188 -1199

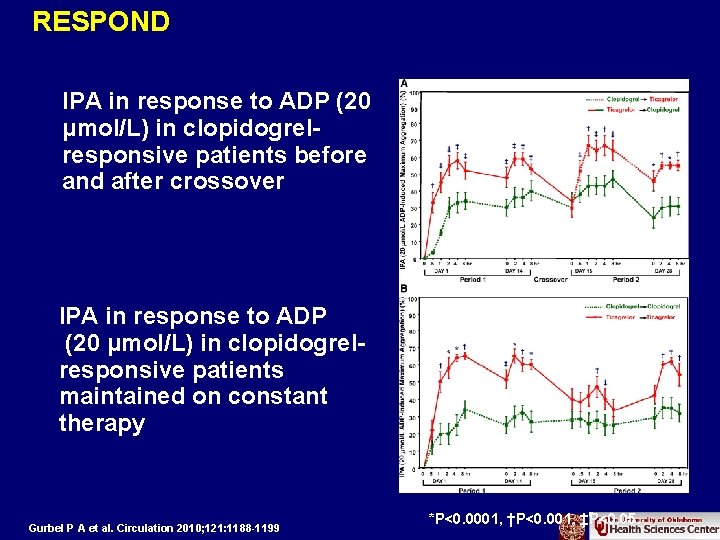
RESPOND IPA in response to ADP (20 μmol/L) in clopidogrelresponsive patients before and after crossover IPA in response to ADP (20 μmol/L) in clopidogrelresponsive patients maintained on constant therapy Gurbel P A et al. Circulation 2010; 121: 1188 -1199 *P<0. 0001, †P<0. 001, ‡P<0. 05.

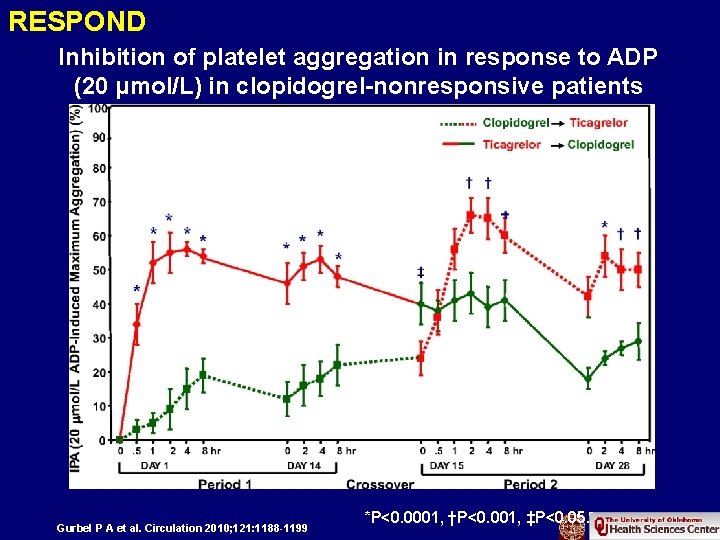
RESPOND Inhibition of platelet aggregation in response to ADP (20 μmol/L) in clopidogrel-nonresponsive patients Gurbel P A et al. Circulation 2010; 121: 1188 -1199 *P<0. 0001, †P<0. 001, ‡P<0. 05.

RESPOND Nonresponders Cohort: • The proportion of clopidogrel nonresponders who achieved >10% final extent IPA on ticagrelor and clopidogrel was similar • A greater proportion of clopidogrel nonresponders achieved >10% maximum extent IPA on ticagrelor compared with clopidogrel (100% vs. 75% of patients, respectively; p=0. 005)

RESPOND • A greater proportion of clopidogrel nonresponders achieved >30% and >50% maximum extent IPA on ticagrelor (75% and 13% of patients, respectively; p<0. 001) versus clopidogrel (13% and 0% of patients, respectively; p=0. 046) • When switched from clopidogrel to ticagrelor, platelet aggregation decreased from 59± 9% to 35± 11% (p<0. 0001) • When switched from ticagrelor to clopidogrel, platelet aggregation increased from 36± 14% to 56± 9% (p <0. 0001).

PLATO Clopidogrel Received Prior to Index Event ¨ Clopidogrel-exposed patients, both those on maintenance treatment with clopidogrel and those who received an open-label loading dose of clopidogrel prior to randomization, were eligible for inclusion into the PLATO trial ¨ PLATO was not designed or powered to demonstrate the efficacy or safety of ticagrelor compared with clopidogrel specifically in those patients who received clopidogrel prior to randomization into the PLATO trial.

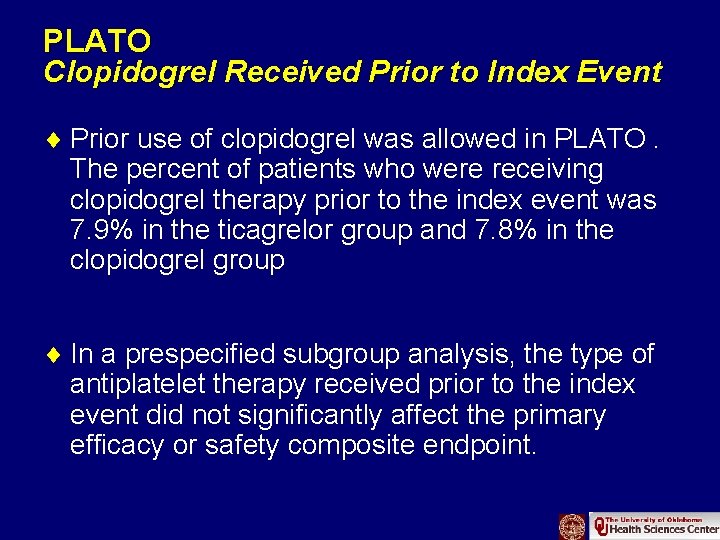
PLATO Clopidogrel Received Prior to Index Event ¨ Prior use of clopidogrel was allowed in PLATO. The percent of patients who were receiving clopidogrel therapy prior to the index event was 7. 9% in the ticagrelor group and 7. 8% in the clopidogrel group ¨ In a prespecified subgroup analysis, the type of antiplatelet therapy received prior to the index event did not significantly affect the primary efficacy or safety composite endpoint.

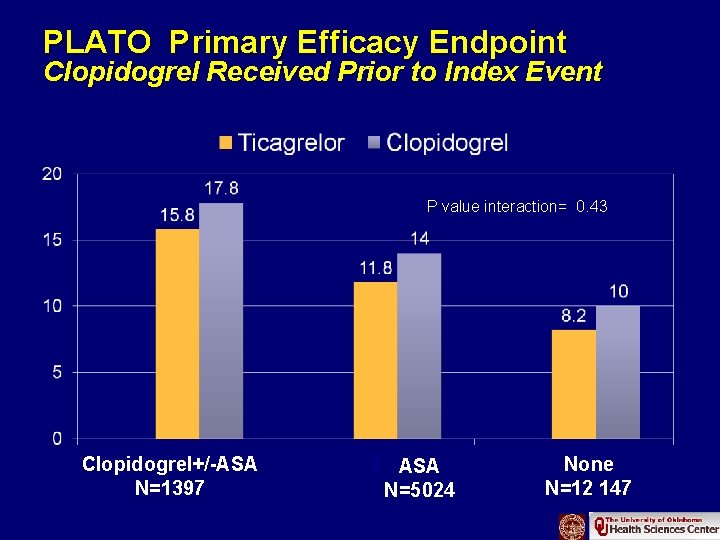
PLATO Primary Efficacy Endpoint Clopidogrel Received Prior to Index Event P value interaction= 0. 43 Clopidogrel+/-ASA N=1397 ASA N=5024 None N=12 147

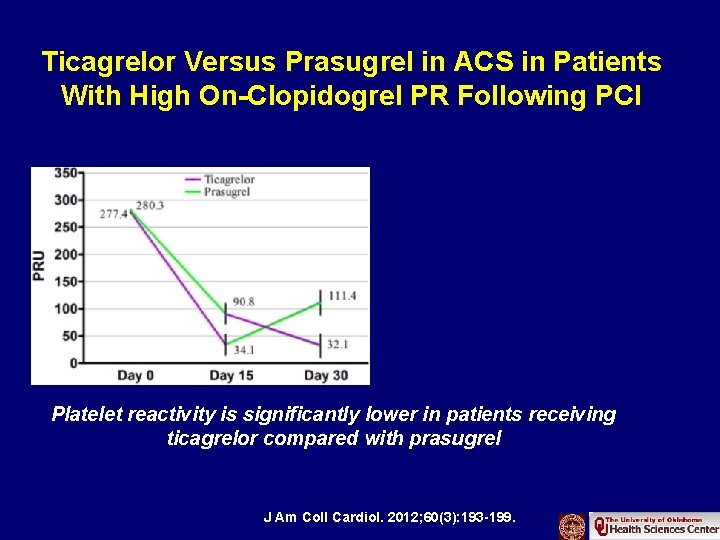
Ticagrelor Versus Prasugrel in Acute Coronary Syndrome Patients With High On-Clopidogrel Platelet Reactivity Following Percutaneous Coronary Intervention: A Pharmacodynamic Study J Am Coll Cardiol. 2012; 60(3): 193 -199.

Ticagrelor Versus Prasugrel in ACS in Patients With High On-Clopidogrel PR Following PCI Platelet reactivity is significantly lower in patients receiving ticagrelor compared with prasugrel J Am Coll Cardiol. 2012; 60(3): 193 -199.

How to Switch from Clopidogrel to Ticagrelor ¨ Patients can be transitioned from clopidogrel to ticagrelor without interruption of antiplatelet effect ¨ Patients with ACS who have received a LD of clopidogrel may be started on ticagrelor ( 180 mg LD and continue treatment with 90 mg twice daily) ¨ Transitioning from clopidogrel to ticagrelor resulted in an absolute IPA increase of 26. 4% and from ticagrelor to clopidogrel resulted in an absolute IPA decrease of 24. 5% ¨ In ACS patients with HTPR on clopidogrel post PCI, ticagrelor produces higher platelet inhibition when compared to prasugrel

How to Switch from Clopidogrel to Prasugrel ¨ Patients can be transitioned from MD clopidogrel to MD prasugrel without interruption of antiplatelet effect ¨ Transitioning from MD clopidogrel to LD prasugrel results in profound IPA and “acute abolition” of poor clopidogrel responders ¨ Platelet reactivity with Prasugrel 60 mg LD added to clopidogrel 600 mg LD was no different than Prasugrel 60 mg alone in patients with ACS undergoing PCI ¨ In patients with HTPR after PCI, prasugrel is more effective compared to high clopidogrel in reducing PR, particularly in CYP 2 C 119*2